Removal of Bi (III) with Adsorption Technique Using Coconut Shell Activated Carbon
2012-02-14SARTAPEAshishMANDHAREAniruddhaSALVIPrathmeshPAWARDattatrayaRAUTPrakashANUSEMansingandKOLEKARSanjay
SARTAPE Ashish, MANDHARE Aniruddha, SALVI Prathmesh, PAWAR Dattatraya,RAUT Prakash, ANUSE Mansing and KOLEKAR Sanjay,*
1 Analytical Chemistry Laboratory, Department of Chemistry, Shivaji University, Kolhapur-416 004, India
2 Department of Environmental Science, Shivaji University, Kolhapur-416 004, India
1 INTRODUCTION
The level of natural resources of bismuth is about 2×10-7. The world production of bismuth is about 5000 tones per year. Much of the bismuth produced in the U.S. is obtained as byproduct of refining lead,copper, tin, silver and gold ores. Bismuth and its compounds are used in semiconductor, cosmetics, alloys and metallurgical additives as well as in the preparation and recycling of uranium nuclear fuel [1, 2]. The inorganic bismuth salts are used for medical treatment.Bismuth containing compounds are being used for different medicinal purposes, especially for the treatment of syphilis, gastritis, and ulcer. As the use of bismuth in medicines is increasing, it has spread in the environment, and the exposure of organisms to bismuth increases. A number of toxic effects in humans are attributed to bismuth compounds such as nephropathy,osteoarthropathy, hepatitis, and neuropathology [3].Bismuth contamination is becoming an environmental problem [4]. Therefore, the removal of bismuth is essential for elimination of its contamination.
There are several techniques for removal of Bi(III) from water, including liquid-liquid extraction [5],solid phase extraction [6], flotation [7] and adsorption[8]. The experimental studies on Bi (III) adsorption by using resins [9] and the electrode surface of metals,such as Pt (111) and Pt (100) [10], Pt (110) [11], Au [12]and Si (001) [13], have been reported.
Amongst the many methods available for the removal of heavy metals from aqueous solutions, such as electrochemical precipitation, ion exchange, ultrafiltration, and reverse osmosis, adsorption techniques have been shown to be a feasible option, both technically and economically [14]. Especially, if the adsorbent is inexpensive and readily available, adsorption process provides an attractive alternative. Activated carbons are the effective adsorbents for many pollutant compounds (organic, inorganic, and biological) of concern in water and wastewater treatment. The major use of activated carbon is in solution purification and for the removal of color, odors and other objectionable impurities from liquids, water supplies as well as vegetable and animal oils [15]. Activated charcoal derived from coconut shell was found to be a good non conventional adsorbent used for the removal of Cd (II) [16],Pb [17] and cationic dye [18] from aqueous solutions.The green coconut shell was used, as it is, for separation of Cr (VI), Cr (III), As (V) and Cd (II) [19]. Coconut shell as well as coconut husk was reported for removal of Cr (VI), Zn (II) and Ni (II) [20].
In present paper, we report the adsorption study of bismuth on low cost, easily and abundantly available adsorbent, activated carbon developed from coconut shell. The influence of adsorption time, adsorbent dosage, shaking speed etc. is studied. From experimental data the isotherm models, kinetic model,and the thermodynamic parameters are investigated for adsorption of Bi (III).
2 MATERIALS AND METHOD
2.1 Preparation of materials
All chemicals used were of S. D. Fine Chem. Ltd.(India). The standard solution with 1 mg·mL-1of Bi (III)was prepared from bismuth nitrate and the required solutions were prepared from dilutions with stock solution.
The adsorbent was prepared from dry coconut shells. The shells were crushed into small pieces,washed with double distilled water, and dried in oven at 383 K for 24 h. 25 g dried sample was put in a container and 50 ml concentrated H2SO4was added as impregnating reagent, then kept for 24 h. After the acid was leached out the sample was washed with distilled water till neutral pH. For complete formation as carbaneous material, it was kept in muffle furnace, for 1 h at 573 K. Dried coconut shell activated carbon(CSAC) powder was then sieved through BSS-25.
2.2 Characterization of adsorbent
The developed adsorbent CSAC was characterized with Fourier transform infra red spectroscopy(FTIR) (Perkin Elmer Spectrum 100), and scanning electron microscopy (SEM) (JEOL-JSM 6360 unit,Japan). The FTIR spectrum shows the peaks at 3310.05,2899.46, 1703.10, 1608.48, 1167.01, and 1034.86 cm-1for functional groups O H stretch, C H, C O stretch, C C, S O stretch, and C O stretch, respectively (Fig. 1). These functional groups are responsible for the better adsorption of Bi (III).
Figure 2 shows the SEM images of CSAC before and after adsorption confirming the porous nature. The result of C, H, N, S analyzer (Euro EA, Elemental Analyzer) indicates the major formation of carbon.The adsorption depends on surface area of activated carbon. The surface area and pore diameter of the adsorbents were measured with a NOVA (Quantachrome)surface area analyzer using a Brunauer-Emmett-Teller(BET) nitrogen adsorption technique. The total pore volume was also determined. The BET surface area analysis indicates that the adsorbent has large surface area. The percentage amount of elements, surface area and physical properties are summarized in Table 1.
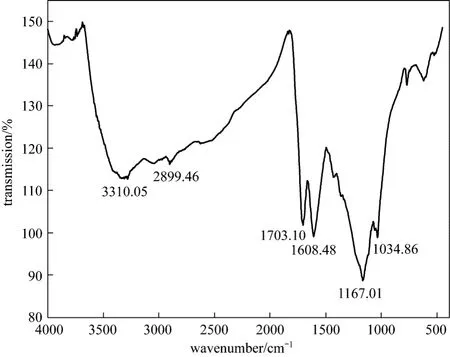
Figure 1 FTIR spectrum of CSAC

Figure 2 SEM images of CSAC before/after adsorption of Bi (III)

Table 1 Characteristics of coconut shell activated carbon
2.3 Batch adsorption experiment
The batch experiment was carried out by using orbital shaker with Erlenmeyer flasks. The agitation was conducted at constant temperature (299±2) K for predetermined period. The adsorption was studied by varying the variables such as initial concentration of metal ion, agitation period, and speed. The medium was maintained acidic, pH 2, throughout the study,since Bi (III) precipitates at pH values higher than 2.5 in aqueous solutions [8]. The concentrations of Bi (III)in residual solutions were analyzed by using AAS(Perkin Elmer, A Analyzer 300). The initial concentration of Bi (III) for experiment was varied from 250 to 1000 mg·L-1and an adsorbent mass from 0.1 to 0.7 g.The kinetic and thermodynamic study was done for different time intervals. For the thermodynamic study temperature was varied from 303 to 323 K. The equilibrium adsorption capacity was evaluated using the following equation,
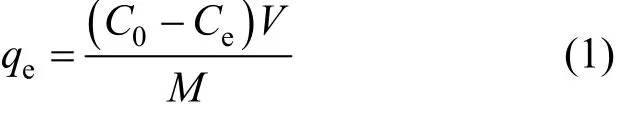
where,qe(mg·g-1) is the equilibrium adsorption capacity,C0andCeare the initial and equilibrium concentrations (mg·L-1) of Bi (III) ion solution,Vis the volume, andMis the mass of adsorbent.
3 RESULTS AND DISCUSSION
3.1 Effect of time on the adsorption
The adsorption of Bi (III) was influenced by time.Fig. 3 shows the result at (299±2) K, 160 r·min-1agitation speed, and initial Bi (III) concentration of 250 mg·L-1. The maximum removal, 98.72%, is obtained at equilibrium time 240 min and the adsorption is 17.62% mg·g-1. There was no further adsorption as time increased, thus further study was carried out in 240 min.

Figure 3 Effect of time on removal and amount adsorbed of Bi (III), on CSAC [C0=250 mg·L-1, T=(299±2) K, CSAC=0.7 g, 160 r·min-1]
3.2 Effect of initial concentration
The removal of Bi (III) is dependent on the initial concentration. Fig. 4 shows that the initial concentration increases from 250 mg·L-1to 1000 mg·L-1, the amount adsorbed increases from 17.62 mg·g-1to 53.47 mg·g-1with the pH value, temperature and other conditions kept constant throughout the study.
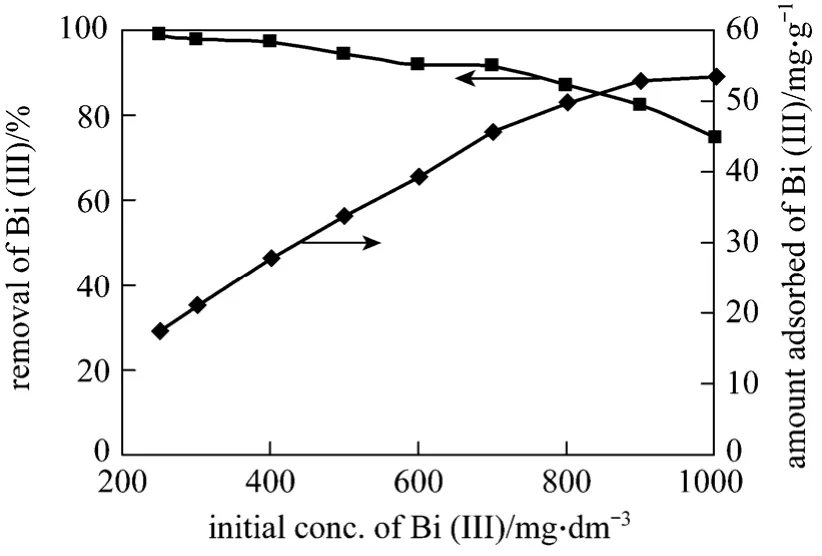
Figure 4 Effect of initial concentration of Bi (III) on amount adsorbed of Bi (III) on CSAC [time=240 min, T=(299±2) K,CSAC=0.7 g, 160 r·min-1]
The adsorption is rapid in the early stage and then attains an asymptotic value for longer adsorption time. The percentage removal of Bi (III) decreases with the increase of initial Bi (III) concentration. It may be due to an increase in the number of Bi (III)ions for the fixed amount of CSAC. The amount of Bi(III) adsorbed per unit mass of activated carbon increases with Bi (III) concentration, may be due to the complete utilization of adsorbent surface and active sites available.
3.3 Effect of adsorbent dosage and agitation speed
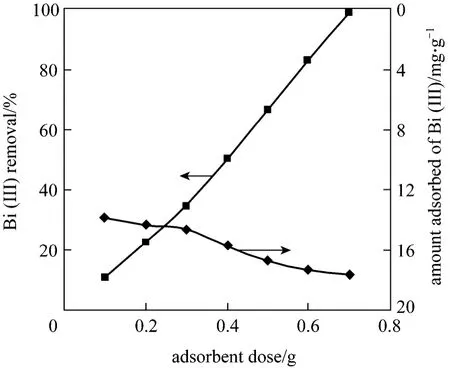
Figure 5 Effect of adsorbent dosage on removal and amount adsorbed of Bi (III) on CSAC [C0=250 mg·L-1,time=240 min, T=(299±2) K, 160 r·min-1]
Figure 5 shows the effect of adsorbent dosage.The removal of Bi (III) is 98.72% with 0.7 g CSAC and the amount adsorbed is 17.62 mg·g-1. Bi (III) removal efficiency increases with the increase of adsorbent dose. As contact surface of adsorbent particles and adsorption sites increases, the adsorption efficiency also increases.
Figure 6 shows the effect of agitation speed on the adsorption of Bi (III) under controlled temperature 160 r·min-1is appropriate.
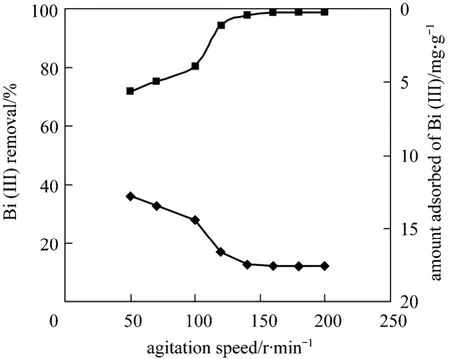
Figure 6 Effect of agitation speed on removal and amount adsorbed of Bi (III) on CSAC [C0=250 mg·L-1, time=240 min,T=(299±2) K, CSAC=0.7 g]
3.4 Adsorption isotherm
An adsorption isotherm, which describes the relation between the activity of adsorbent and the quantity of adsorbate on the surface at constant temperature,is usually employed to describe the adsorption. The L-shaped isotherm shows more affinity between adsorbent and adsorbate [21]. The adsorption isotherm of Bi (III) on CSAC adsorbent is shown in Fig. 7. The Langmuir and Freundlich isotherm models were used in this work.
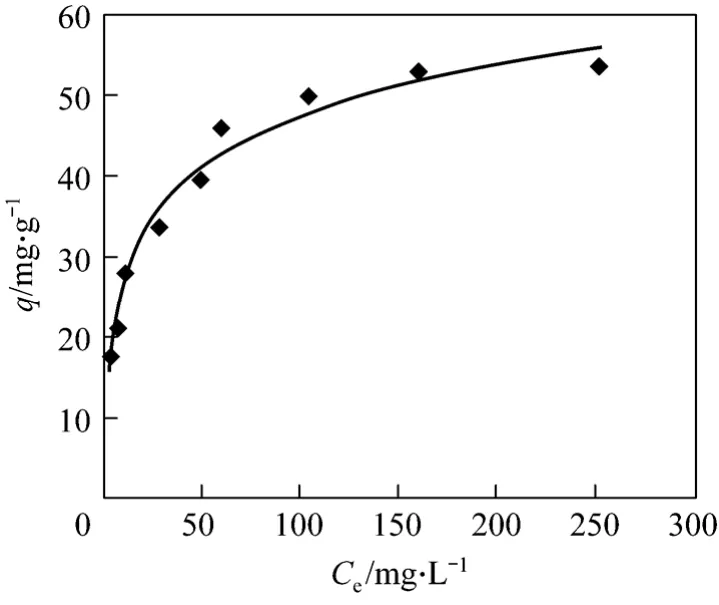
Figure 7 Adsorption isotherm for Bi (III) adsorption on CSAC
The Langmuir model supposes a monolayer adsorption with a homogeneous distribution of adsorption sites and adsorption energy, without interactions between the adsorbed molecules. It is in good agreement with a wide variety of experimental data [22, 23].

whereqmis the maximum loading capacity,Ceandqeare the equilibrium adsorbate concentrations in the aqueous and solid phases, respectively, andbis the equilibrium constant related to the energy of adsorption [24]. The essential characteristics of the Langmuir isotherm can be described by a dimensionless equilibrium parameter,RL[25],

In the present study, the computed values ofRL(Table 2) are in the range of 0 to 1, indicating that the adsorption process is favorable for CSAC adsorbent for the removal of Bi (III) ions. The values ofqmandbare evaluated from the plot (Fig. 8) and are given in Table 2.The high value of correlation coefficientR2indicates a good relation between the parameters and confirms the monolayer adsorption of Bi (III) onto the adsorbent surface.

Table 2 Langmuir and Freundlich constant for the adsorption of Bi (III) on CSAC
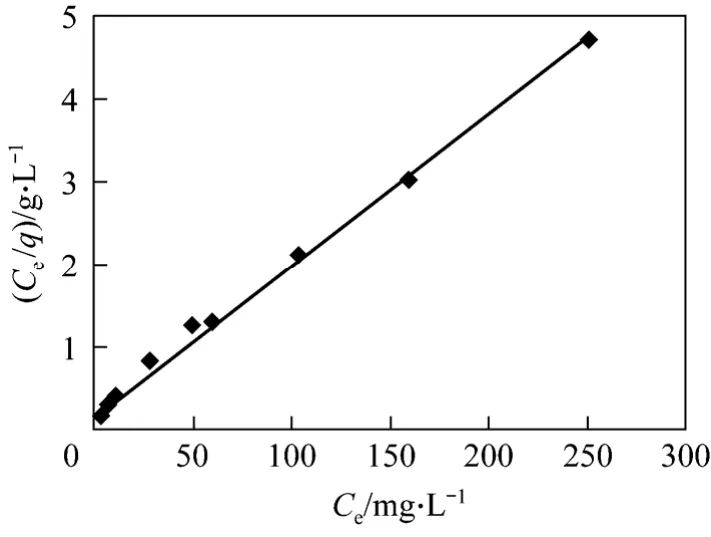
Figure 8 Langmuir isotherm for adsorption of Bi (III) on CSAC
The linear form of the Freundlich isotherm [23] is
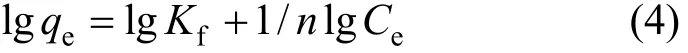
where,qeis the amount adsorbed (mg·g-1),Ceis the equilibrium concentration of adsorbate (mg·L-1),Kfandnare Freundlich constants. The values ofKfandncan be calculated from the intercept and slope (Fig. 9)and presented in Table 2. The results show that the adsorption fits the Langmuir equation better.
3.5 Adsorption kinetics

Figure 9 Freundlich isotherm for adsorption of Bi (III) on CSAC
Kinetics of sorption describes the solute uptake rate, which in turn governs the residence time of sorption reaction. The linear form of pseudo first-order equation (Lagergren equation) is generally expressed as follows,

where,qeandqtare the amount of adsorption at equilibrium (mg·g-1) and at timet(min) respectively, andk1is the rate constant of the pseudo first-order adsorption process. When the values of lg(qe-qt) are correlated witht, a linear relationship is obtained (Fig. 10),from whichk1andqecan be determined (Table 3)from the slope and intercept, respectively [26].
The Lagergren plot (Fig. 10) is for the adsorption of Bi (III) at 0.7 g dosage of CSAC, at the initial concentration of metal ion to be 250 mg·L-1, constant temperature of (299±2) K and agitated with fixed 50 ml quantity and 160 r·min-1.
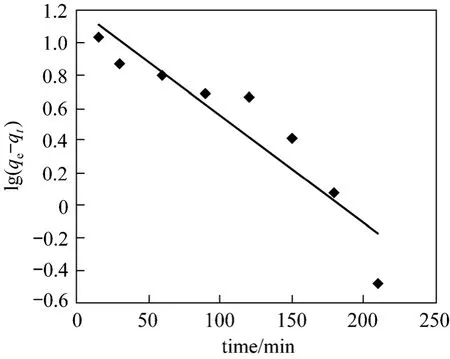
Figure 10 Pseudo first-order for adsorption of Bi (III) on CSAC
Thek1andqevalues are found to be 0.66×10-3min-1and 16.17 mg·g-1, while the experimental value ofqeis 17.62 mg·g-1. The correlation coefficients for the pseudo first-order kinetic model obtained at all the studied concentrations are low and the calculatedqevalues from this model are not reasonable, indicating that this adsorption system does not follow a pseudo first-order reaction.
The rearranged linear form of pseudo second-order adsorption kinetic rate equation is expressed [27] as,
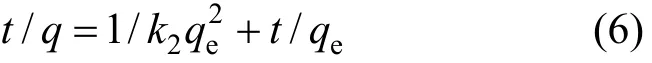
where,k2is the rate constant of pseudo second-order adsorption (g·mg-1·min-1). The rate parametersk2andqecan be obtained from the intercept and slope of the plot oft/qtvs.t(Fig. 11) [28]. The values obtained from the graphs for both adsorption models are given in Table 3. The results show that the pseudo second-order is more appropriate than the pseudo first-order.
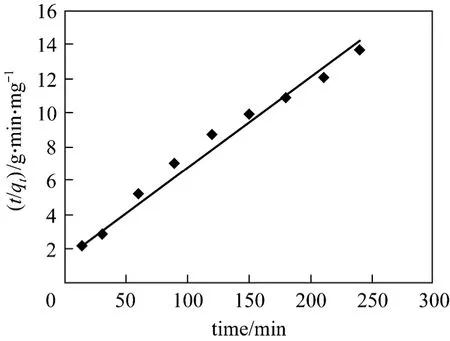
Figure 11 Pseudo second-order for adsorption of Bi (III)on CSAC
3.6 Intraparticle diffusion
The most commonly used technique for identifying the mechanism involved in the sorption process is by fitting the experimental data in an intraparticle diffusion plot. A good fit to the experimental data means that, the sorption rate is governed by intraparticle diffusion, which is the rate-limiting step. According to Weber and Morris, an intraparticle diffusion coefficientkidis defined

Thekid(mg·g-1·min-1/2) value can be obtained from the slope of the plot ofqt(mg·g-1)versust1/2(Fig. 12).
The slope of the linear portion of the plot is defined as the intraparticle diffusion parameterkid, while the intercept reflects the boundary layer effect. Thelarger the intercept, the greater the contribution of the surface adsorption in the rate limiting step. Higher values of the intraparticle rate constants illustrate an enhancement in the rate of adsorption, as well as a better adsorption mechanism. However, these plots indicate that the intraparticle diffusion is not the only rate controlling step because it does not pass through the origin. The rate constant of intraparticle diffusion is given in Table 3. The diffusion process is controlled by the diffusion of ions within the adsorbent [29-31].
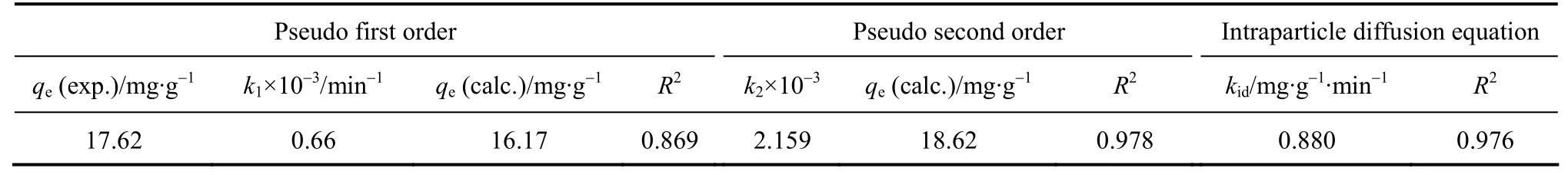
Table 3 Kinetic parameters for the effect of temperature on removal of Bi (III)
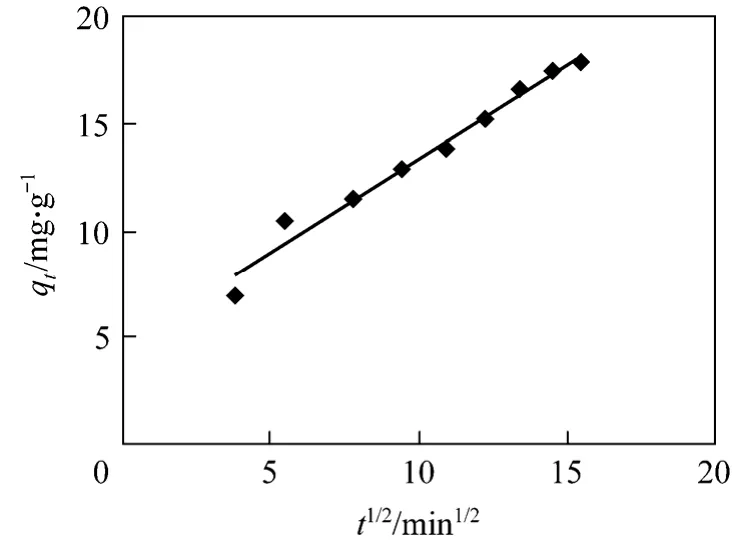
Figure 12 Intraparticle diffusion for adsorption of Bi (III)on CSAC
3.7 Effect of temperature
Figure 13 shows that the adsorption capacity of Bi (III) onto CSAC increase with temperature. The adsorption capacity increases from 54.9 to 59.09 mg·g-1for the initial concentration of 1000 mg·L-1at pH 2.0.

Figure 13 Effect of temperature on amount adsorbed of Bi (III) on CSAC (C0=1000 mg·L-1, time=240 min, CSAC=0.7 g, 160 r·min-1)
Temperature plays a major role in the adsorption of heavy metals on adsorbent. The magnitude of the heat effect for the adsorption process is one of the most important criteria for the efficient removal of heavy metals from the water. The adsorption capacity of carbon increases with temperature from 303 to 323 K.The equilibrium constantKcis evaluated at each temperature using the following relationship,

where,bCAeis the amount adsorbed on solid at equilibrium andCeis the equilibrium concentration.
The standard Gibb’s free energy (ΔGӨ) is calculate as,

where,Tis the temperature in Kelvin andRis the gas constant. Thermodynamic parameters such as change in free energy (ΔGӨ) (kJ·mol-1), enthalpy (ΔHӨ)(kJ·mol-1) and entropy (ΔSӨ) (J·mol-1·K-1) are determined using the following equation,
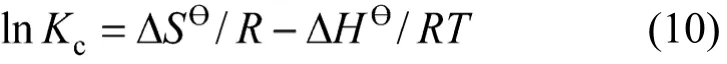
where, ΔHӨand ΔSӨare obtained from the slope and intercept of the van’t Hoff’s plot of lnKcversus1/Tas shown in Fig. 14. Positive value of ΔHӨindicates that the adsorption process is endothermic. The negative values of ΔGӨ(Table 4) reflect the feasibility of the process and the value becomes more negative as temperature increases, indicating that the adsorption is highly favorable and spontaneous. The positive value of standard ΔSӨentropy shows the increased disorder and randomness at the solid solution interface of bismuth ion and CSAC. The enhancement of adsorption capacity of the activated carbon at higher temperatures is attributed to the enlargement of pore size and activation of the adsorbent surface. The enrichment in the adsorption capacity may be due to the chemical interaction between adsorbates and adsorbent, creating some new adsorption sites or increasing the rate of intraparticle diffusion of Bi (III) ions at higher temperatures [32, 33].
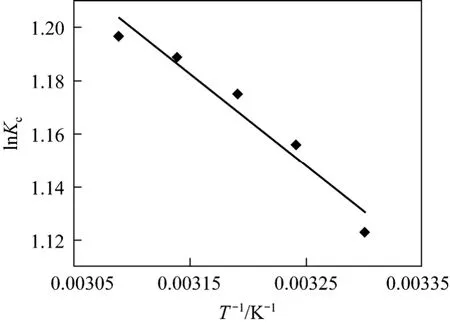
Figure 14 vant Hoff’s plot of adsorption of Bi (III) on CSAC
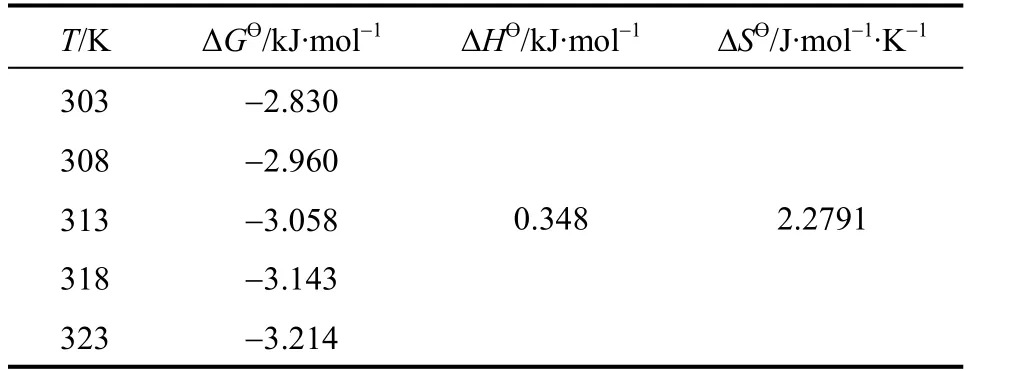
Table 4 Thermodynamic parameters of CSAC
4 CONCLUSIONS
The adsorbent was easily prepared from dry coconut shell, which is abundantly available waste. It was developed as porous, effective and economically affordable activated carbon and its formation was confirmed with various characterizations such as C, H,N, S analyzer, SEM, FTIR and BET surface area analysis. The coconut shell activated carbon has a high capacity to adsorb Bi (III) ions from aqueous solutions with amount adsorbed from 17.62 mg·g-1to 53.47 mg·g-1as the initial concentration increased up to 1000 mg·L-1. All adsorptions were carried out in acidic pH and the required period was only 4 h. Removal percentage was significant, i.e. 98.72%. The adsorption isotherm is an L shaped curve, which indicates less competition between adsorbent and adsorbate.The models of adsorption, Langmuir and Freundlich were used, among which the Langmuir equation shows more applicability to the experimental data than the Freundlich isotherm. The rate of adsorption was also investigated with kinetic study and it was found that the experimental data fits better in pseudo second order than pseudo first order. The adsorption was feasible, spontaneous and endothermic, which was confirmed by the evaluation of thermodynamic parameters viz. ΔHӨ, ΔGӨand ΔSӨ. The results show that the activated carbon from coconut shell can be effectively applied for the removal of Bi (III) from aqueous solutions. Easy and inexpensive availability and suitability for the production of activated carbon from coconut shell, makes it one of the biomass wastes that can be effectively utilized for removal Bi (III) from aqueous solutions.
ACKNOWLEDGEMENTS
The authors acknowledge the DST-FIST, UGC-SAP facilities, Department of Chemistry, Shivaji University,Kolhapur. Authors are grateful to Professor G. S. Gokavi for his valuable suggestions.
NOMENCLATURE
b Langmuir adsorption intensity constant, mg-1
CAeamount adsorbed on solid at equilibrium, mg·L-1
Ceequilibrium concentration of Bi (III) ion solution, mg·L-1
C0initial concentration of Bi (III) ion solution, mg·L-1
c constant
ΔGӨstandard Gibbs energy of adsorption, kJ·mol-1
ΔHӨstandard enthalpy of adsorption, kJ·mol-1
Kcequilibrium constant
KfFreundlich multilayer adsorption capacity, mg·g-1
kidintraparticle diffusion rate, mg·g-1·min-1/2
k1rate constant for pseudo first-order adsorption
k2rate constants of pseudo second-order adsorption
M mass of adsorbent, g
qeamount adsorbed on adsorbent, mg·g-1
qmLangmuir monolayer adsorption capacity, mg·g-1
qtamount adsorbed at time t, mg·g-1
RLdimensionless equilibrium parameter
R2correlation coefficient
ΔSӨstandard entropy of adsorption, J·mol-1·K-1
T temperature, K
t time, min
V volume of the solution, L
1 Mane, C.P., Anuse, M.A., “Studies on liquid-liquid extraction and recovery of Bi (III) from succinate media using 2-octylaminopyridine in chloroform”, J. Chin. Chem. Soc., 55 (4), 807-817 (2008).
2 Guo, H., Li, Y., Xiao, P., He, N., “Determination of trace amount of bismuth(III) by adsorptive anodic stripping voltammetry at carbon paste electrode”, Anal. Chim. Acta, 534, 143-147 (2005).
3 Rao, G.P.C., Rao, M.M., Veni, S.S., Seshaiah, K., Ramesh, A.,Murthy, K.S., “Determination of bismuth in natural water samples by ICP-AES after preconcentration on dithiocarbamates-coated amberlite XAD-7”, Intern. J. Environ. Anal. Chem., 86 (6), 443-452(2006).
4 Mueller, R.L., “Bismuth—An environmental pollutant of the future”,Zentralbl Hyg Umweltmed, 189 (2), 117-124 (1989).
5 Throat, R.B., Burungale, A.S., Kadam Patil, N.B., “Liquid liquid extraction and separation of bismuth(III) with N-n-hexylaniline”,Rasayan, J. Chem., 2 (1), 1-8 (2009).
6 Tokman, N., Akman, S., “Determination of bismuth and cadmium after solid-phase extraction with chromosorb-107 in a syringe”, Anal.Chim. Acta, 519 (1), 87-91 (2004).
7 Ghazy, S.E., Mostafa, G.A., “Separation of Cd(II), Hg(II), Bi (III)and Sb(III) from drinking and river waters by flotation”, Can. J.Anal Sci. Spectros., 53 (1), 28-35 (2008).
8 Wang, R., Liao, X., Zhao, S., Shi, B., “Adsorption of bismuth(III) by bayberry tannin immobilized on collagen fiber”, J. Chem. Technol.Biotechnol., 81, 1301-1306 (2006).
9 Sahan, S., Saçmaci, S., Sahin, U., Ulgen, A., Kartal, S., “An on-line preconcentration/separation system for the determination of bismuth in environmental samples by FAAS”, Talanta, 80 (5), 2127-2131(2010).
10 Smith, S.P.E., Abrun, H.D., “The co-adsorption of UPD copper and irreversibly adsorbed bismuth on Pt(111) and Pt(100) electrodes”, J.Phys. Chem. B, 103, 6764-6769 (1999).
11 Godfrey, D.C., Hayden, B.E., Murray, A.J., Parsons, R., Pegg, D.J.,“Bismuth adsorption on Pt( 110) and the coadsorption of carbon monoxide”, Surface Science, 294, 33-42 (1993).
12 Puckrin, E., Slavin, A.J., “Adsorption of bismuth onto Au(111) surface”, Phys. Rev. B, 41 (8), 4970-4976 (1990).
13 Qian, Y., Bedzyk, M.J., Lyman, P.F., Lee, T.L., Tang, S., Freeman,A.J., “Structure and surface kinetics of bismuth adsorption on Si(001)”, Phys. Rev. B, 54 (7), 4424-4427 (1996).
14 Huang, G., Zhang, H., Shi, J.X., Langrish, T.A.G., “Adsorption of chromium(vi) from aqueous solutions using cross-linked magnetic chitosan beads”, Ind. Eng. Chem. Res., 48, 2646-2651 (2009).
15 Khalkhali, R.A., Omidvari, R., “Adsorption of mercuric ion from aqueous solutions using activated carbon”, Pol. J. Envi. Studies, 14(2), 185-188 (2005).
16 Gaikwad, R.W., “Removal of Cd (II) from aqueous solution by activated charcoal derived from coconut shell”, EJEAFChe, 3 (4),702-709 (2004).
17 Gueu, S., Yao, B., Adouby, K., Ado, G., “Kinetics and thermodynamics study of lead adsorption on to activated carbons from coconut and seed hull of the palm tree”, Int. J. Environ. Sci. Tech., 4 (1),11-17 (2007).
18 Low, K.S., Lee, C.K., “The removal of cationic dyes using coconut husk as an adsorbent”, Pertanika, 13 (2), 221-228 (1990).
19 Pino, G.H., L. Mesquita, M.S., Torem, M.L., “Biosorption of heavy metals by powder of green coconut shell”, Sep. Sci. Technol., 41,3141-3153 (2006).
20 Olayinka, K.O., Alo, B.I., Adu, T., “Sorption of heavy metals from electroplating effluents by low cost adsorbents II: Use of waste tea,coconut shell and coconut husk”, J. Applied Sci., 7 (16), 2307-2313(2007).
21 Sparks, D.L., Environmental Soil Chemistry, 2nd edition, Academic Press, Elsevier Science, California (2003).
22 Al-Masri, M. S., Amin,Y., Al-Akel, B., Al-Naama, T., “Biosorption of cadmium, lead, and uranium by powder of poplar leaves and branches”, Appl. Biochem. Biotechnol., 160 (4), 976-987 (2010).
23 Bansal, R.C., Goyal, M., Activated Carbon Adsorption, CRC Press,Taylor & Francis Group, Boca Raton (2005).
24 Prasad, M., Saxena, S., “Sorption mechanism of some divalent metal ions onto low-cost mineral adsorbent”, Ind. Eng. Chem. Res., 43,1512-1522 (2004).
25 Kannan, N., Veemaraj, T., “Removal of lead(II) ions by adsorption onto bamboo dust and commercial activated carbons -a comparative study”, E. J. Chem., 6 (2), 247-256 (2009).
26 Kumar, P.S., Gayathri, R., “Adsorption of Pb2+ions from aqueous solutions onto bael tree leaf powder: Isotherms, kinetics and thermodynamics study”, J. Eng. Sci. Technol., 4 (4), 381-399 (2009).
27 Demirbasa, E., Kobyab, M., Senturkb, E., Ozkan, T., “Adsorption kinetics for the removal of chromium (VI) from aqueous solutions on the activated carbons prepared from agricultural wastes”, Water SA, 30 (4), 533-540 (2004).
28 Babu, B. V., Gupta. S., “Removal of Cr (VI) from waste water using activated tamarind seeds as an adsorbent”, J. Environ. Eng. Sci., 7,553-557 (2008).
29 Arivoli, S., Martin, P., Prasath, D., Thenkuzhali M., “Adsorption of chromium ion by acid activated low cost carbon”, EJEAFChe, 6 (9),2323-2340 (2007) .
30 Arivoli, S., Hema, M., Karuppaiah, M., Saravanan, S., “Adsorption of chromium ion by acid activatedlow cost carbon-kinetic, mechanistic, thermodynamic and equilibrium studies”, E. J. Chem., 5 (4),820-831(2008).
31 Acharya, J., Sahu, J.N., Sahoo, B.K., Mohanty, C.R., Meikap, B.C.,“Removal of chromium(VI) from wastewater by activated carbon developed from tamarind wood activated with zinc chloride”, Chem.Eng. J., 150, 25-39 (2009).
32 Karthikeyan, T., Rajgopal, S., Miranda, L. R., “Chromium(VI) adsorption from aqueous solution by Hevea Brasilinesis sawdust activated carbon”, J. Hazard. Mat. B, 124, 192-199 (2005).
33 Meena, A.K., Rajgopal, C., Kiran, G.K.M., “Removal of heavy metal ions from aqueous solutions using chemically (Na2S) treated granular activated carbon as an adsorbent”, J. Sci. Ind. Res., 69, 449-453(2010).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Phenol Oxidation by Combined Cavitation Water Jet and Hydrogen Peroxide*
- Venting Design for Di-tert-butyl Peroxide Runaway Reaction Based on Accelerating Rate Calorimeter Test
- Effect of Return Sludge Pre-concentration on Biological Phosphorus Removal in a Novel Oxidation Ditch*
- Separation of α-Tocopherol with a Two-Feed Simulated Moving Bed*
- Experimental and CFD Studies on the Performance of Microfiltration Enhanced by a Turbulence Promoter*
- Pervaporation of Aqueous Solution of Acetaldehyde Through ZSM-5 Filled PDMS Composite Membrane*
