Immobilization of β-Cyclodextrin as Insoluble β-Cyclodextrin Polymer and Its Catalytic Performance*
2012-02-14JIANGHongguo江红果YANGZujin杨祖金ZHOUXiantai周贤太FANGYanxiong方岩雄andJIHongbing纪红兵
JIANG Hongguo (江红果), YANG Zujin (杨祖金), ZHOU Xiantai (周贤太), FANG Yanxiong(方岩雄),** and JI Hongbing (纪红兵),**
1 Faculty of Chemical Engineering and Light Industry, Guangdong University of Technology, Guangzhou 510006,China
2 School of Chemistry and Chemical Engineering, Sun Yat-sen University, Guangzhou 510275, China
1 INTRODUCTION
Cyclodexrtrin (abbreviated as CD) is cyclic oligosaccharide composed of six, seven or eight glucopiranose units linked by glycosidic bonds (as α, β and γ-CD, respectively) [1]. They have a hollow truncated cone shape that is hydrophilic at the periphery and hydrophobic in the central cavity. It is known that β-cyclodexrtrins (abbreviated as β-CD) can form inclusion complexes with different guest molecules in aqueous solution or in the solid state [2]. Hence, β-CD has been widely employed in various organic reactions e.g. oxidation, reduction, ring opening and hydrolysis in aqueous solution [3]. However, for the reactions catalyzed by β-CD in previous works [4-11], generally large amount of solvent is required for extracting organic compounds from the mixture and recycling β-CD catalyst, making these processes less benign.Therefore, immobilization of β-CD is crucial for the industrial applications of those reaction systems.
Insoluble β-CD polymer (abbreviated as β-CDP)is drawing much attention due to its recyclability, easy recovery and cost-effectiveness [12]. It has been widely used in many fields, e.g. chemistry (organic and polymer synthesis) [13-16] and catalysis (oxidation, reduction,substitution reaction and so on) [17-19]. Two synthetic methods have been applied to prepare insoluble β-CD polymer. One is that β-CD molecules are attached as pendent groups on other polymer chains via radical polymerization of the functional β-CD monomers such as acrylolyl cyclodextrin (β-CD-A) [20]. Through the reaction of β-CD molecules with bifunctional crosslinking agents such as epichlorohydrin (abbreviated as EPI) is another way to prepare β-CDP [21, 22].
The preparation [23-27], characterization [28, 29],properties [30, 31] and application [32, 33] of β-CD polymers have been well investigated. The β-CD polymers also showed excellent catalytic activity for the oxidation of benzyl alcohol, which is comparable with β-CD [5]. Properties of the insoluble β-CD polymers would be influenced by the preparation process.However, few publications were reported on polymerization process in order to obtain insoluble polymers of different crosslinking degrees. In this paper, the controllable preparation of insoluble β-cyclodextrin polymers and precise tuning of their performances were well demonstrated by Fourier Transform Infrared(FTIR) and Thermogravimetry (TG). These catalysts are metal-free, making these processes more environmentally-friendly.
2 EXPERIMENTAL
2.1 Materials and reagents
β-CD (≥99.0%, Shanghai Boao Corp , China),epichlorohydrin (EPI, analytical grade, Tianjin Damao Chemical Reagent Factory, China). All other chemicals were purchased from Guangzhou Chemical Reagent Factory, China. All reagents and solvents were of analytical grade and used without further purification unless indicated.
2.2 Polymer synthesis
According to previous reports [22, 25, 27], a typical procedure for preparing β-CD polymer was described as follows. β-CD (5 g, 0.44 mmol) was mixed with 8 ml NaOH (50%, by mass) solution and mechanically stirred for 20 min till β-CD was dissolved completely. Then, 15 ml EPI was added in dropwise as the mixture was heated gently up to 65 °C. The reaction mixture was polymerized at 65 °C under vigorous stirring (200 r·min-1). After stirring for about 1-2 h, precipitate could be observed, and the viscosity of the solution was also increased. The solution was mixed with 100 ml acetone, and the insoluble polymers were poured into water. The resultant product was filtrated, and further washed with acetone in a Soxhlet extractor for 24 h. After drying in vacuum oven at 80 °C for 12 h, the polymer product was crushed and granulated to 160-250 μm in diameter.
2.3 Characterization
The FTIR spectra of the samples were measured by KBr pellet. All the infrared spectra were recorded on a Bruker TENSOR 37 FTIR spectrometer with the wavenumber ranging from 400 to 4000 cm-1and resolution of 4 cm-1. The X-ray powder diffraction (XRD)patterns were measured on a Rigaku Denki MAX III diffractometer with Na-filtered Cu Kαradiation. Stepscans were recorded for all samples in theθrange of 5°-80°. The XRD peaks were analyzed using the Rigaku program ‘JADE-5’. Thermogravimetry Analysis(TGA) experiments were performed on a Netzsch STA-449C thermal analysis system. The flow rate of nitrogen was about 40 ml·min-1and a heating rate of 10 °C·min-1was employed. For TG-FTIR measurements, the transfer line and the head of the TG balance were heated at a constant temperature of 320 °C and the spectral region was 4000-400 cm-1.
2.4 Measurement of β-CD content in β-CDP
β-CD content of water-insoluble polymers was determinedviaelemental analysis on a Vario EL analyzer (Elementar Analysensysteme, Hanau, Germany).The results were corrected taking into account the humidity of the samples, obtained from thermogravimetry analysis (Netzsch, STA-449C) as previously reported [34]. For water-soluble polymers, the content of β-CD was measured using the determination of reducing sugars with tetrazolium blue after acidic hydrolysis, as previously reported [35].
2.5 Solubility of β-CDP
The β-CD polymer was dispersed in different solvents (water, methanol, ethanol, acetone, ethyl acetate, hexane, 0.5 mol·L-1sulfuric acid, 1 mol·L-1sodium hydroxide). Each sample (3 g) was dispersed in 25 g solvent at 60 °C for 4 h with magnetic stirring.Then the mixture was centrifuged at 10000 r·min-1for 15 min. The supernatant was taken and dried in a vacuum drier, and the mass of the remained polymer was measured for the calculation of solubility.
2.6 Catalytic performance
A typical experiment was carried out in a 100 ml three-necked flask fitted with a reflux condenser and magnetic stirrer. 2.615 g β-cyclodextrin polymer was dispersed in 25 ml water at 50 °C. Then, 0.108 g benzyl alcohol and 5 ml 7.5% NaClO were added slowly while stirring for 3.0 h. The reaction mixture was extracted by ethyl acetate and subsequently analyzed by GC (Agilent 7890) with naphthalene as an internal standard.
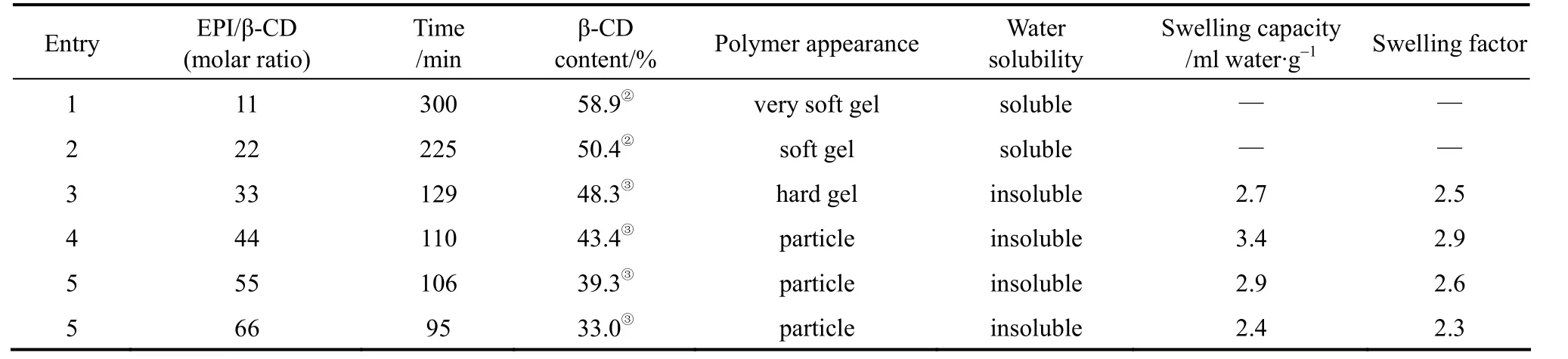
Table 1 Effect of the EPI/β-CD molar ratio on the polymerization products① EPI/β-CD=44/1(molar ratio, mass ratio=3.6/1), 65 °C.
② Based on the calculation method from literature[35].
③ Based on the calculation method from literature[34].
3 RESULTS AND DISCUSSION
3.1 Studies on polymerization reaction
3.1.1Effect of molar ratio of EPI to β-CD on the polymerization
A series of β-CD polymers were prepared with different molar ratios of EPI to β-CD, and the results are summarized in Table 1. Insoluble polymers could not be obtained as the molar ratio of EPI/β-CD was less than 22, even though the reaction time was prolonged to 300 min (Entry 1). From soluble products to insoluble products, the critical molar ratio of EPI to β-CD was 33 (Entry 3). It could also be known that the polymerization time and β-CD content of the polymers decreased with increasing molar ratio of EPI to β-CD.The β-CD polymers produced with high EPI/β-CD ratios are a three-dimensional network mixture containing CD units joined by single or multiple glyceryl links. It is insoluble in aqueous solutions, but swells strongly. The result is in good agreement with previous reports [10].
The FTIR spectra of the β-CD polymers with different molar ratios of EPI to β-CD were presented in Fig. 1. With increasing EPI/β-CD, the band of O H stretching vibration at 3422 cm-1for the hydroxyl groups of the polymers weakened gradually, accompanying with the enhancement of the asymmetric stretching vibration of CH2at 2876 cm-1, which indicated that the reaction of the hydroxyl groups of β-CD with EPI. Meanwhile, the intensity of C O C stretching at 1036 cm-1decreased with the increasing molar ratio of EPI to β-CD, demonstrating that the crosslinking degree increased by the increasing amount of EPI. Therefore, it is possible to obtain a series of β-CD polymers with different crosslinking degrees by varying the molar ratio of EPI to β-CD.
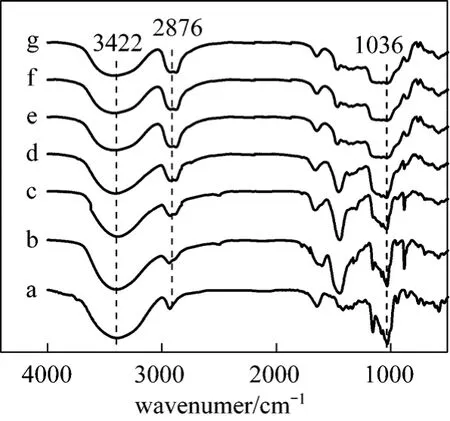
Figure 1 FTIR spectra of β-CD (a) and β-CDP prepared with different molar ratios of EPI/β-CD: (b) EPI/β-CD=11∶1, (c) EPI/β-CD=22∶1, (d) EPI/β-CD=33∶1, (e)EPI/β-CD=44∶1, (f) EPI/β-CD=55∶1, (g) EPI /β-CD=66∶1
The TG curves for the β-CDPs were presented in Fig. 2. A typical TG curve (a) with a large mass loss peak at 310 °C, corresponding to the decomposition of β-CD, was for pure β-CD. Meanwhile, the characteristic thermal profiles of cross-linked products (b-g)were distinguishable and the mass loss peaks were shifted more than 310 °C for the insoluble polymers.Such results indicated that the stability of the insoluble polymers was improved significantly after polymerization. The result shows that the molar ratio of EPI/β-CD has significant influence on the structure of final products. From the TG analysis, the decomposition temperature of β-CDP of EPI/β-CD=44 is 380°C, which is higher than those of other molar ratios,indicating the optimal molar ratio of EPI/β-CD is 44.
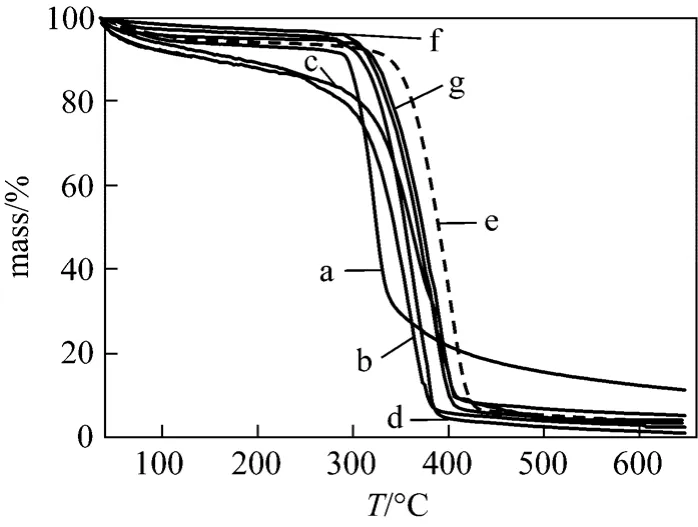
Figure 2 TG curves of β-CD (a) and β-CDP prepared with different molar ratio of EP/β-CD: (b) EPI/β-CD=11∶1, (c)EPI/β-CD=22∶1, (d) EPI/β-CD=33∶1, (e) EPI/β-CD=44∶1, (f) EPI/β-CD=55∶1, (g) EPI/β-CD=66∶1
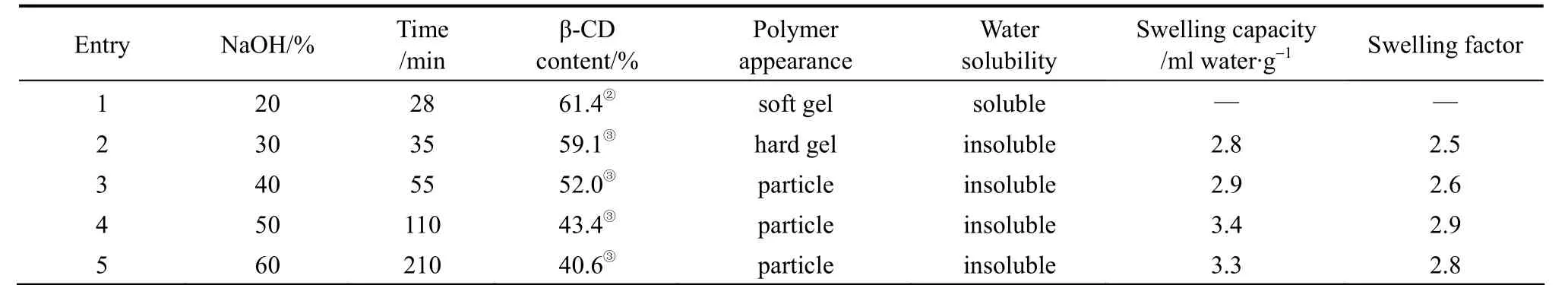
Table 2 Effect of NaOH concentration on the polymerization① EPI/β-CD=44/1(molar ratio, mass ratio=3.6/1), 65°C.
② Based on the calculation method from literature[35].
③ Based on the calculation method from literature[34].
3.1.2Effect of NaOH concentration on the polymerization
The effects of NaOH concentration on the synthesized polymers have been investigated, and the results were summarized in Table 2.
As shown in Table 2, it seemed that the polymerization was closely related with the NaOH concentration. The use of 20% NaOH solution tends to accelerate the reaction between β-CD and EPI to form water soluble polymer (Entry 1). Moreover, when the polymerization was carried out in the presence of 20% NaOH,the reaction rate was 5-10 times faster than that with 50% or 60% NaOH (Entries 4, 5). It indicated that low concentration favored to soluble linear polymer instead of insoluble crosslinked one. However, insoluble polymers could be produced when the concentration of NaOH was over 30% (Entry 2). It could be attributed to the favorable deprotonation of C-2 or C-3 carbon atoms in β-CD glucopyranose units with increasing NaOH concentration, which resulted in the cross-linking agent which could react easily with β-CD [36]. The higher the concentration, the less the insoluble of β-CDP in water was. As the NaOH concentration was up to 60%, β-CD could not dissolved completely, and the β-CD content in the polymers also decreased to 40%.
The FTIR spectra for the prepared β-CD polymers with different NaOH concentration were presented in Fig. 3. The variation of characteristic peaks is similar to that for different molar ratio of EPI/β-CD,as discussed above. With increasing NaOH concentration, linear structure polymer was further cross-linked into three-dimensional network.
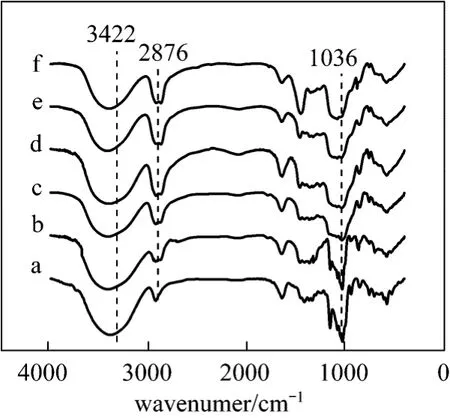
Figure 3 FTIR spectra of β-CD (a) and β-CDP prepared with different NaOH mass concentration: (b) 20%, (c) 30%,(d) 40%, (e) 50%, ( f) 60%
The TG curves of β-CDP prepared were presented in Fig. 4. As discussed above, the insoluble polymer prepared with 50% NaOH concentration exhibited better thermal stability than the others since β-CD is entrapped into crosslinked network of insoluble polymers. Therefore, the optimal NaOH concentration for the crosslinking reaction is 50%.
3.1.3Effect of reaction temperature on the polymerization

Figure 4 TG curves of β-CD (a) and β-CDP prepared with different NaOH concentrations: (b) 20%, (c) 30%, (d)40%, (e) 50%, (f) 60%
The effect of temperature was also investigated,and the results were summarized in Table 3. As shown in Table 3, it seemed that polymerization was not significantly dependent on reaction temperatures. Insoluble polymers could be obtained even if the temperature was as low as 35 °C (Entry 1). However, the reaction rate dramatically changed when the polymerizations were carried out at different temperature. The reaction rate increased rapidly with raising temperature. The time required for crosslinking decreased from 520 min to 30 min while the reaction temperature rose from 35 °C to 75 °C. Therefore, the higher temperature is helpful for the polymerization. However, from the viewpoint of the volatility of EPI, the optimal reaction temperature was 65 °C.
Figure 5 showed the FTIR spectra of polymers produced at different temperature. From Fig. 5, it could be found that the variation of characteristic peaks for these polymers were not obvious, which again demonstrated that structure of the β-CD polymers was not significantly related to synthesized temperatures.
The TG curves of β-CDP prepared at different temperature were given in Fig. 6. The conclusion that polymerization was not significantly dependent on the reaction temperature could also be confirmed from Fig. 6.
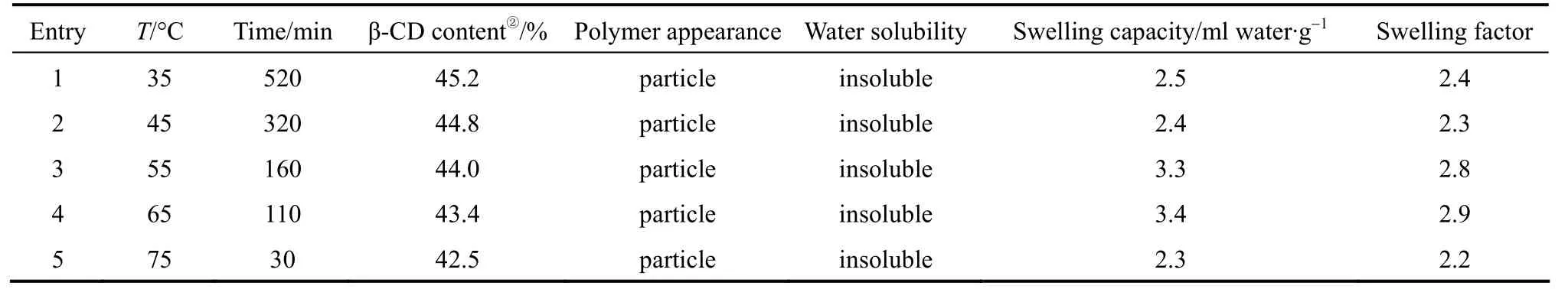
Table 3 Effect of reaction temperature on the polymerization① EPI/β-CD=44/1(molar ratio, mass ratio=3.6/1), 65 °C.
② Based on the calculation method from literature[34].
3.2 Plausible mechanism for the synthetic reaction
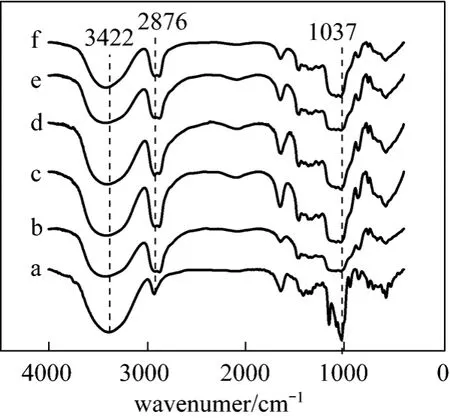
Figure 5 FTIR spectra of β-CD (a) and β-CDP prepared at different temperature: (b) 35 °C, (c) 45 °C, (d)55 °C, (e) 65 °C, (f) 75 °C
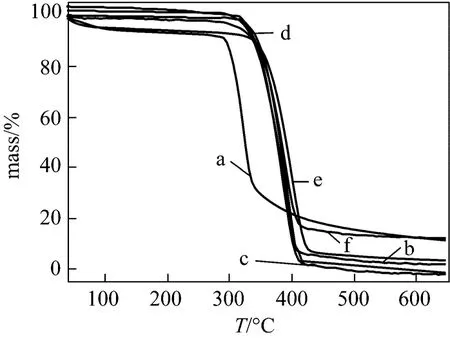
Figure 6 TG curves of β-CD (a) and β-CDP prepared at different temperature: (b) 35 °C, (c) 45 °C, (d) 55 °C, (e)65 °C, (f) 75 °C
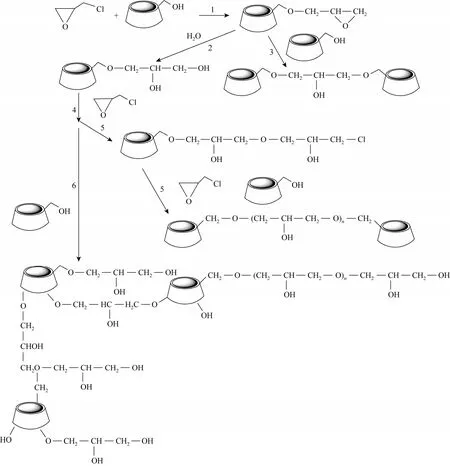
Figure 7 Schematic illustration of reaction between β-CD and EPI
β-CD polymers were prepared from the reaction between β-CD and epichlorohydrin in alkaline solution. The plausible reaction processes were presented in Fig. 7. Firstly, β-CD molecules reacted with epichlorohydrin (Reaction 1). The side chain obtained could further react in two different ways: (a) the epoxyde ring could react with another hydroxyl group of another β-CD molecule (Reaction 3); (b) the epoxyde ring was hydrolysed (Reaction 2), and the product could react with another EPI (Reaction 4), then polymerized with another β-CD molecules (Reaction 5).When the molar ratio of EPI/β-CD was less than 33,structure of β-CD polymers remained straight chain and was soluble in water. Otherwise, EPI could react with CD molecules and/or itself (polymerization step)(Reaction 6). The resulting polymer was a mixture containing CD units joined by repeating glyceryl linkers. A number of CD rings were interconnected and a three-dimensional network was formed. The matrix of the β-CD polymers became insoluble. Both soluble and insoluble polymers could be obtained respectively if the experimental conditionsi.e. concentration of β-CD in NaOH solution, molar ratio of EPI/β-CD,temperature, reaction time and also the NaOH concentration in the solution were well controlled [21, 37].
3.3 Characterization
3.3.1FTIR measurement
The FTIR spectra for the prepared β-CDP with different reaction time were presented in Fig. 8. The FTIR spectra of β-CDP (2, 3, 4) are different from that of β-CD (1). The stretching vibration of O H at 3422 cm-1gradually reduced and the asymmetric stretching vibration of CH2at 2876 cm-1intensified with raising temperature. Such changes are obvious evidences for crosslinking between β-CD and epichlorohydrin during the reaction. Furthermore, comparing with β-CD, the significantly weaker and wider absorption of C O and C O C at 1036 cm-1also indicated the occurrence of the cross-linked products. It was found that the absorption status of C O and C O C at 1036 cm-1is an indication for its solubility. When the product is soluble, where the shape and intensity of the peak at 1036 cm-1will consistent with that of β-CD, otherwise, the absorption of C O and C O C will become weaker and wider. It maybe due to that the chain length increased with the prolonged reaction time, in which the structures of polymer underwent a transition from linear chains to three-dimensional networks, leading to the product change from soluble to insoluble. The results were in good agreement with Delval’s reports [38].
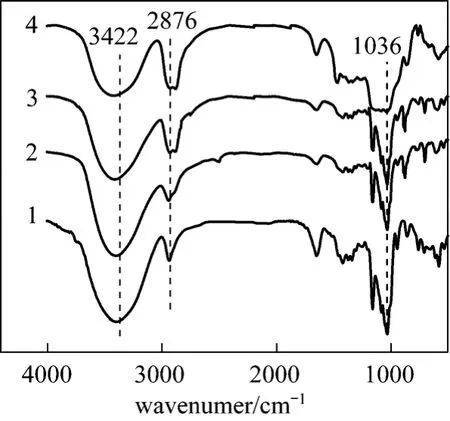
Figure 8 FTIR spectra of β-CDP with different synthetic time (Reaction conditions: Molar ratio of EPI to β-CD was 44∶1, the concentration of NaOH was 50%, 65 °C)1—0 min (β-CD); 2—30 min; 3—60 min; 4—110 min (final product)
3.3.2XRD measurement
Figure 9 shows the XRD patterns of β-CD (1),unpurified β-CDP (3) and purified β-CDP (2). As it can be observed, the diffraction pattern of β-CD showed several sharp peaks for a crystalline compound. While for β-CDP, a mild hump ranging from 15°-25°, pointing to the amorphous nature for the polymer. The XRD results suggested the cross-linkage between EPI and β-CD. The characteristic peaks in the unpurified β-CDP,were in good agreement with β-CDP. But the typical peaks associated with NaCl crystalline were observed either. For purified β-CDP, its X-ray pattern does not show these NaCl peaks, indicating NaCl byproduct has been removed completely. Accordingly, filtering and careful wash were necessary for removing residual linker and byproducts from the polymer [39].3.3.3TG-FTIR measurement

Figure 9 X-ray diffraction curves (Reaction conditions of β-CDP: Molar ratio of EPI to β-CD was 44∶1, the concentration of NaOH was 50%, 65 °C)1—β-CD; 2—β-CDP; 3—unpurified β-CDP; □ NaCl
The TG curves for β-CDP and β-CD was shown in Figs. 2 and 10, respectively. The decomposition stages, the corresponding temperature ranges and estimated mass loss for β-CD and β-CDP were listed in Table 4.
The first stage for thermal decomposition of β-CD was related to dehydration within 40-140 °C, the corresponding mass loss was 13%. The second stage was related to the decomposition of framework and mass loss of 75% was observed. However, β-CDP exhibited only a single decomposition stage ranging from 310-450 °C, and mass loss of 95% were depicted.
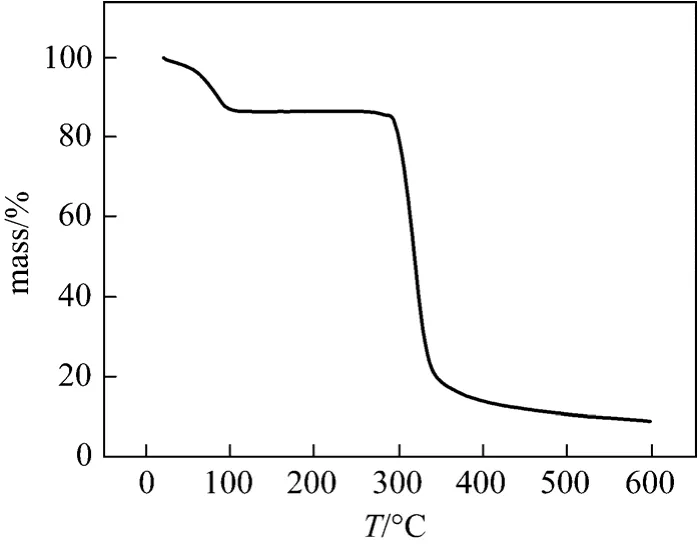
Figure 10 TG Curves of β-CD
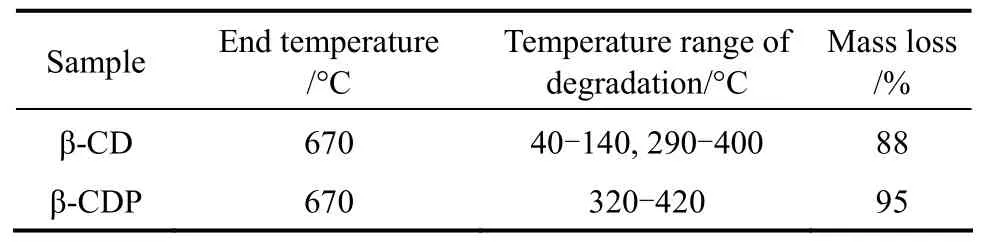
Table 4 End of degradation temperature of different samples① Reaction conditions of β-CDP: Molar ratio of EPI to β-CD was 44∶1, concentration of NaOH was 50%, 65 °C. β-CDP/solvent=1/8.33 (by mass).
The gaseous products emitted from TG analysis were detected by Fourier transform infrared spectroscopy (FTIR). The temperature dependence of intensity agrees well with the temperature dependence of DTG curve, and the intensity of gas emission reaches its maximum at 320 °C and 410 °C for β-CD and β-CDP,respectively. They are the different temperatures of the maximum loss rate during pyrolysis and combustion.The spectrograms at 320 °C and 410 °C in β-CD and β-CDP residues pyrolysis are shown in Fig. 11. As to β-CD, the main compounds emitted at 320 °C are identified as carbon dioxide (CO2), which indicated carbon dioxide was emitted in the pyrolysis and combustion of β-CD. While for β-CDP, the characteristic peak of CO2appeared at 385 °C. Compared with β-CD, the intensity at 2900 cm-1corresponded to the CH-stretching vibration for β-CDP was significantly increased, which demonstrated that the length of the polymer chain was longer than the carbon chain of β-CD.From the comparison of the TG and TG-FTIR profiles,β-CDP presented higher thermal stability due to the association with EPI, such similar behavior was observed in the copolymers such as poly(N-vinylimidazole-coethyl methacrylate) [40].
3.3.4Solubility of β-CDP
The solubility of β-CDP in various solvents, including acidic and basic solution in Table 5 suggest that the solubility of β-CD polymer was lower than 0.2% in all solvents studied, whether they are polar,non-polar, or strong acid and base solutions. It seemed the solubility of the polymer in sulfuric acid (Entry 8)and alcohols (Entries 4 and 5) were higher than in other solvents. From Table 5, it could be observed that the polymer has some characteristics such as highly hydrophilic, excellent swells in aqueous solutions and insoluble in organic solvents. The soluble fractions could be oligomers or unpolymerizations β-CD derivatives, and the majority of the polymer was comprised of insoluble fractions. A number of β-CD rings are interconnected and a three-dimensional network is formed [41].
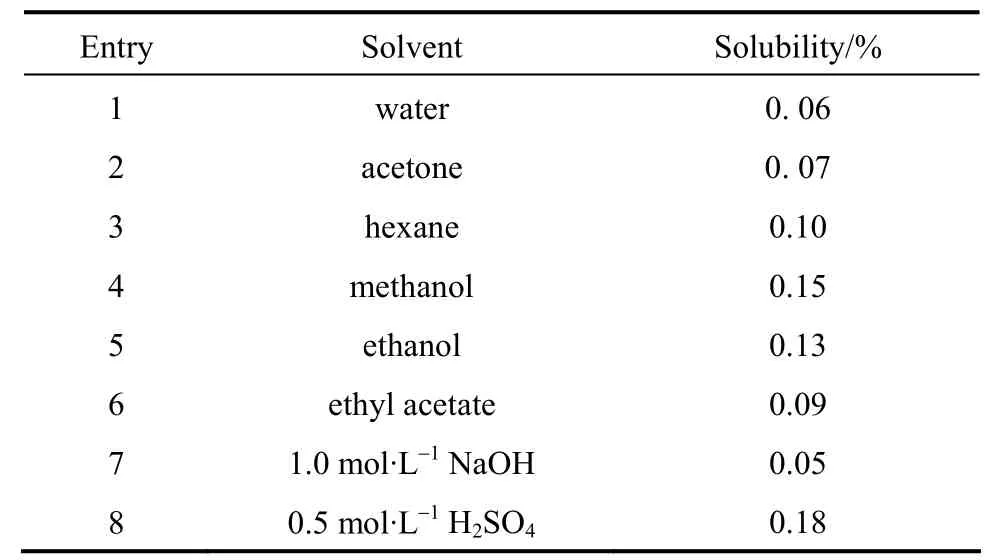
Table 5 Solubility of β-CDP in different solvents① Reaction conditions of β-CDP: Molar ratio of EPI to β-CD was 44∶1, concentration of NaOH was 50%, 65 °C. β-CDP/solvent=1/8.33 (by mass).
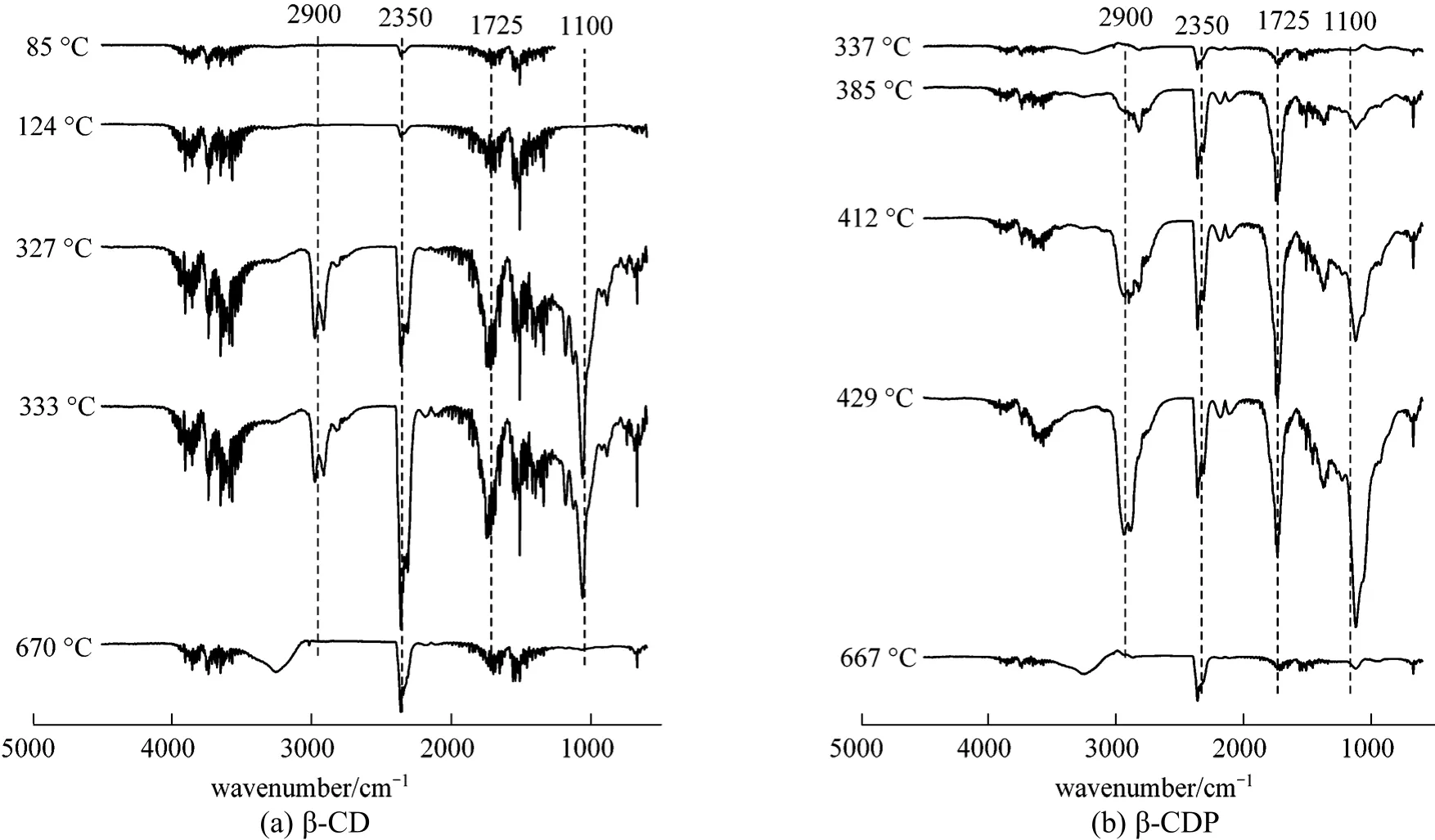
Figure 11 FTIR spectra of gases released by heating of β-CD (a) and β-CDP (b) at different temperatures (Reaction conditions of β-CDP: Molar ratio of EPI to β-CD was 44∶1, the concentration of NaOH was 50%, 65 °C)

Figure 12 Oxidation of benzyl alcohol to benzaldehyde in the presence or absence of β-CDP [Reaction condition: benzyl alcohol (1 mmol), β-CDP (2.000 g, EPI to β-CD=44∶1), 2 ml NaClO of 7.5% (by mass), temperature (50 °C), 200 r·min-1]
3.4 Catalytic activity
The oxidations of benzyl alcohol were employed for evaluating catalytic activity of β-CDP, as illustrated in Fig. 12. In a control experiment, the oxidation of benzyl alcohol was conducted in the absence of β-CDP. The yield of benzaldehyde was only 8% after 60 min reaction without β-CDP. However, benzyl alcohol was oxidized to benzaldehyde with the yield of 65% and maximal yield (ca. 72%) was obtained with 2 g of β-CD, indicating yield of benzaldehyde increased with increasing the amount of β-CD. The results demonstrate that β-CD is crucial for the oxidation of benzyl alcohol and are in good agreement with the previous report [10], and also confirm that β-CDP could effectively catalyze the oxidation of benzyl alcohol. It should be noted that the immobilized β-CDP has overcome the shortcoming related to the recycle of β-CD.
4 CONCLUSIONS
Insoluble β-CDP was synthesized by β-CD using EPI as crosslinking agent under basic conditions. The preparation of insoluble β-CDP by carefully controlling polycondensation has been investigated. The polymerization degree was closely related to the initial molar ratio of EPI/β-CD, NaOH concentration and reaction temperature. Influencing factors were investigated and the optimal polymerization conditions were molar ratio of EPI to β-CD was 44∶1, reaction temperature was 65 °C and the concentration of NaOH was 50%. The characterization of FTIR, TGA,XRD and TG-FTIR demonstrated that EPI crosslinked well β-CD, and the polymer possessed a remarkably high swelling capacity in water. The polymer presented excellent thermal stability, insolubility and catalytic activity for the oxidation of benzyl alcohol to benzaldehyde.
1 Connors, K.A., “The stability of cyclodextrin complexes in solution”,Chem.Rev., 97, 1325-1357 (1998).
2 Szejtli, J., “Introduction and general overview of cyclodextrin chemistry”,Chem.Rev., 98, 1743-1753 (1998).
3 Ji, H.B., Hang, L.Q., Shi, D.P., Zhou, X.T., “β-Cyclodextrin as supermocecular catalyst in liquid- phase organic synthesis”,Chin.J.Org.Chem., 28 (12), 2072-2080 (2008).
4 Chen, H.Y., Ji, H.B., “Alkaline hydrolysis of cinnamaldehyde to benzaldehyde in the presence of β-cyclodextrin”,AIChE J., 56 (2),466-476 (2010).
5 Shi, D.P., Ji, H.B., “β-Cyclodextrin promoted oxidation of aldehydes to carboxylic acids in water”,Chin.Chem.Lett., 20, 139-142(2009).
6 Surendra, K., Krishnaveni, N.S., Rao, K.R., “A simple biomimetic protocol for the oxidation of alcohols with sodium hypochlorite in the presence of β-cyclodextrin in water”,Can.J.Chem., 82 (7),1230-1233 (2004).
7 Reddy, M.A., Surendra, K., Bhanumathi, N., Rao, K.R., “Highly facile biomimetic regio- selective ring opening of epoxides to halohydrins in the presence of β-cyclodextrin”,Tetrahedron, 58 (30),6003-6008 (2002).
8 Chan, W.K., Yu, W.Y., Che, C.M., “A cyclodextrin-modified ketoester for stereo-selective epoxidation of alkenes”,J.Org.Chem.,68, 6576-6582 (2003).
9 Ji, H.B., Hu, X.F., Shi, D.P., Zhong, L., “Controllable oxidation of sulfides to anlfoxides and sulfones with aqueous hydrogen peroxide in the presence of β-cyclodextrin”,Russ.J.Org.Chem., 42 (7),959-961(2006).
10 Ji, H.B., Shi, D.P., Zhong, L., Wang, L.F., “Transition metal-free and substrate-selective oxidation of alcohols using water as an only solvent in the presence of β-cyclodextrin”,Tetrahedron Lett., 46 (14),2517-2520 (2005).
11 Ji, H.B., “Highly shape-selective, biomimetic, and efficient deprotection of carbonyl compounds masked as ethylene acetals or dioxolanes produced from 1,2-ethanediol”,Eur.J.Org.Chem., 18,3659-3662 (2003).
12 Crini, G., Morcellet, M., “Synthesis and applications of adsorbents containing cyclodextrins”,J.Sep.Sci., 25, 789-813 (2002).
13 Jeromin, J., Ritter, H., “Cyclodextrins in polymer synthesis: free radical poly-merisation of cyclodextrin complexes of cyclohexyl and phenyl methacrylate in aqueous medium”,Macromol.Rapid Commun.,19, 377-379 (1998).
14 Seo, T., Kajihara, T., Iijima, T., “The synthesis of poly(allylamine)containing covalently bound cyclodextrin and its catalytic effect in the hydrolysis of phenyl esters”,Macromol.Chem., 188, 2071-2082(1987).
15 Komiyama, M., “Selective syntheses using cyclodextrins as catalysts.V: para-selective hydroxymethylation of phenol by formaldehyde using hydroxypropyl cyclodextrins”,J.Am.Chem.Soc., 11, 2031-2034(1989).
16 Veglia, A.V., Rossi, D.R.H., “Selectivity in the iodination of phenol in the presence of β-cyclodextrin”.J.Org.Chem., 53, 5281-5287(1988).
17 Easton, C.J., Licon, S.F., Barr, L., “Molecular reactors and machines:applications, potential, and limitations”,Chem.Eur.J., 10, 3120-3128(2004).
18 Breslow, R., Dong, S.D., “Biemimetic reactions catalyzed by cyclodextrins and their derivatives”,Chem.Rev., 98, 1997-2011 (1998).
19 Martel, B., Morcellet, M., “Cyclodextrin-poly(vinylamine) systems-II. catalytic hydrolysis of p-nitrophenyl acetate”,Eur.Polym.J.,31, 1089-1093 (1995).
20 Harada, A., Fure, M., Nozakura, S., “Incusion of aromatic compounds by a β-cyclodextrin-epichlorohydrin polymer”,Polym.J., 13,777-781 (1981).
21 Crini, G., Bertini, S., Torri, G., “Sorption of aromatic compounds in water using insoluble cyclodextrin polymers”,J.Appl.Polym.Sci.,68, 1973-1978 (1998).
22 Renard, E., Deratani, A., Volet, G., Sebille, B., “Preparation and characterization of water soluble high molecular weight β-cyclodextrinepichlorohydrin polymers”,Eur.Polym.J., 33 (1), 49-57 (1997).
23 Solms, J., Egli, R.H., “Harze mit einschlusshohlraumen von cyclodextrin-struktur”,Helv.Chim.Acta, 48 (6), 1225-1228 (1965).
24 Harada, A., Furue, M., Nozakura, S.J., “Optical resolution of mandelic acid derivatives by column chromatography on crosslinked cyclodextrin gels”,Polym.Sci.Polym.Chem.Ed., 16 (1), 189-196 (1978).
25 Sreenivasan, K., “Solvent effect on the interaction of steroids with a novel methyl β-cyclodextrin polymer”,J.Appl.Polym.Sci., 68 (11),1857-1861 (1998).
26 Zohrehvand, S., Evans, C.H., “2-Naphthol-containing β-cyclodextrinepichlorohydrin copolymers: synthesis, characterization and fluorescence studies”,Polym.Inter., 54 (5), 744-753 (2005).
27 Nozaki, T., Maeda, Y., Kitano, H., “Cyclodextrin gels which have a temperature respon- siveness”,J.Polym.Sci.Pol.Chem., 35 (8),1535-1541 (1997).
28 Crini, G., Janus, L., Morcellet, M., Torri, G., “Macroporous polyamines containing cyclo- dextrin: synthesis, characterization and sorption properties”,J.Appl.Polym.Sci., 69 (7), 1419-1427 (1998).
29 Szejtli, J., Fenyvesi, E., Zsadon, B., “Cyclodextrinpolymere”,Starch,30 (4), 127-131 (1978).
30 Crini, G., Cosentino, C., Bertini, S., Naggi, A., Torri, G., Vecchi, C.,Janus, L., Morcellet, M., “Solid state NMR spectroscopy study of molecular motions in cyclomaltoheptaose (β-cyclodextrin) crosslinked with epichlorhydrin”,Carbohydr.Res., 308 (1-2), 37-45 (1998).
31 Crini, G., Bourdonneau, M., Martel, B., Piotto, M., Morcellet,M.,Richert, T., Vebrel, J., Torri, G., Morin, N., “Solid-state NMR characterization of cyclomaltoheptaose (β-cyclodextrin) polymers using high-resolution magic angle spinning with gradients”,Appl.Polym.Sci., 75 (10), 1288-1295 (2000).
32 Yu, J.C., Jiang, Z.T., Liu, H.Y., Liu, J., Zhang, L.Z., “β-Cyclodextrin epichlorohydrin copolymer as a solid-phase extraction adsorbent for aromatic compounds in waters samples”,Anal.Chim.Acta, 477 (1),93-101 (2003).
33 Romo, A., Penas, F.J., Isasi, J.R., “Sorption of dibenzofuran derivatives from aqueous solutions by β-cyclodextrin polymers: An isosteric heat approach”,J.Colloid.Interf.Sci., 279 (1), 55-60 (2004).
34 Vélaz, I., Isasi, J.R., Sánchez, M., Uzqueda, M., Ponchel, G., “Structural characteristics of some soluble and insoluble β-cyclodextrin polymers”,J.InclusionPhenom.Mol.Recognit.Chem., 57 (1-4), 65-68 (2007).
35 Yudiarto, A., Kashiwabara, S., Tashiro, Y., Kokugan, T., “Separation of structural isomers using soluble β-cyclodextrin polymer by ultrafiltration”,Sep.Pur.Technol., 24 (1-2), 243-253 (2001).
36 Gaidamauskas, E., Norkus, E., Butkus, E., “Deprotonation of β-cyclodextrin alkaline solutions”,Carbohydr.Res., 344 (2), 250-254(2009).
37 Hanabusa, K., Tsutsumi, H., Kurose, A., Shirai, H., Hayakawa, T.,Hojo, N., “Synthesis of poly(amino acid)s on molecular assemblies formed by functional active esters of amino acids”,J.Polym.Sci.A Polym.Chem., 27 (5), 191-195 (1989).
38 Delval, F., Crini, G., Bertini, S., Crini, N.M., Badot, P.M., Vebrel, J.,Torri, G., “Characterization of crosslinked starch materials with spectroscopic techniques”,J.Appl.Polym.Sci., 93 (6), 2650-2663(2004).
39 Flores, J., Jiméne, V., Belmar, J., Mansilla H.D., Alderete J. B., “Inclusion complexation of phenol derivatives with a β-cyclodextrin based polymer”,J.Incl.Phenom.Macrocycl.Chem., 53 (1-2), 63-68(2005).
40 Pekel, N., Sahiner, N., Güven, O., Rzaev, Z.M.O., “Synthesis and characterization ofN-vinylimidazole-ethyl methacrylate copolymers and determination of monomer reactivity ratios”,Eur.Polym.J., 37(12), 2443-2451 (2001).
41 Girek, T., Shin, D.H., Lim, S.T., “Polymerization of β-cyclodextrin with maleic anhydride and structural characterization of the polymers”,Carbohydr.Polym., 42 (1), 59-63 (2000).
猜你喜欢
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Phenol Oxidation by Combined Cavitation Water Jet and Hydrogen Peroxide*
- Venting Design for Di-tert-butyl Peroxide Runaway Reaction Based on Accelerating Rate Calorimeter Test
- Effect of Return Sludge Pre-concentration on Biological Phosphorus Removal in a Novel Oxidation Ditch*
- Separation of α-Tocopherol with a Two-Feed Simulated Moving Bed*
- Experimental and CFD Studies on the Performance of Microfiltration Enhanced by a Turbulence Promoter*
- Pervaporation of Aqueous Solution of Acetaldehyde Through ZSM-5 Filled PDMS Composite Membrane*
