Electrostatic Enhancement of Coalescence of Oil Droplets(in Nanometer Scale) in Water Emulsion
2012-02-14HOSSEINIandSHAHAVI
HOSSEINI M.* and SHAHAVI M.H.
Faculty of Chemical Engineering, Babol Noshirvani University of Technology, Babol, Iran
1 INTRODUCTION
Since 1980s, attention has been focused on emulsions with droplet size in the nanometer range, which is referred to as nanoemulsions (droplet size in the nanometric scale typically in the range of 20-200 nm)[1-4]. Although emulsion systems have developed in industrial applications, the formation of an emulsion is not desirable in many chemical industries. There are two forms of emulsions: oil-in-water (o/w) and waterin-oil (w/o). Demulsification of o/w emulsions can be accelerated by chemical and physical methods. Thanks to economic and especially environmental aspects, the related technique is more suitable than chemical or other mechanical methods. Chemical methods include the use of demulsifiers and emulsifiers [5-7]. The physical methods are considered to increase the contact frequency of oil droplets with study on dynamic interfacial mechanisms and drop size [8-10], to use microwave demulisification [11, 12], membrane or fabrics filtration [13, 14] and electric field [15-26].
Because of the simplicity of the apparatus and process for demulsification, the electrical one would be one of the best ways to destroy o/w emulsions. The separation of water in crude oil emulsion with non-uniform electric field (positive DEP) has been studied and the effects of temperature, time, voltage and all kinds of electrical current were considered [23].The electrostatic separation of water from crude oil is one of the key technologies in the petroleum industry.
Rapid demulsification of dense oil-in-water emulsion by low external electric field has been investigated [24], however, the electrical demulsification of o/w emulsions and the physical effect of a low electric field on the interfacial properties of oil droplets have not been studied in advance. In this research, demulsification of fried oil in water under non uniform electric field (DEP) and the effects of different voltages and temperatures have been reported.
2 DIFFERENT KINDS OF ELECTRIC FIELD
2.1 Uniform electric field (electrophoresis)
In this kind of electric field, both electrodes were settled in the emulsion container and the electric current is created between two electrodes (Fig. 1). The drops will be absorbed towards the electrodes with opposite charge.
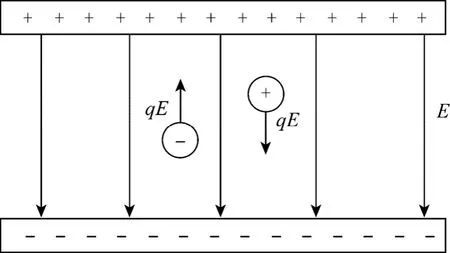
Figure 1 Schematic diagram of a uniform electric field
2.2 Non-uniform (radial) electric field
In this kind of electric field, one electrode is settled inside the emulsion and the other electrode is immersed in 30 % acid container (electrolyte solution).When current electricity passes through two electrodes,the field is created in a radial fashion towards of the electrode [23-26].
3 DIELECTROPHORESIS
Dielectrophoresis (DEP) is defined as the movement of the substance which is imputed for the effects of polarization in a non-uniform electric field.
The positive DEP (p-DEP) is a kind of non-uniform electric field and is related to the migration of microparticles which enters an area in the high electric field regardless of the nature of the charge of the particles,leading to the coalescence of droplets and formation of larger droplets [26]. The negative DEP (n-DEP) is a complete reverse phenomenon and it occurs in particles that are less polarizable than the surrounding medium [27].

Figure 2 Two different particles are in a non-uniform electric field
In Fig. 2, the particle on the right is more polarizable than the surrounding medium and is attracted towards the strong field at the pin electrode (p-DEP),whilst the particle of low polarizability on the left is directed away from the strong field region (n-DEP) [27].
In this research a sinusoidal form of current was created under different temperatures and voltages in glass container which is observable and controllable from outside (Figs. 3-4). The particles migrate to the electrode with the weakest degree of electric field(n-DEP).
Two points must be considered:
(1) Glass should be thick and durable enough against the particle hits.
(2) Glass should not be too thick otherwise it would cause excessive electric field loss.
According to Eq. (1), the relative dielectric constant must be high enough to be effective to move the particles. A dispersed drop with dielectric constantεdis surrounded by continuous phase with dielectric constantεc[26].

In Eq. (1), d and c refer to dispersed phase and continuous phase, respectively.
DEP-forceFdifor cylindrical container (co-centre)has been estimated as follows [26]:
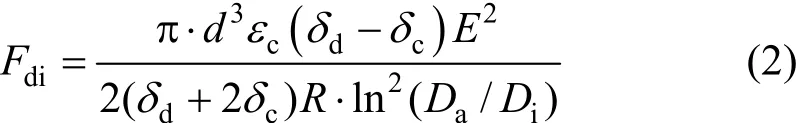
The DEP-forceFdiaccording to Eq. (2) depends on the following parameters:
(1) The force of DEP depends on the ratio of external glass diameterDa(30% acid container) to central glass diameterDi(emulsion container). Eq. (2)shows that the force of DEP will be weaker when the above mentioned ratio is increased, thus the higher electric field strength is required (Fig. 4).
(2) Dielectric constantεin continuous phase depends on the type of emulsion.
(3) When the drops diameter (d) is small, the force of DEP will be weaker.
(4) The difference of electrical permeability(δd-δc) between dispersed and continuous phase depends on the kind of emulsion. When the particle effective permittivity and/or conductivity are smaller than those of the medium, theFdiwill tend to move the particle towards the weakest, or the minimum region of the electric field (Fig. 2). This phenomenon is called n-DEP [27].
(5) The distance between the center of oil drops and central electrode is defined asR. When this distance is increased, the effect of electrical field intensity from central electrode to farther drops will be decreased.When glass diameterDi(emulsion container) is increased, the higher electric field strength is required.
If an o/w emulsion is placed in a non-uniform electric field (DEP), oil particles are charged negative regardless of the kind of the charge (positive or negative).They migrate to the electrode with the weakest degree of electric field (n-DEP). As illustrated in Fig. 2, the more the particles get themselves away from the center,the weaker theFdiforce.
4 MATERIALS AND METHODS
4.1 Preparation of emulsion and the measurement of separation efficiency
The liquid oil of sunflower (of Ladan Company of Iran) was used in this study. The emulsion was prepared by using Hielscher UP400S ultrasonic processor (20 min, cycle time 0.5 and amplitude 100 %) and by adding 2% (by volume) oil and 1% (by volume)sodium dodecyl sulfate (SDS) as a surfactant in the water. To calculate the separation efficiency, at first water was poured into a 4 cm diameter pipe separator(glass container), then it was added to 2% (by volume)of oil. The height of oil was measured by a ruler before being emulsified. After the glass container underwent the ultrasound process, the emulsion was placed under the electric field. The separation took about 10 minutes then the height of the oil was measured in isolation.The separation efficiency can ultimately be calculated by having these amounts.
4.2 Laboratory set up
Separator apparatus involves three concentric glass cylinders. As shown in Fig. 3, it is controllable and observable from outside. The central cylinder (diameter of 4 cm, height of 32 cm) is emulsion container and the middle cylinder (diameter of 7 cm, height of 32 cm) is 30% acid container. The outer cylinder (diameter of 9 cm, height of 32 cm) is thermal converter. Electrodes are separated in here. A metallic electrode was used into the central container. A small wire was used into the middle container (30% acid container). A schematic representation of the systems is shown in Fig. 4.
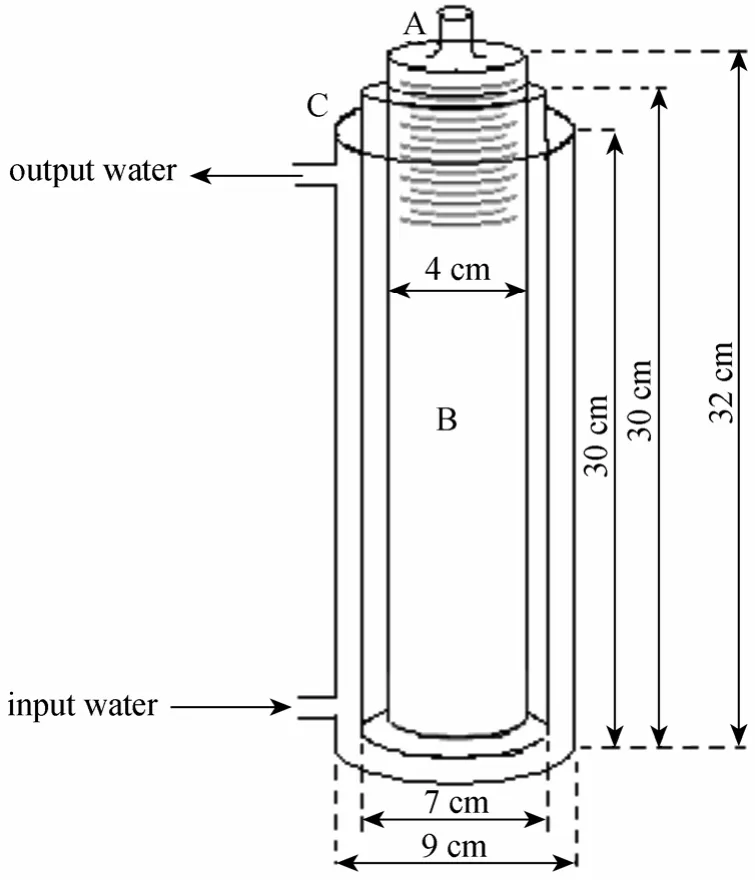
Figure 3 Schematic diagram of the electrical separation instrument
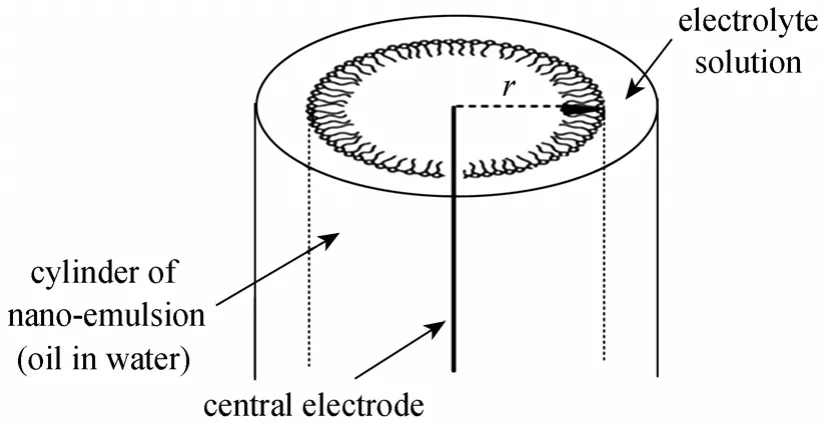
Figure 4 Schematic diagram of the laboratory experience
4.3 Metallic electrode features
E7010-A1 steel metallic electrode with diameter of 1 cm and height of 35 cm was used.
4.4 Particle size analyzer
The dispersions were characterized in terms of particle size and size distribution. Particle-size analysis measurements were performed using a ZetaSizer Nano ZS, Model ZEN 1600 (Malvern Instrument Ltd., Malvern, UK).
4.5 Ultrasonic homogenizer
The disintegration by ultrasound was performed with an ultrasonic processor (UP400S; Dr. Hielscher,Germany) operating at a nominal power of 400 W and 24 kHz. The sonotrode had a diameter of 14 mm.
5 RESULTS AND DISCUSSION
5.1 Effect of voltage magnitude on separation rate
In this study like another studies [23], some items such as voltage, time and temperature has been optimized. Fig. 5 illustrates the effect of voltage magnitude on separation efficiency in constant temperature (38 °C).The curve shows that higher voltages lead to stronger electric field. In this method the greatest electric field is in the vicinity of metallic electrode in the center of the separator (Fig. 4). Separation efficiency at the first couple of minutes is higher than the last time. Figs. 5 and 6 show that the voltages more or less than 3000 V do not have significant effect on increasing of separation efficiency. Thus, 3000 V was chosen as the optimum voltage. It started to be emulsified again by using voltage higher than the optimum one. The maximum separation efficiency of 85% is obtained at the optimum temperature 38 °C and optimum voltage 3000 V.
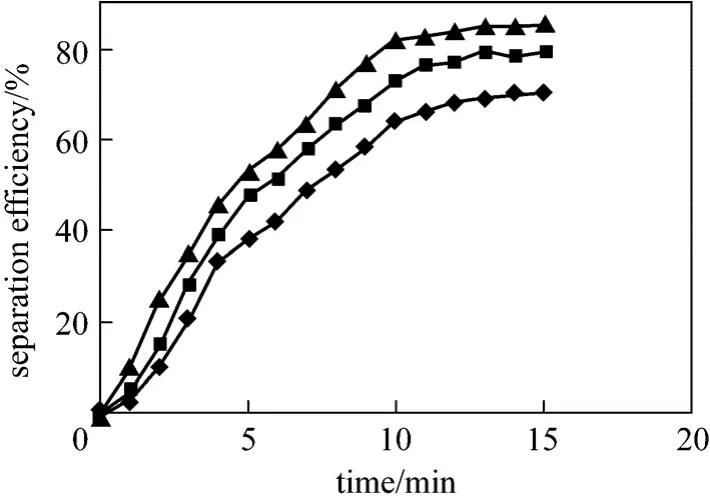
Figure 5 Effect of different voltage on separation efficiency at optimum temperature (38 °C)◆ 1000 V; ■ 2000 V; ▲ 3000 V
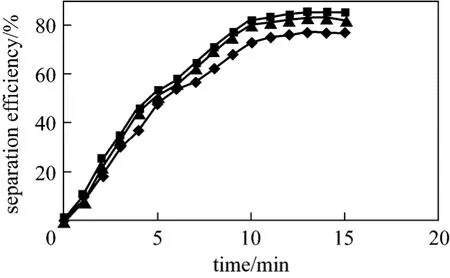
Figure 6 Effect of different voltage on separation efficiency at optimum temperature (38 °C)◆ 3200 V; ■ 3000 V; ▲ 2900 V
5.2 Effect of temperature on separation efficiency
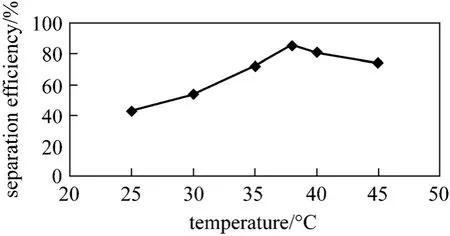
Figure 7 Thermal shift effects on separation efficiency(Separation time for each test is 15 min)
The effect of temperature on separation efficiency is considerable. Fig. 7 illustrates thermal shift effects on separation efficiency. The separation efficiency increases significantly with increasing temperature from 22 to 38 °C, while it is insignificant from 38 to 60 °C. As the temperature reaches higher than 65 °C,the separation efficiency decreases. By increasing temperature, oil viscosity is decreased and the effective hits are reduced despite of the increasing number of hits, resulting in a decrease in efficiency. At temperature higher than optimum one, the mobility of particles is increased and the viscosity is decreased. As a result,there are no any more effective hits. Therefore, it is started to emulsify again and the efficiency is decreased.
5.3 Analysis of particle size
The oil particles sizes have been shown as approximately 76 nm using a ZetaSizer Nano ZS, Model ZEN 1600 (Malvern Instrument Ltd.) (Fig. 8).
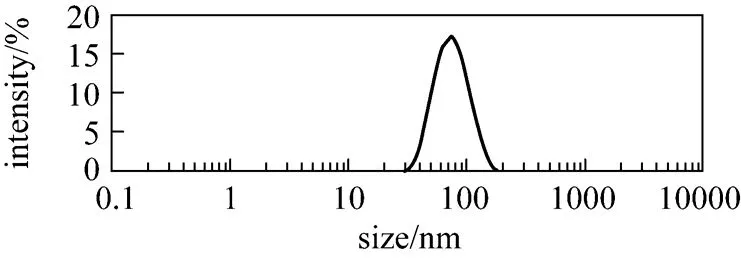
Figure 8 Average size of oil particles using a ZetaSizer Nano ZS, Model ZEN 1600 (Malvern Instrument Ltd., Malvern, UK)
6 CONCLUSIONS
By non-uniform electrical field (n-DEP), the particle effective permittivity and/or conductivity are smaller than those of the medium and p-DEP occurs in particles that are more polarizable than the surrounding medium [27]. The negativeFdiwill tend to move the particle towards the weakest or minimum region of the electric field. Droplets concentration is higher in faraway of central of container where the weakest electrical intensity exists and droplets have more contact to each other in comparison with uniform electric field. As a result, the more particles contact with each other and the higher efficiency of separation can be achieved. The probability of electrical short circuit would be weaker in comparison with uniform electric field because two separated electrodes are used here.Voltage, temperature and time are extremely important parameters for separation rate. Optimum temperature and voltage is approximately 38 °C and 3000 V. The highest separation efficiency in this condition is 85%.Above the optimum voltage, the effect of voltage is negligible because it has been started to emulsify again. Within temperature range of 38 to 60 °C, the increase in temperature has no significant effect on separation efficiency. Within the range of 60 to 70 °C,an increase in temperature has negative effect on separation efficiency because it is started to emulsify again which is similar to higher voltage.
NOMENCLATURE
Daouter cylinder diameter which contains the 30% acid, mm
Diinner cylinder diameter which contains the sample, mm
ddroplet diameter, mm
Ecfield intensity in continuous phase, V·m-1
Edfield intensity in dispersed phase, V·m-1
Rdistance between droplet and the central electrode, mm
δcconductivity in continuous phase, S·m-1
δdconductivity in dispersed phase, S·m-1
εcdielectric constant in dispersed phase, C2·N-1·m-2
εddielectric constant in continuous phase, C2·N-1·m-2
1 Rao, J., McClements, D.J., “Food-grade microem., nanoemulsions and emulsions: Fabrication from sucrose monopalmitate & lemon oil”,Food Hydrocolloids, 25 (6), 1413-1423 (2011).
2 Solans, C., Izquierdo, P., Nolla, J., Azemar, N., Garcia-Celma, M.J.,“Nano-emulsions”,Colloid & Interface Science, 10 (34), 102-110(2005).
3 Qian, C., McClements, D.J., “Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: Factors affecting particle size”,Food Hydrocolloids, 25 (5),1000-1008 (2011).
4 Tadros, T., Izquierdo, P., Esquena, J., Solans, C., “Formation and stability of nano-emulsions”,Advances in Colloid and Interface Science, 108, 303-318 (2004).
5 Chen, C.M., Lu, C.H., Chang, C.H., Yang., Y.M., “Influence of pH on the stability of oil-in-water emulsions stabilized by a splittable surfactant”,Colloids Surf.A, 170, 173-179 (2000).
6 Zaki, N.N., Abdel-Raouf, M.E., Abdel-Azim., A.A., “Propylene oxide-ethylene oxide block copolymers as demulsifiers for water-in-oil emulsions Monatsh”,Chem.Eng., 127, 621-629 (1996).
7 Zaki, N.N., Abdel-Raouf, M.E., Abdel-Azim., A.A., “Propylene oxide-ethylene oxide block copolymers as demulsifiers for water-in-oil emulsions Monatsh”,Chem.Eng., 127, 1239-1245 (1996).
8 Demetriades, K., Coupland, J.N., McClements, D.J., “Physical properties of whey protein stabilized emulsions as related to pH and NaCl”,J.FoodSci., 62, 342-347 (1997).
9 Kim, Y.H., Wasan, D.T., Breen, P.J., “A study of dynamic interfacial mechanisms for demulsification of water-in-oil emulsions”,Colloids Surf.A, 95, 235-241 (1995).
10 Mason, S.L., May, K., Hartland, S., “Drop size and concentration profile determination in petroleum emulsion separation”,Colloids Surf.A, 96, 85-92 (1995).
11 Fang, C.S., Chang, B.K.L, Lai, P.M.C., Klaila, W., “Microwave demulisification”,Chem.Eng.Commun., 73, 227-239 (1988).
12 Fang, C.S., Lai, P.M.C., “Microwave heating and separation of water-in-oil emulsions”,J.Microw.Power Electromagn.Energy, 30,46-57 (1995).
13 Sun, D., Duan, X., Li, W., Zhou, D., “Demulsification of water-in-oil emulsion by using porous glass membrane”,J.Membr.Sci, 146,65-72 (1998).
14 Briscoe, B.J., Luckham, P.F., Jayarajah, J.N., Akeju, T., “Separation of emulsions using fibrous fabric”, Colloids Surf., 163, 151-164 (2000).15 Haifeng, C., Ye, P., “Polarization characteristic of droplet of water-in-oil emulsion in a high uniform electric field”, J. Modelling,Simulation and Optimization, 20, 139-142 (2008).
16 Hirato, T., Koyama, K., Tanaka, T., Awakura, Y., Majima, H., “Demulisification of water-in-oil emulsion by an electrostatic coalescence method”, Mater. Trans. JIM, 32, 257-263 (1991).
17 Wang, S.S., Lee, C.J., “Demulsification of water-in-oil emulsions by use of a high voltage AC field”, Sep. Sci. Technol., 29, 159-170(1994).
18 Tsuneki, I., “Electrical demulsification of oil-in-water emulsion”,Colloids Surf., 302, 581-586 (2007).
19 Beetge, J.H., Horne, B.O., “Chemical demulsifier development based on critical electric field measurements”, SPE J., 13 (3),346-353 (2008).
20 Lu, G., Lu, Q.H., Li., P.S., “Break-down of liquid membrane emulsion under high electric field”, J. Membr. Sci., 128, 1-6 (1997).
21 Eoe, J.S., Ghadiri, M., Sharif, O.A., Williams, T.J., “Electrost. enhancement of coalescence of water droplets in oil. A review of the current understanding”, Chem. Eng. J., 84, 173-192 (2001).
22 Ichikawa, T., “Electrical demulsification of oil-in-water emulsion”,Colloids and Surfaces A, Physicochem. Eng. Aspects, 302, 581-586(2007).
23 Alinezhad, K., Hosseini, M., Mowagharnegad, K., Salehi, M., “Exprimental and modeling approach to separation of water in crude oil emulsion under non-uniform electrical field”, Korean J. Chem. Eng.,27 (1), 198-205 (2010).
24 Ichikawa, T., Itoh, K., Yamamoto, S., Sumita, M., “Rapid demulsification of dense oil-in-water emulsion by low external electric field”,Colloids and Surfaces A, Physicochem. Eng. Aspects, 242, 21-26(2004).
25 Pohl, H.A., “Some effects of non-uniform fields on dielectrics”, J.Appl. Phys, 29, 1182-1188 (1958).
26 Draxler, J., Fluessige Membranen fuer die Abwasserreinigung,Dbv-Verlag, Graz (1992).
27 Flavio, H., Morales, F., Duart, J., Samitier-Marti, J., “Bacterial handling under the influence of non-uniform electric fields: dielectrophoretic and electrohydrodynamic effects”, Anais Annals of the Brazilian Academy of Sciences, 80 (4), 627-638 (2008).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Phenol Oxidation by Combined Cavitation Water Jet and Hydrogen Peroxide*
- Venting Design for Di-tert-butyl Peroxide Runaway Reaction Based on Accelerating Rate Calorimeter Test
- Effect of Return Sludge Pre-concentration on Biological Phosphorus Removal in a Novel Oxidation Ditch*
- Separation of α-Tocopherol with a Two-Feed Simulated Moving Bed*
- Experimental and CFD Studies on the Performance of Microfiltration Enhanced by a Turbulence Promoter*
- Pervaporation of Aqueous Solution of Acetaldehyde Through ZSM-5 Filled PDMS Composite Membrane*
