Optimization of Parameters for Melt Crystallization of p-Cresol*
2012-03-22CONGShan丛山LIXingang李鑫钢WUJun邬俊andXUChangchun许长春
CONG Shan (丛山), LI Xingang (李鑫钢), WU Jun (邬俊) and XU Changchun (许长春), **
1 School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China
2 National Engineering Research Center of Distillation Technology, Tianjin University, Tianjin 300072, China
1 INTRODUCTION
p-Cresol is an important organic raw material and its further process produces a series of derivatives.p-Cresol and its derivatives are widely used in medicine,agricultural chemicals, dyestuff, spice, cosmetics, food,polymer material, etc. Recently, more high-purity p-cresol is needed in international and domestic markets. Therefore, the separation and purification of crude p-cresol call for a high attention. At present, from various synthetic methods the products are basically a mixture of o-, m-, and p-cresol. Further separation and purification are needed to obtain the required methyl phenol. By conventional distillation, o-cresol can be effectively separated from the mixed cresol. However, the boiling points of m-cresol and p-cresol are very close, with a difference of 0.3 °C only, so it is difficult to obtain highpurity p-cresol by conventional distillation method.
The melt crystallization, a clean technology with low energy consumption for the separation of organics(e.g. close boiling hydrocarbons, isomers, heat sensible materials) without using solvent [1], has found its wide applications in chemical, pharmaceutical, food and material industries. The principle of melt crystallization is to cool a melt in a controlled way to crystallize its fraction [2]. Studies have showed that this technology can serve as an appealing alternative to conventional separation processes, such as distillation and adsorption, particularly when a high purity of product is desired [3, 4]. It differs from other crystallization methods in that the operating temperature is close to the melting temperature of the main component, which enables the use of extra purification methods around the melting temperature (e.g. washing with the pure melts and sweating) and prevents the degradation of thermally sensitive substances [5].
This technology has been used for the refining of a number of compounds such as naphthalene, bisphenol, benzene dichloride and mono-chloroacetic acid[6], but its application for industrial purification of p-cresol (widely used as disinfectant and deodorizer)is less reported. The reason may be that the scale-up of melt crystallization is limited by the difficulty in determination of operation parameters in the process design [5]. A detailed knowledge of these parameters(e.g. crystallization time, cooling rate, sweating time)is useful for the optimization of industrial crystallization processes. Generally, purification efficiency of dynamic processes is much higher than that of static processes.For example, a pulsation of aeration to the melt enhances the crystallization efficiency, especially when the impurity content in the feed is low [7]. However, it remains unclear to which extent the aeration flux (or gas velocity), which affects turbulent flow and thereby the heat and mass transfer in a dynamic melt crystallization process, influences the separation and purification of p-cresol. A suitable aeration condition would allow high degree of separation and low consumption of energy and resource. Meanwhile, the cooling rate in the process needs to be optimized as a high cooling rate may cause the crystals to grow rapidly, leading to imperfect crystals and a highly porous layer containing a relatively high volumetric fraction of melt [8].Additionally, the sweating process, which is a special operation in melt crystallization and used to partially melt the crystal layer to get rid of fluid impurities, is a temperature induced purification step. Parameters to be considered include sweating temperature, sweating time and temperature increasing rate.
In this study, we report the optimal operation parameters including gas velocity, cooling rate, crystallization rate, operating time and temperature in sweating process for melt crystallization of p-cresol, which are necessary information for the design of industrial purification.
2 EXPERIMENTAL
2.1 Experimental set up

Figure 1 Experimental set-up for p-cresol crystallization
The p-cresol crystallization was carried out in a crystallizer, which was made of glass and fitted with crystallization tube (Ф=50 mm), gas tube (Ф=10 mm) and jacket tube (Fig. 1). The time reply and solenoid valve were used to control pulse-bubbling. Water circulation system was set by connecting the jacket tube with cold water tank and thermostatic water bath.Cold or hot water was pumped through the system,which was manually controlled by valves.
3.1 Crystallization process
The meltp-cresol was loaded into the crystallizer in batch, where hot water was pumped through the water circulation system to keep thep-cresol at melt state for 15 min. Then the crystallizer was cooled down from 40 °C at 0.4-1.5 °C·min-1by cool water bath. Nitrogen was fed through the gas tube at 5 min after the crystal formed in the crystallization tube.When the crystal scale reached a specified value, nitrogen was shut off. The mother liquor was collected from the bottom of the crystallizer by opening the bottom valve. After the crystallization for about 40 min, sweating process was carried out by increasing water temperature from 25 °C to 36 °C at heating rate of 0.2-0.5 °C·min-1. The sweated liquid was collected at the bottom of the crystallizer. The bottom valve was then shut off and water temperature was increased until all thep-cresol remaining in the crystallizer was melted. The bottom valve was open again and the final product was collected for analysis.
2.2 Analytical method
The concentration ofp-cresol in feed, mother liquor, sweated liquid and final product were determined by Agilent gas chromatography equipped with a flame ionization detector (GC-FID). N2(1 ml·min-1)was used as the carrier gas. Splitless injection with a sample volume of 1 μl and the injector temperature of 230 °C were applied. The oven and FID detector temperature was set as 135 °C and 220 °C, respectively.The crystal separation degree (CSD) was determined by the concentration difference betweenp-cresol in final product and in mother liquor.
3 RESULTS AND DISCUSSION
3.1.1Influence of aeration on crystal separation degreeThe CSD under different aeration conditions is shown in Fig. 2. The aeration is beneficial to crystallization compared with static operation without aeration, so the CSD is improved from 0.7% to 1.9% [Fig.2 (a)]. This may be due to that the increased pressure after aeration enhances the heat and mass transfer near the crystal layer, improving the product purity. The CSD is also influenced by aeration mode and gas velocity [Fig. 2 (b)]. Generally, CSD with the pulsation is 28% to 73% higher than that in continuous mode,attributed to the formation of Taylor bubble in the
pulsed aeration mode [9]. Although the turbulence is strengthened and local dynamic pressure is increased by continuous aeration, the major influence region is only near the central gas tube while the pressure near the inner wall of the crystallizer is still relatively low.In contrast, the dynamic pressure distribution resulted from Taylor bubble is more stable and the high pressure region extends to the inner wall of the crystallizer with the pulsed aeration applied. Moreover, the extrusion between bubbles leads to the formation of liquid membrane zone, which attenuates the laminar inner layer between the melted and crystalloidp-cresol. This would increase the heat and mass transfer rates by decreasing the thickness of boundary layer. Therefore,pulsed aeration is more preferable than continuous aeration. In both aeration modes, the CSD increases and then decreases with the increase of gas velocity[Fig. 2 (b)]. The optimal gas velocity is found at 90 L·h-1, with which the CSD is 2.6% and 1.9% in pulsed and continuous mode, respectively. Higher gas velocity (>90 L·h-1) is adverse for crystallization because (i)exorbitant turbulence would result in a large amount of suspended minor crystal and (ii) too fast mass transfer would lead to the incorporation of impurity into the crystal and reduce the separation efficiency.


Figure 3 Influence of cooling rate on crystal scale (a), average concentration of crystal layer (b), and crystal separation degree (c)
3.1.2Influence of cooling rate on crystal scale, layer concentration and separation degree
Figure 3 indicates that the crystal scale is significantly increased as the cooling rate increases from 0.4 to 1.5 °C·min-1, but the average concentration of crystal layer decreases. It is a trade-off between the yield and quality of crystal to determine the optimal cooling rate in the application. A fast cooling rate increases the temperature difference between the solution and cooling material, which increases the supersaturation degree of solution and decreases the purity of crystallization product. On the other hand, a low cooling rate may improve the purity but requires long operation duration, which is unfavourable for economic reasons.Based on the above results, the recommended cooling rate is between 0.6 and 0.8 °C·min-1with the crystal scale and average concentration of crystal layer approximately 0.7 and 0.992, respectively, after 40 min.Under this condition, the CSD (~2.0%) is the maximum [Fig. 3 (c)].
3.1.3Influence of crystal scale and feed concentration on separation degree
The crystal scale increases as the final temperature decreases. Table 1 shows the effect of crystal scale on the separation degree. Overall, the CSD decreases by 22% as the final crystallization temperature decreases from 25 °C to 5 °C. The difference in CSD is insignificant when the crystal scale changes from 52% to 71%, but further increase in crystal scale (or decrease in final temperature) results in sharp decrease of CSD, because faster growth of crystal in the crystallizer will increase the thickness of the crystal layer,resulting in higher resistance for heat and mass transfer and reducing the product purity. Therefore, the crystal scale in industrial application is recommended as 70%.
Table 1 Crystal scale and separation degreeversusfinal temperature (crystallization time: 40 min;initial temperature: 40 °C)
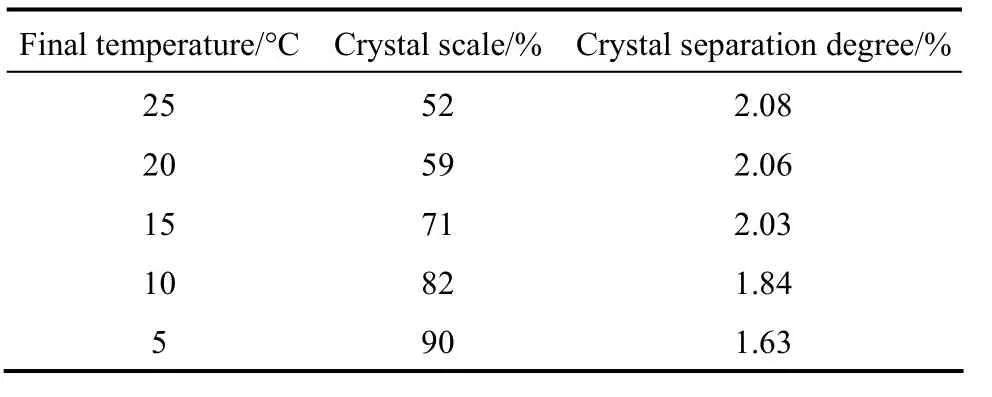
F i n a l t e m p e r a t u r e/°C C r y s t a l s c a l e/% C r y s t a l s e p a r a t i o n d e g r e e/%2 5 5 2 2.0 8 2 0 5 9 2.0 6 1 5 7 1 2.0 3 1 0 8 2 1.8 4 5 9 0 1.6 3
Table 2 indicates that CSD is sensitive to the feed concentration. A slight increase of feed concentration(<0.2%) results in significant (>40%) increase of CSD,which implies the melt crystallization technique is especially applicable for the solution containing high concentrationp-cresol. This also shows how to improve the separation efficiency for the feed with less purity.
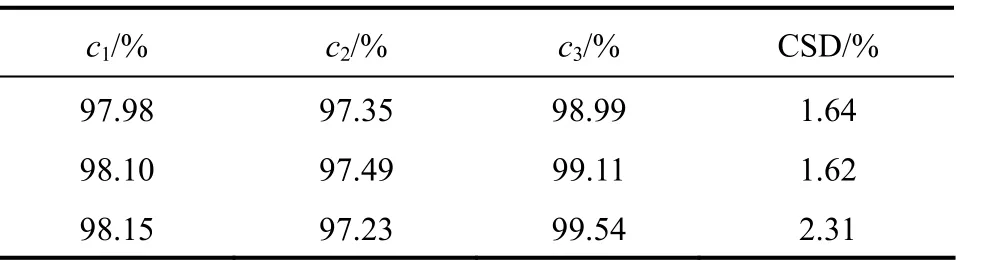
Table 2 Crystal separation degree versus feed concentration
3.2 Sweating process

Figure 4 Changes in crystal layer temperature (a), sweat cumulant (b), and sweat average concentration (c) with time at different sweating temperature■ 30 °C; ● 32 °C; ▲ 34 °C
Figure 4 shows the effect of temperature and time on sweating. The crystal layer temperature decreases as sweating temperature increases, reaching a plateau after 40 min. The sweat cumulant increases quickly at initial stage, but changes little after 40 min. Higher sweating temperature results in higher sweat cumulant because of higher local melt coefficient at higher temperature. The effect of sweating temperature on the sweat average concentration is similar but less compared to that on the sweat cumulant. Table 3 demonstrates the increase of product purity and CSD as sweating time increases. However, the sweat liquid also increases significantly with time, which may reduce the yield of product. The overall results suggests that the optimal sweating time is about 40 min. On this basis,the heating rate would be 0.2-0.3 °C⋅min-1.
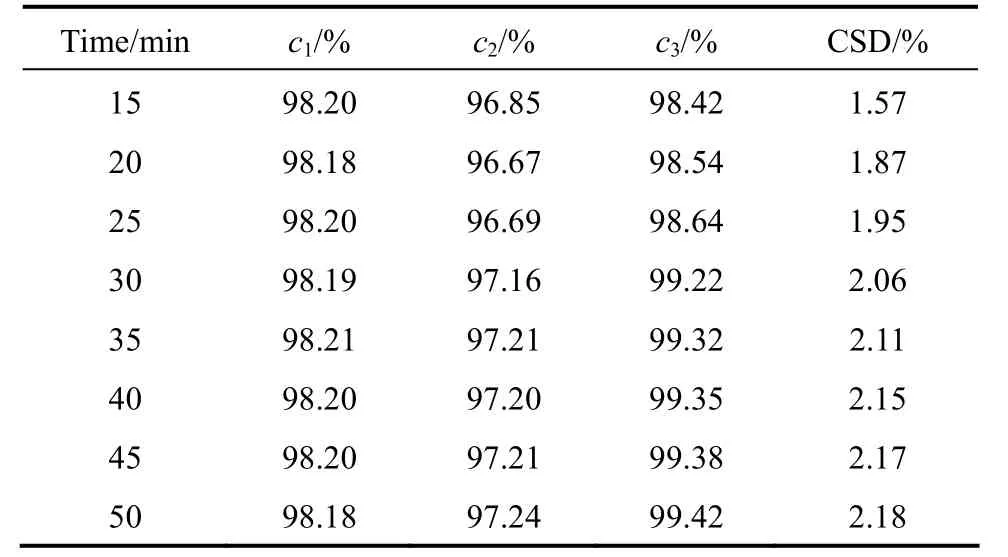
Table 3 Crystal separation degree and concentration versus sweating time (initial temperature: 25 °C;final temperature: 36 °C)
4 CONCLUSIONS
This study demonstrates that the pulsed aeration is better than continuous aeration for the melt crystallization ofp-cresol, in which the crystal separation degree was increased by up to 73% and the nitrogen consumption was reduced significantly. The optimal gas velocity in the pulsed aeration was 90 L·h-1. The recommended cooling rate in the melt crystallization and temperature increasing rate in the sweating process were 0.6-0.8 °C·min-1and 0.2-0.3 °C⋅min-1, respectively. The optimal crystallization and sweating time was 40 min when the crystal scale was controlled around 70%. The results can provide guidance and reference for process design and industrial applications of refining high-purityp-cresol melt crystallization.
1 Kim, K. J., Ulrich, J., “A quantitative estimation of purity and yield of crystalline layers concerning sweating operations”,Journal of Crystal Growth, 234 (2/3), 551-560 (2002).
2 Konig, A., Stepanski, M., Kuszlik, A., Keil, P., Weller, C., “Ultra-purification of ionic liquids by melt crystallization”,Chem.Eng.Res.Des., 86 (7), 775-780 (2008).
3 Chaleepa, K., Szepes, A., Ulrich, J., “Dry fractionation of coconut oil by melt crystallization”,Chem.Eng.Res.Des., 88 (9),1217-1222 (2010).
4 Arkenbout, G., Melt Crystallization Technology, Technomic Publishing, Lancaster (1995).
5 Guardani, R., Neiro, S., Bülau, H., Ulrich, J., “Experimental comparison and simulation of static and dynamic solid layer melt crystallization”,Chem.Eng.Sci., 56 (7), 2371-2379 (2001).
6 Zhong, W., Cao, G., “Advances in research and application of melt crystallization technology”,Sino—Global Energy, 15 (6), 95-99(2010). (in Chinese)
7 Ulrich, J., Ozoguz, Y., “Progressive freezing and sweating in a test unit”,Journal of Crystal Growth, 99 (1-4), 1134-1137 (1990).
8 Scholz, R., Wangnick, K., Ulrich, J., “On the distribution and movement of impurities in crystalline layers in melt crystallization processes”,Journal of Physics D:Applied Physics, 26, B156-B161(1993).
9 Pinto, A.M.F.R., Coelho Pinheiro, M.N.C., Campos, J.B.L.M.,“Coalescence of two gas slugs rising in a co-current flowing liquid in vertical tubes”,Chem.Eng.Sci., 53 (16), 2973-2983 (1998).
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- HPLC Analysis of Egg Yolk Phosphatidylcholine by Evaporative Light Scattering Detector
- Removal of Organic Matter and Ammonia Nitrogen inAzodicarbonamide Wastewater by a Combination of Power Ultrasound Radiation and Hydrogen Peroxide*
- A Geometric Approach to Support Vector Regression and Its Application to Fermentation Process Fast Modeling*
- Three Dimensional Numerical Simulation of Convection-Condensation of Vapor with High Concentration Air in Tube with Inserts*
- Nanoparticle Migration in a Fully Developed Turbulent Pipe Flow Considering the Particle Coagulation*
- Regeneration of Spent Activated Carbon by Yeast and Chemical Method*
