Separation of α-Tocopherol with a Two-Feed Simulated Moving Bed*
2012-02-14WEIFeng危凤SHENBo沈波CHENMingjie陈明杰ZHOUXianbo周先波andZHAOYingxian赵迎宪
WEI Feng (危凤)**, SHEN Bo (沈波), CHEN Mingjie (陈明杰), ZHOU Xianbo (周先波) and ZHAO Yingxian (赵迎宪)
Ningbo Institute of Technology, Zhejiang University, Ningbo 315100, China
1 INTRODUCTION
The simulated moving bed (SMB) developed by Universal Oil Products [1] is a continuous adsorption technique. As illustrated in Fig. 1, the SMB connects several chromatographic columns end to end. Four ports for the feed, desorbent, raffinate and extract are switched periodically in the direction of liquid flow,simulating the counter-current movement of stationary phase against mobile phase. This merit makes the SMB overtake batch chromatography in terms of the solvent consumption and productivity for binary separations [2-9]. However, not all columns in SMB are effective for the separation. Some columns only act as expensive vessels to “store” the solution containing a single component or the pure solvent. For example, at the beginning of a switch interval as shown in Fig. 1, the two columns in zone I store the strong retained solute B, the last column in zone III and the first column in zone IV store the weak retained solute A, and the last column in zone IV stores the pure solvent. Obviously, only the three columns in zones II and III are used to separate the mixture.
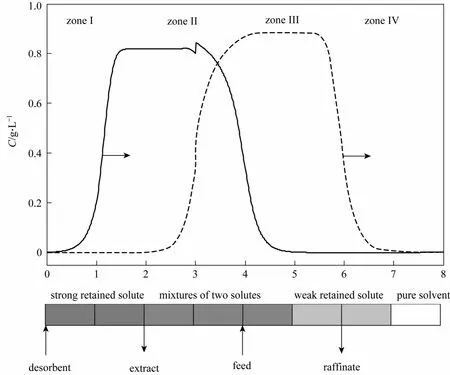
Figure 1 The concentration profile in four-zone SMB at the beginning of a switch interval
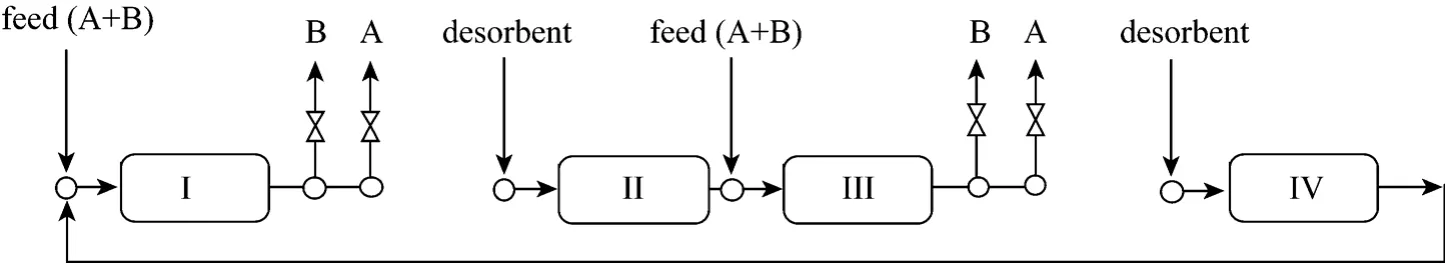
Figure 2 The scheme of two-feed SMB
In this work, we propose a SMB with two feeds to overcome the above disadvantage. As shown in Fig. 2, zones I and II are disconnected from zones III and IV. Two feeds are added at the inlets of zones I and III, while two desorbents are pumped into zones II and IV. The SMB is actually a combination of two-zone SMBs proposed by Lee [10]. The tail of B and the front of A will elute out of zones I and III orderly during each switch interval. Consequently, products B and A are alternately collected from the two outlets of zones I and III. Such modification may maximize the number of columns used for separations and double the productivity.
In this study, the SMB with two feeds will be investigated in detail. The separation process for ideal and linear adsorption will be analyzed according to the movement of concentration band. α-tocopherol will be separated from its homologue mixture to evaluate the feasibility of the two-feed SMB.
2 ANALYSIS OF THE SMB WITH TWO FEEDS
The two-feed SMB is a combination of a pair of two-zone SMBs, one with zones II and III, and another with zones I and IV. The two SMBs have the same operating conditions,i.e.,FIIidentical toFIV,andFIIIidentical toFI. Thus, only the analysis on zones II and III is given.
For simplicity, we only consider the ideal and linear adsorption. In such case, the moving velocity of the boundary of a solute band, front or tail, is [11-13]

whereFis the liquid flow rate,dthe column inner diameter,εthe column voidage, andKthe adsorption equilibrium constant of solute.
In the two-feed SMB, the moving distance of the front of B in zone III in each switch interval Δtshould not be larger than the column lengthL,
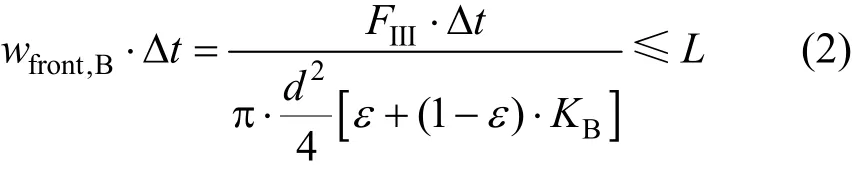
The tail of A in zone II should not be smaller thanL,
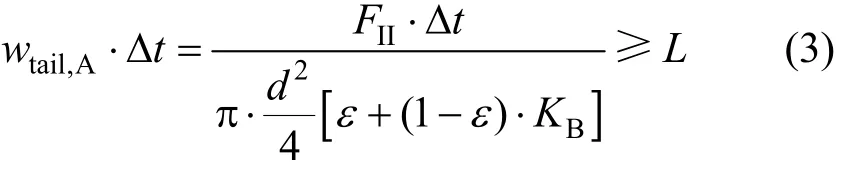
Inequalities (2) and (3) can be rewritten as

whereVcolis the column volume andVR,iis the retention volume of theith solute. Inequalities (4a) and (5a)are the same as those given by Triangle Theory [14-18],defining a separation region in the plane ofmII-mIIIand giving the initial effective operating conditions.
The tail of B in zone II in the (n-1)th switch interval is switched into zone I and continues to move toward the outlet of zone I in thenth switch. An effective separation requires that the front of A does not overtake the tail of B in zone I in the present switch,that is
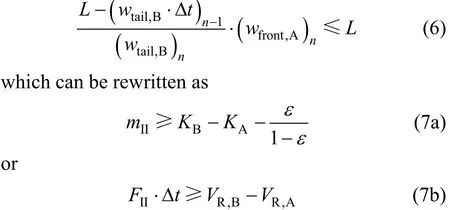
Thus,FIIis limited by two inequalities (4) and (7). Ifis determined by inequality (4). As a result, the feed rate is identical to that in the conventional four-zone SMB. If
is dependent on inequality (7). In this case, in comparison with the four-zone SMB,FIIhas to be decreased, leading to the reduction in feed. However, SMB is normally suitable for difficult separations with selectivity ranging from 1.3 to 2.0 [19], so inequality (4) is stricter than inequality (7)in many situations, making the feed rate not change.Furthermore, the two-feed SMB can process two feeds simultaneously, potentially doubling the productivity.
3 EXPERIMENTAL
The two-feed SMB was modified from an SMB system (Pilot System CSEP®C916, Knauer, German)by disconnecting zone I from zone II and zone III from zone IV. The eight columns (10 mm×100 mm)were packed with Sinochrom ODS-BP (10 μm, Elite,Dalian, China) and the mobile phase was methanol.An analytical liquid chromatography system (K501 pump, K2501 UV detector, Knauer, and Agilent TC-C18 column, Agilent Technologies, USA) was used to analyze the products from SMB.
Separation of α-tocopherol from its homologue mixture was conducted to investigate the feasibility of the two-feed SMB. According to the elution order, the compounds in the raw materials were labeled as compound 1, 2, 3, 4, 5, and 6, as illustrated in Fig. 3. The most retained solute 6 is α-tocopherol while the other solutes are not identified. Due to the very low contents,solutes 1 and 5 were not considered in the separation,and the mixture (2, 3 4, and 6) was separated into non-α-tocopherol (2 + 3 + 4) and α-tocopherol (6).
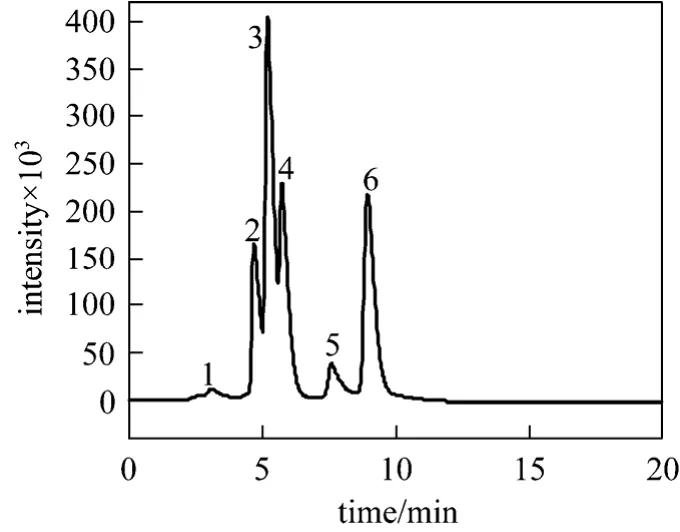
Figure 3 The chromatogram of raw materials on the ODS column with the mobile phase of methanol
According to the separation task, the key components are solutes 2, 4 and 6, which determines the flow rates in SMB. Their retention volumes measured are listed in Table 1.
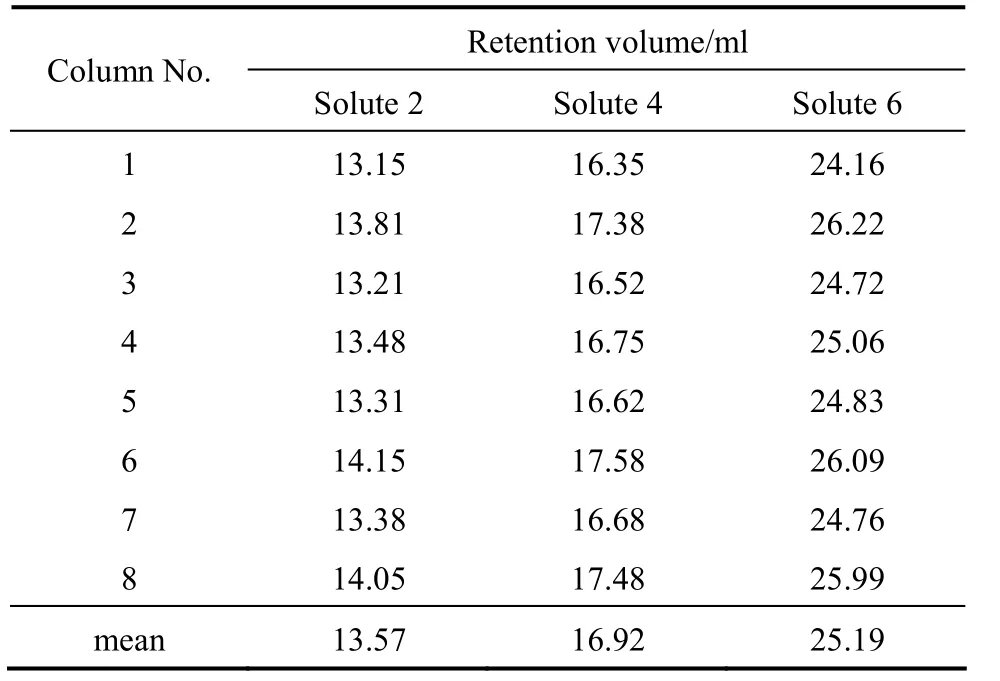
Table 1 Retention volume of key components in raw material
4 RESULTS AND DISCUSSION
4.1 Separations by conventional four-zone SMB
Using a thought similar to inequalities (4) and (5),flow rates in zones I to IV can be determined as follows.

According to inequalities (8)-(11), initial operating conditions can be obtained. It should be noted that inequalities (8)-(11) hold true only for ideal and linear adsorptions. In the cases of non-ideal and nonlinear adsorptions, the separating conditions should be tuned through experiments using a procedure of trial and error. We investigated the separations at two feed concentrations, 10 and 50 mg·ml-1. The operating conditions and the separation performance are listed in Table 2.
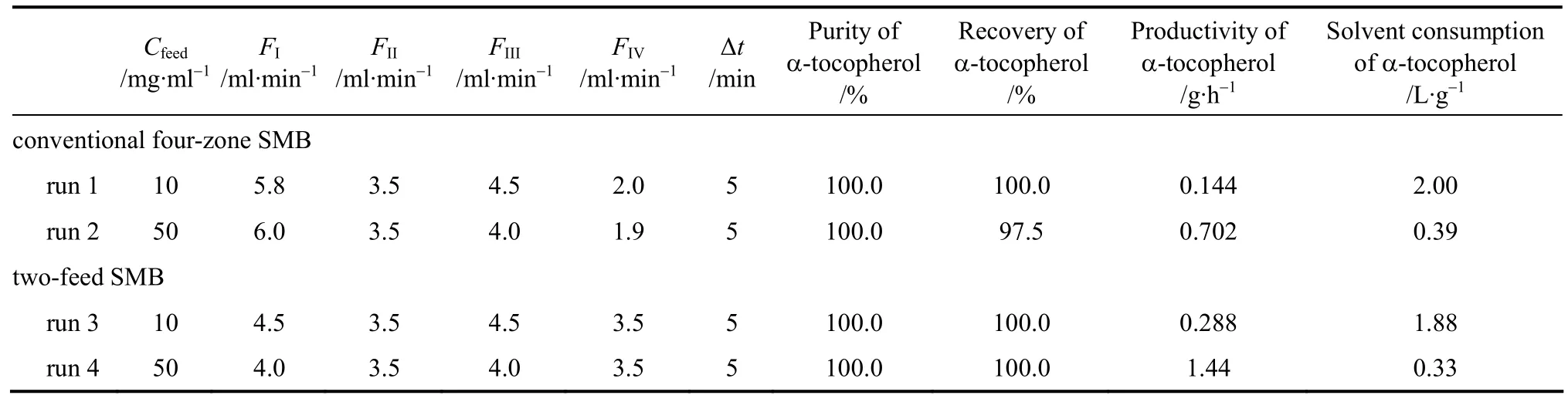
Table 2 Separation of α-tocopherol by simulated moving beds

Figure 4 Concentration profiles in runs 1 and 2strong retained solute; weak retained solute
The outlet concentrations of the eight columns at the end of a switch interval were analysed after reaching cyclic steady state. As shown in Fig. 4, run 1 is very close to the optimal operation. The two tails of α-tocopherol and non-α-tocopherol move from the head to the end of the first column in zones I and II,while the two fronts move from the head to the end of the last column in zones III and IV separately. However, run 2 is not optimized.
4.2 Separations by the two-feed SMB
For the separation of the most retained solute from a multicomponent mixture, the two restrictions of inequalities (4) and (7) are modified as

Inequalities (4c) and (7c) mean that the tail of the weak retained solute 4 in zone II should move a distance longer thanL, and the front of the weakest retained solute 2 does not overtake the tail of the last retained solute 6 in zone III.
From the retention volumes listed in Table 1,inequality (4c) has a stricter limitation than inequality(7c). Thus,FIIandFIIIin the four-zone SMB can be copied to zones II and III as well as zone IV and I in the two-feed SMB, as listed in columns 4 and 5 in Table 2.
The streams from zones I and III after 60 switches were detected separately. As shown in Fig. 5, the effluent curves at the outlets of zones I and III change periodically. The effluent concentration is reduced at first and then increased in each switch interval, indicating that different solutes flow out of zones I and III orderly. The stream out of zone I in run 3 in the 6th switch interval and the stream out of zone III in run 4 in the 1st switch interval are cut to three fractions. The analysis of the fractions (see Fig. 6) confirms that different solutes flow out of zone I and III orderly in each switch interval. In run 3, fraction 1 flowing out in 0-1.7 min (the residence time in the tube is deducted)only contained α-tocopherol, fraction 2 in 1.7-2.4 min contains no solutes, and fraction 3 in 2.4-5.0 min contains non-α-tocopherol. In run 4, α-tocopherol flows out in 0-1.8 min, and non-α-tocopherol flows out in 2.3-5 min. Consequently, two products can be obtained simultaneously from the two outlets of zones I and III in each switch interval. Both the purity and the recovery are 100.0%.
The second fraction containing no solutes can be reused, reducing the solvent consumption greatly. For example, in run 3, the ratio of the desorbent to the feed is reduced from 4.5 to 3.87, and the value of run 1 is somewhat larger than 3.8.
The homogeneity of the column set has large effect on the separation performance, especially in the case of high feed concentrations. As shown in Fig. 5(c), the tail of α-tocopherol is overlapped with the front of non-α-tocopherol in the 2nd, 5th, 7th and 8th switch intervals. In such case, the stream is also cut to three fractions, but the middle fraction contains the front of the weak retained solute and the tail of the strong retained solute. In order to keep the high purities in the first and last fractions, the beginning and end of the middle fraction should be moved up and postponed, respectively, reducing the recovery.
It is interesting that there is always a peak at about 2.6-2.8 min in each switch interval (Fig. 5). The equipment has a 64-way switch valve designed for 16 columns, but only 8 columns are used. Thus, there is a tube between every two columns and the valve has to switch at a half of the original designed switch interval. In the middle of the original designed switch interval, a tube located at the inlet of zone II or IV is switched to the outlet of zone I or III. Accordingly, the concentration of the effluent from zones I and III is decreased to zero suddenly and then increased immediately as shown in Fig. 5.
5 CONCLUSIONS

Figure 5 Effluent curves at the outlets of zones I and III
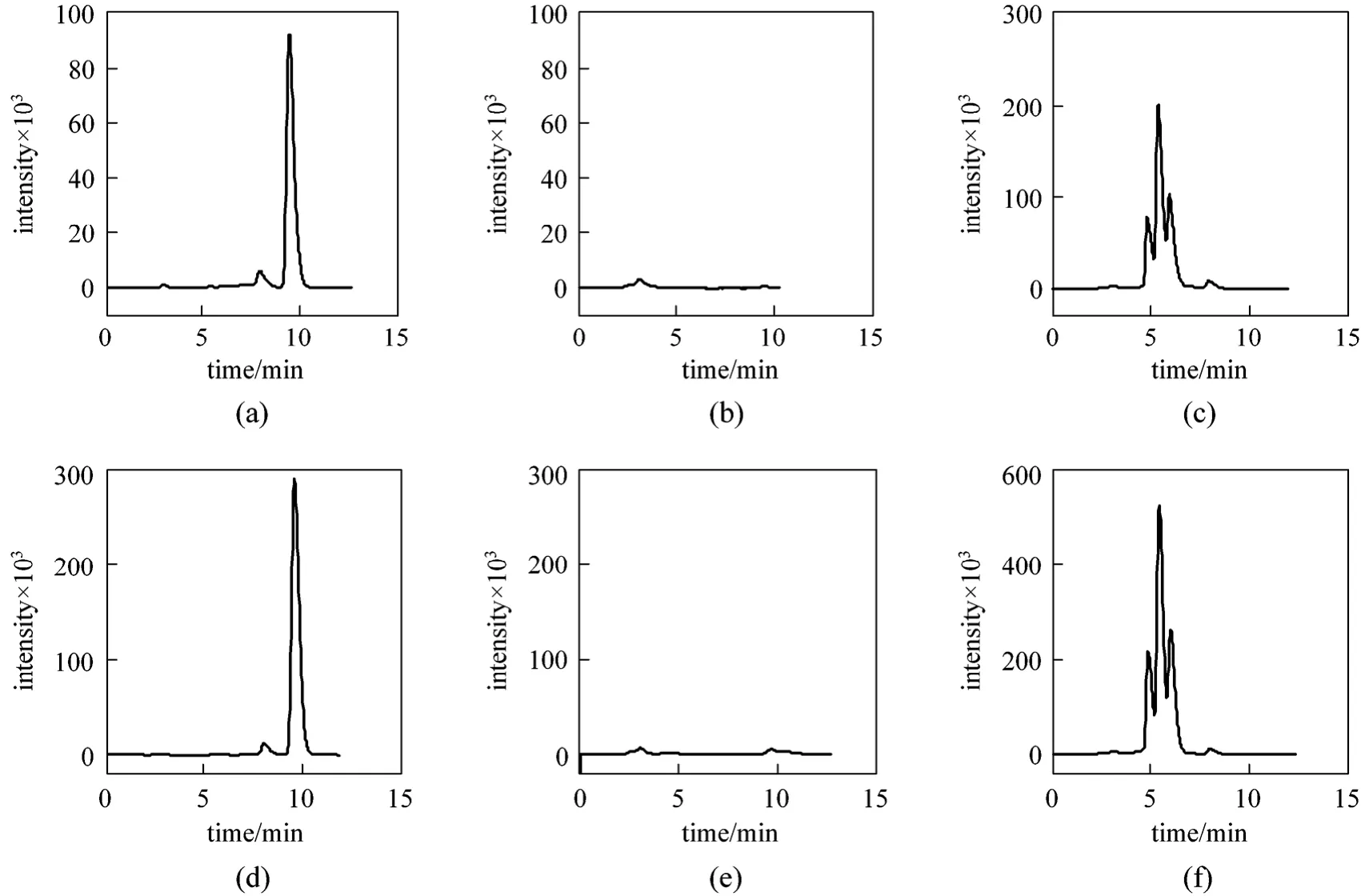
Figure 6 Chromatograms of fractions 1 to 3 collected in runs 3 (a to c) and 4 (d to f)
A two-feed SMB was proposed by disconnecting zone I from II and zone III from IV in a four-zone SMB. Two feeds were added to zones I and III, and two desorbents to zones II and IV separately. The movement of concentration bands was investigated.The modified SMB separated α-tocopherol from its homologue mixtures successfully. It was found that at the outlets of zones I and III, the tail of the strong retained solutes and the front of the weak retained solutes eluted out orderly. Thus, the stream out of zone I or zone III in each switch interval could be cut to three fractions. The first fraction only contained the strong retained solute, the middle fraction contained no solute, and the last fraction contained the weak retained solute. The two-feed SMB could double the productivity, and the solvent consumption could be reduced by reusing the middle fraction.
1 Broughton, D.B., Gerhold, C.G., “Continuous sorption process employing fixed bed of sorbent and moving inlets outlets”, U.S. Pat.,2985589 (1961).
2 Rajendran, A., Paredes, G., Mazzotti, M., “Simulated moving bed chromatography for the separation of enantiomers”, J. Chromatogr.A, 1216, 709-738 (2009).
3 Seidel-Morgenstern, A., Keβler, L.C., Kaspereit, M., “New developments in simulated moving bed chromatography”, Chem. Eng.Technol., 31, 826-837 (2008).
4 Lü, Y.B., Wei, F., Shen, B., Ren, Q.L., Wu, P.D., “Modeling, simulation of a simulated moving bed for separation of phosphatidycholine from soybean phospholipids”, Chin. J. Chem. Eng., 14, 171-177(2006).
5 Wei, F., Zhao, Y.X., “Comparison of enantioseparation of omeprazole by simulated moving bed and batch chromatography”, J. Chem.Eng. Chin. Univer., 23, 303-308 (2009).
6 Cong, J., Lin, B., “Separation of liquiritin by simulated moving bed chromatography”, J. Chromatogr. A, 1145, 190-194 (2007).
7 Pynnonen, B., “Simulated moving bed processing: escape from the high-cost box”, J. Chromatogr. A, 827, 143-160 (1998).
8 Juza, M., Mazzotti, M., Morbidelli, M., “Simulated moving-bed chromatography and its application to chirotechnology”, Trends Biotechnol., 18, 108-118 (2000).
9 Lin, B.C., Simulated Moving Bed Chromatography Technology,Chemical Industry Press, Beijing (2007). (in Chinese)
10 Lee, K., “Two-section simulated moving bed process”, Sep. Sci.Technol., 35, 519-534 (2000).
11 Rhee, H.K., Aris, R., Amundson, N.R., “First-order partial differential equations I: Theory and application of single equations”, Dover Publications, New York (1986).
12 Helfferich, F.G., Whitley, R.D., “Non-linear waves in chromatography II. Wave interference and coherence in multicomponent systems”, J. Chromatogr. A, 734, 7-47 (1996).
13 Guiochon, G., Felinger, A., Shirazi, D.G., Katti, A.M., “Fundamentals of preparative and nonlinear chromatography”, Elsevier Inc.,San Diego (2006).
14 Storti, G., Baciocchi, R., Mazzotti, M., Morbidelli, M., “Design of operating conditions of simulated moving bed adsorptive separation units”, Ind. Eng. Chem. Res., 34, 288-301(1995).
15 Migliorini, C., Mazzotti, M., Morbidelli, M., “Continuous chromatographic separation through simulated moving beds under linear and nonlinear conditions”, J. Chromatogr. A, 827, 161-173 (1998).
16 Mazzotti, M., “Design of simulated moving bed separations: Generalized Langmuir Isotherm”, Ind. Eng. Chem. Res., 45, 6311-6324(2006).
17 Paredes, G., Rhee, H.K., Mazzotti, M., “Design of simulated-movingbed chromatography with enriched extract operation (EE-SMB):Langmuir Isotherms”, Ind. Eng. Chem. Res., 45, 6289-6301 (2006).
18 Mazzotti, M., “Equilibrium theory based design of simulated moving bed processes for a generalized Langmuir isotherm”, J. Chromatogr. A, 1126, 311-322 (2006).
19 Schulte, M., Strube, J., “Preparative enantioseparation by simulated moving bed chromatography”, J. Chromatogr. A, 906, 399-416(2001).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Phenol Oxidation by Combined Cavitation Water Jet and Hydrogen Peroxide*
- Venting Design for Di-tert-butyl Peroxide Runaway Reaction Based on Accelerating Rate Calorimeter Test
- Effect of Return Sludge Pre-concentration on Biological Phosphorus Removal in a Novel Oxidation Ditch*
- Experimental and CFD Studies on the Performance of Microfiltration Enhanced by a Turbulence Promoter*
- Pervaporation of Aqueous Solution of Acetaldehyde Through ZSM-5 Filled PDMS Composite Membrane*
- Effects of CO2 Dilution on Methane Ignition in Moderate or Intense Low-oxygen Dilution (MILD) Combustion: A Numerical Study*
