Issues in Freeze Drying of Aqueous Solutions*
2012-02-14WANGWei王维CHENMo陈墨andCHENGuohua陈国华
WANG Wei (王维), CHEN Mo (陈墨) and CHEN Guohua (陈国华)**
1 College of Life Science, Dalian Nationalities University, Dalian 116600, China
2 Department of Chemical Engineering, Dalian University of Technology, Dalian 116024, China
3 Department of Chemical and Biomolecular Engineering, the Hong Kong University of Science and Technology,Hong Kong, China
1 INTRODUCTION
There is a large demand for rapid development of new pharmaceutical products with ever increasing concerns on health. Among pharmaceutical unit operations, drying in general is an expensive one. Of all drying operations, freeze drying is the most expensive,both in terms of capital and operation costs [1]. There are a number of reasons why the freeze drying method is used to a great extent in industry. The most important one, common to all industrial sectors, is that the products freeze dried are the most often sensitive to heat and cannot be dried with other drying techniques due to their high operating temperatures. Development of freeze dried oral and injectable pharmaceutical products has traditionally been a process of trial and error, with respect to both the formulation composition and the process conditions during freeze drying[2]. Conservative freeze drying conditions result in long processing time, excessive energy consumption and more production cost. The main focus on optimization of the freeze drying cycle in the formulation and process development is to minimize drying time,while maintaining an acceptable product quality.
The increased importance of freeze drying as a unit operation of pharmaceuticals has gained a worldwide interest of research. A growing body of literature demonstrates that the scientific approach can result in improved product quality with minimum trial and error empiricism. Although the modern industry has benefited from existing knowledge of freeze drying,fundamentals in some areas are still undetermined. It is necessary to establish a systematical understanding of the physical chemistry of freezing and freeze drying,the material science and mechanisms of heat and mass transfer involved in different drying stages. This paper presents an overview of relevant literatures concerning these areas.
2 DESCRIPTION OF FREEZE DRYING
Figure 1 shows the diagram of an industrially based freeze dryer for pharmaceuticals. It consists of a drying chamber, a vacuum pump, a condenser, a compressor and a controller, in addition to the auxiliaries.A typical freeze drying process may proceed as follows: liquid material or aqueous solution is filled into partially stopped glass vials, the vials are placed on the cooled shelves within the freeze dryer as shown in Fig. 2 [3]. The shelf temperature is reduced and the material is frozen to a uniform, preset temperature.After freezing, pressure in the drying chamber is then lowered to a level less than the vapor pressure of ice at local temperature to initiate freeze drying. Moisture removal of freeze drying is mainly by sublimation. In order for sublimation to occur, energy must be supplied to balance the latent heat, ΔH of ice sublimation.The shelves are warmed to a temperature high enough for effective sublimation, but not so high as to melt the frozen material in contact with the bottom of the vials. The freeze drying separation process generally involves three stages: freezing, primary drying and secondary drying. During the primary drying, water vapor is progressively removed from the frozen material by sublimation whilst the shelf temperature is controlled at a constant, low temperature. The secondary drying is initiated by increasing the shelf temperature usually to above room value and reducing the chamber pressure further so that the absorbed water within the semi-dried material can be removed until the residual water content decreases to the desired level.

Figure 1 Schematic diagram of a pharmaceutical freeze dryer
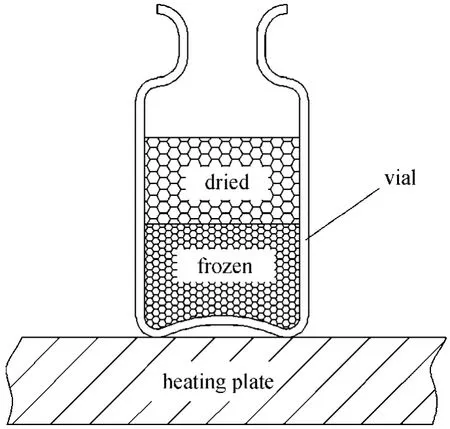
Figure 2 A filled vial on the shelf under freeze drying
3 FREEZING
Freezing is the first step of a freeze drying process, and the performance of the overall freeze drying process depends significantly on this stage. At the end of the freezing step, about 65%-90% of the initial moisture is in the frozen state and the rest remains at the adsorbed state in many cases [4]. The freezing temperature, freezing rate and supercooling degree are all important factors influencing the overall drying time and product quality. Based on the physical and chemical properties of material, the freezing protocol can be optimized to produce the most favorable freeze drying results in terms of both high product quality and short drying time [5]. The characteristics of the frozen matrix strongly affect drying rates at primary and secondary stages [6].

Figure 3 Different freezing behavior of an aqueous solution
It is generally accepted that the liquid material being frozen displays one of two different types of freezing behaviors as shown in Fig. 3: the liquid phase suddenly solidifies (eutectic formation) at a temperature depending on the nature of solids in solutions, or the liquid phase does not solidify (glass formation),but rather it becomes more and more viscous until it finally takes the form of a very stiff substance, and becomes a highly viscous liquid [4].
3.1 Freeze concentration
If a solution is cooled below the normal freezing point without freezing, the solution is said supercooled. For aqueous solution, the supercooling temperature can be in a range of 10 to 15 °C below 0 °C[7], depending on the nucleation temperature of ice.Following the time scale of freezing, a sudden increase in temperature,Tf, indicates the crystallization of ice due to the release of latent heat as shown in Figs. 4 and 5 [7]. For the first case, crystalline components, which have the least solubility in the formulation, form a mixture with crystalline water, and the temperature increases to the eutectic crystalline temperature,Te. A eutectic is defined as an intimate physical mixture of two or more crystalline solids,having then the same physical properties as if it were one component [7]. However, a multi-component mixture often presents noTe[8], because in this freezing stage, the molecules diffusion is reduced dramatically, which, on the other hand, is essential for crystallization [9]. Therefore, one of the most important parameters to optimize the freeze drying process is the reversible transition between viscous and glassy state[10], termed the glass transition temperature of the freeze-concentrated solution,gT′.

Figure 4 Temperature vs. time for freezing of sodium chloride-water system
Franks illustrated the freezing behavior of a sucrose-water system as shown in Fig. 6 [8]. The solute phase is concentrated from an initial solid content of 5% to about 80%, which suggests that most of separation in the freeze drying process occurs in the freezing stage [11], but still a large fraction of unfrozen water exists [8, 9]. The sucrose-water system does not precipitate as a crystal phase when the solution is cooled down to the eutectic point, but remains in a thermodynamically unstable solution. BelowgT′, the system behaves like a solid [12].
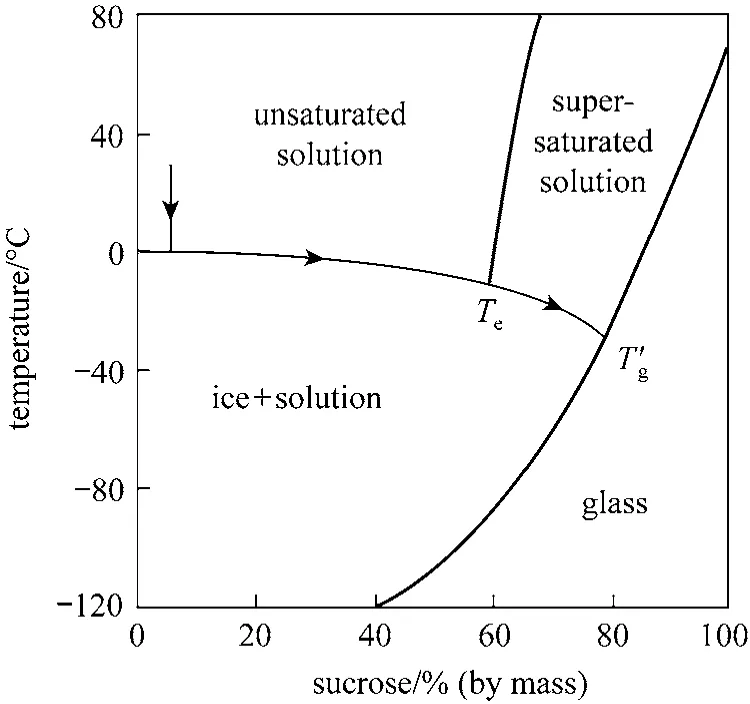
Figure 6 Solid-liquid diagram for sucrose-water system

Figure 5 Temperature vs. time for freezing of an amorphous solute
When the material is further cooled, more liquid water is converted into ice and all interstitial fluid in the vicinity concentrates ultimately until it crystallizes or the viscosity of the system is so high that the system transforms into a solid amorphous state [11].
3.2 Effects of freezing rate
It is well recognized that quick freezing gives small and numerous ice crystals while slow freezing results in large and less numerous crystals. The shape of pores, pore size distribution, and pore connectivity of the porous matrix of the dried layer formed by ice sublimation during the primary drying stage depend on the size of ice crystals during the freezing stage.This dependence is of extreme importance because the mass transfer rate is affected significantly by the porous structure of the dried layer [13, 14]. If the ice crystals are small and discontinuous, the mass transfer rate of vapor in the dried layer is hindered. On the other hand, if large ice crystals are formed and homogeneous dispersion in the frozen solution can be realized, the mass transfer rate could be high and the material could be dried more quickly [15, 16].
Ice crystal size is inversely correlated to the extent of supercooling [6]. A small pore size in the dried layer results in a high resistance to water vapor transport during primary drying [17, 18] and a prolongation of primary drying time. On the other hand, small ice crystals have a large specific surface area, which promotes water desorption during secondary drying[11]. Searles and his co-workers reported that the main determinant of primary drying rate is the ice nucleation temperature, where higher degree of supercooling results in smaller ice crystals, a higher resistance to mass transfer, and a slow drying rate [6, 19].
3.3 Eutectic formation
Freezing history of an aqueous solution can have several possibilities. The simplest one is crystallization of solute from a freeze-concentrated solution to form a simple eutectic mixture. A typical binary eutectic system is the sodium chloride-water system as illustrated in Fig. 7 [2, 20]. Understanding the behavior of this system is useful for a conceptual understanding of material science in freeze drying. Lineabis the freezing point depression curve of water in the presence of sodium chloride, and linebcrepresents the solubility of sodium chloride in water. The intersection of the two lines is the eutectic melting temperature, which for sodium chloride/ice is -21.5°C, and the eutectic composition is about 23.3% (by mass)sodium chloride. Freezing of a 5% solution of sodium chloride in water is described by linedefgh. At room temperature of pointd, the system is entirely liquid.As the solution cools, ice appears at pointein the absence of supercooling. As the system cools, ice continues to crystallize and the solution becomes more concentrated with sodium chloride. At pointf, two phases are present, ice and a freeze concentrated solution of sodium chloride in water. This freeze concentrated solution has the composition given by pointi,which is in equilibrium with ice. At pointg, the solution is saturated with respect to sodium chloride, and solid sodium chloride begins to precipitate. It is only below the eutectic temperature that the system is completely solidified (pointh). Similarly, if the freezing path is across linebcwith an initial concentration of the solution being betweenbandc, the solid sodium chloride precipitates first rather than ice. Other examples of binary eutectic systems are ammonia chloride-water [21] and glycine-water [2], which has similar freezing histories. The relevance of the eutectic temperature to freeze drying is that it represents the maximum allowable temperature in freeze drying,because eutectic melting would form liquid water and destroy the freeze drying process.
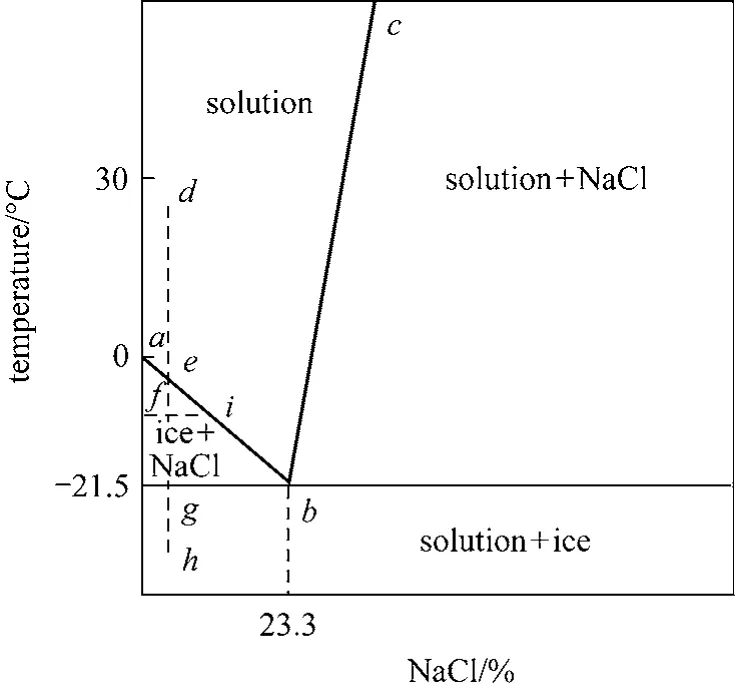
Figure 7 Phase diagram of NaCl-water system
3.4 Glass transition
When a material forms an amorphous phase, it remains as a liquid below the normal freezing point but eventually goes through a rapid increase in viscosity as temperature falls. This transition is defined as the glass transition since the material is glassy. A glass is a true solid that has the chemical composition of the crystalline solid but does not have the ordered molecular structure of the crystalline solid. Fig. 8 displays how a material might form a glass during rapid freezing although it is normally expected to crystallize under slow freezing [22, 23]. The glass transition temperature of a material is strongly dependent on the moisture content. Generally, the highest moisture content at the beginning of drying results in the lowestTg, while the lowest moisture content at the end of drying leads to the highestTg. Thus, there is not one single value ofTgbut rather a range ofTgfor a frozen material corresponding to a range of residual moisture content. At a particular moisture content, the glass transition temperature of a material is termedgT′.
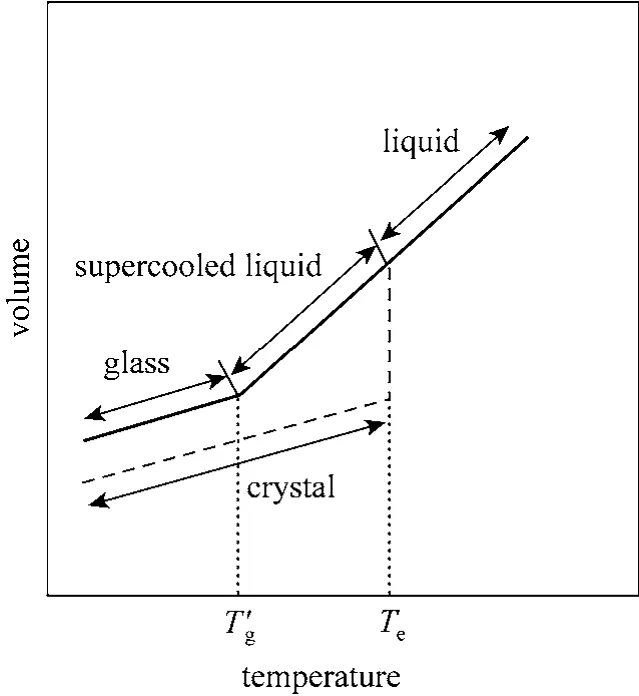
Figure 8 Comparison for a material crystallizing and forming a glassrapid freezing; slow freezing
3.5 Influences of excipients
The use of excipients can help minimize some harmful effects of freeze concentration. Excipients are substances used to facilitate freeze drying of various biological materials, which are usually inactive components like sugars. They are also used to maintain the solid matrix against collapse. Other popular uses of excipients are described in Table 1 [24].
Freezing regime also influences the extent of crystallization and polymorphism of important formulation adjuvant drug, such as mannitol [25]. Crystallization is favored in a formulation when mannitol functions as a bulking agent [11]. Mannitol can gain some advantages like lyophilization at high temperatures and thus shorten freeze drying cycles, and elegant product without defects caused by material collapse [11, 26]. Mannitol used as a stabilizer leads to a system that behaves like a physical mixture, allowing interactions only at the phase boundary [11]. Additionally, it has been observed that different polymorphs of mannitol were obtained at different concentrationsduring freezing. Slow freezing of 10% mannitol solution was demonstrated to yield a mixture of α- and β-polymorphs, and rapid freezing of the same solution resulted in the δ-polymorph [27].
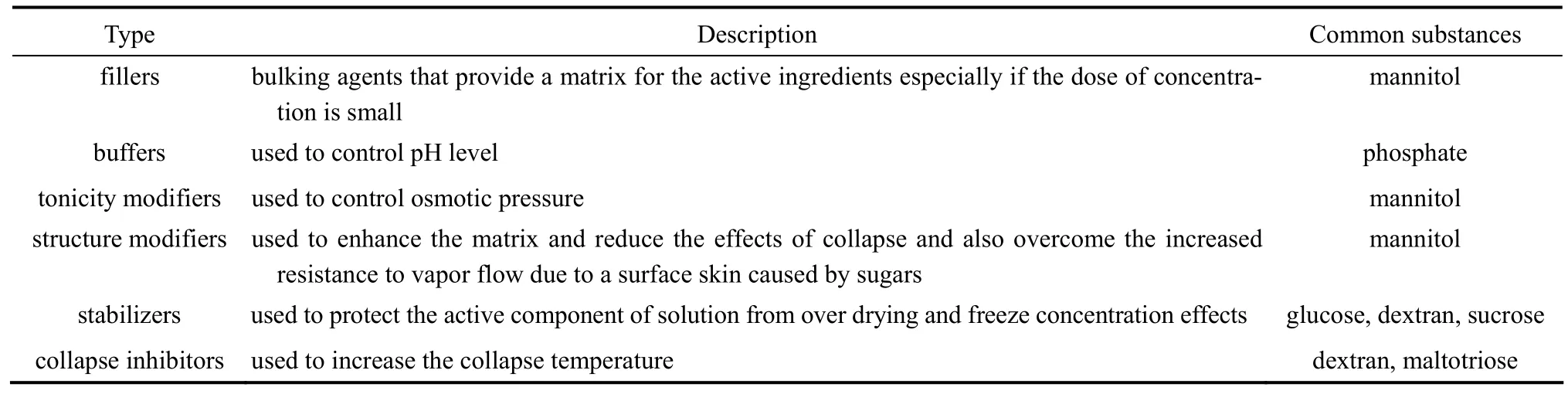
Table 1 Excipients employed in freeze drying
4 PRIMARY DRYING
After the freezing stage, drying chamber is evacuated and the chamber pressure is reduced to a value that allows the ice sublimation to take place.This signifies the start of primary drying stage. Selection of the chamber pressure depends on the ultimate frozen temperature of the material. The pressure must be lower than the vapor pressure of ice in order for sublimation to take place. It was recommended that a reasonable chamber pressure during primary drying should be no more than about one-half and not less than about one-fourth of the vapor pressure of ice at the desired product temperature [2, 26].
As ice crystals sublimate, the sublimation front(sublimation interface), which starts on the outside surface, gradually recedes to the material, and a porous layer of the material remains after front advancing. The heat used for sublimation can be conducted through this dried layer to the sublimation front. The sublimated vapor travels by diffusion and convective flow through the porous layer and enters the drying chamber of freeze dryer. Since water sublimation requires a significant amount of heat, the temperature of material is usually further reduced.
The primary goal of research on freeze drying is to improve process economics by reducing processing time. An important objective is the determination of the drying rate-limiting factors. Heat transfer and mass transfer are the two most likely rate-controlling factors.They depend on operating parameters such as temperature and pressure.
4.1 Structure collapse during freeze drying
Structure collapse refers to a structure loss or damage of the matrix when the sublimation front goes inside the material, which is recognized to be a harmful effect on the freeze drying process. This phenomenon is mainly dependent on the local temperature. The collapse temperature,Tc, is the temperature above which the collapse occurs. It is related to the glass transition temperature,gT′. In one study on collapse,Pikal and Shah found that collapse occurred at a temperature a few degrees higher thangT′ [12]. Wang suggested thatTcis closely related togT′ although the decrease in viscosity atgT′ is not sufficient to cause the structural collapse [28]. Other researchers assumed that they are equally important [5, 29]. Structure collapses due to rigidity decreasing of the solid matrix,which is most likely caused by the viscosity decrease when the local temperature exceeds the value ofgT′.Table 2 lists the collapse and glass transition temperatures of some common pharmaceutical excipients [24,29]. Hatley and Blair presented similar meangT′ data with slight deviation owing to measurement and interpretation differences [30].

Table 2 Collapse and glass transition temperatures of some pharmaceutical excipients
4.2 Mass transfer
The total resistance to mass transfer is the sum of several resistances in series, which are from the partially dried layer, the vial or container, including a partially inserted stopper and the pathway from the chamber to the condenser [7, 11]. The greatest resistance, and therefore the greatest pressure drop, occurs across the dried product layer, where water molecules have to pass the pores and channels which were created during the freezing step to reach the condenser [7].
The diffusion of water vapor in the partially dried layer is one of the major factors affecting the mass transfer rate. The diffusivity is closely related to the pore size. Large ice crystals will be helpful for the movement of water vapor as mentioned in the previous section. The pressure difference is essentially the driving force for the transport of water vapor. The smallest chamber pressure gives the highest ice sublimation rate [26]. In their report of the effect of chamber pressure on heat and mass transfer, Livesey and Rowe noted that the rate-limiting factor in freeze drying changed as drying process proceeds [31]. Initially, the process is limited by heat transfer when the dried layer is thin and an unrealistic heat flux is required to push the sublimation rate to its maximum. After a certain thickness of the dried layer has been developed,the process becomes controlled by mass transfer since,as they claimed, the required heat flux is easily maintained for the decreasing sublimation rate.
4.3 Heat transfer
Process control is related to the control of the product temperature vs. time profile during freeze drying without exceeding the maximum allowable temperature, which is determined by either the Teorof the product [7]. A given product temperature results from a balance between heat transfer rate to the product and the drying rate or mass transfer rate of water vapor, and thus the primary drying stage is a problem coupled heat and mass transfers [11]. The heat supplied to the product is usually by conduction, convection, and/or radiation. Heat transfer from the source to the sublimation interface is an important rate-limiting process during primary drying.
Wolff and his co-workers determined the rate-limiting factors in vial freeze drying of milk [32].By fitting their model to experimental data, they believed that the following three transport parameters affected the drying rate: the water vapor diffusivity in the dried layer, the external mass transfer coefficient and the resistance to the heat transfer from the heated shelf to the frozen material. Since the contact resistance between the vial and the shelf is the most significant barrier to heat transfer, it was determined to be the major rate-limiting factor.
Generally, the effective thermal conductivity comes from two contributions, i.e., the heat conduction and the phase change [33]. When temperature is low, heat conduction plays a major role within the unsaturated region. At high temperature, the phase change is dominant. The controlling step of heat transfer would change from the heat conduction to the phase transition as the moisture content decreases. The main concern for the process in this stage is the collapse temperature, Tc. On the one hand, the temperature of a freeze drying process must be close to Tcto run an effective operation. On the other hand, it cannot exceed this temperature due to the process requirement and product quality concern.
5 SECONDARY DRYING
The secondary drying stage involves the removal of bound water by desorption. The amount of bound water remained is about 10%-35% of the total moisture content [4]. The effect of the bound water on the drying rate and overall drying time is significant. The time required to remove the bound water could be as long as or longer than the time spent for removal of the free water. The governing relation of moisture in porous media during the secondary drying is the adsorption-desorption equilibrium. It depends not only on temperature, but also on moisture content [34, 35].Fakes and co-workers described the moisture sorption behavior of mannitol, anhydrolactase, sucrose,D-(+)-trehalose, dextran 40 and providone (PVP K24)before and after freeze drying in a 10% solution [36].The moisture content of dried product at the end of the secondary drying stage depends on the user requirement or the storage life of products with an acceptable product quality.
The bound water is considered to be either bound at the surface of the crystals in a crystalline product or embedded in the highly viscous amorphous matrix [37].The secondary drying stage for crystalline products may be shortened because higher drying temperature can be applied without the risk of damaging the product [7, 26]. In contrast, glassy products are limited in their drying rate at this stage by the slow molecular diffusion within the dried cake [12]. The amount of residual water may comprise 20% [26] or even up to 40% [7]. Therefore, it is very important to hold the product temperature well below its collapse temperature to prevent cake from deformation [11]. The shelf temperature for amorphous products should be increased slowly (0.1-0.15 °C·min-1), especially in the early stage when the moisture content is high [26].However, Pikal and Shah demonstrated that in the case of maintaining the shelf temperature at the same level as in primary drying for the first few hours, the glass transition temperature rises much faster than the product temperature [12]. This reveals that the shelf temperature during secondary drying should be as high as possible, which is normally in the range of 25 to 50 °C [26]. It is not necessary to change the chamber pressure for secondary drying because the chamber pressure was found to have no measurable influence on drying rate [12].
A strong dependency of the secondary drying rate on the specific surface area is also described in literature similar to the primary drying section [7, 11]. Slow freezing produces large ice crystals with interstitial solid material of low specific surface. Hence, there is a fast drying rate during the primary stage, but conversely a decrease in drying rate during the secondary stage. This is consistent with the observation that the rate-limiting mass transfer process for drying an amorphous solid is either sublimation at the solid-vapor interface or diffusion of vapor within the solid matrix.Thus the mass transfer will be affected by the specific surface area [11, 12]. A desorption process usually requires a raised ambient temperature or a much high vacuum pressure to promote because the amount of the bound water is strongly dependent on the ambient temperature, and the saturation vapor pressure of the bound water is closely related to the residual moisture content.
6 RECENT RESEARCHES AND DEVELOPMENT
Freeze drying is the unique drying process for heat-sensitive materials involving not only in pharmaceuticals and biological products but also in food,as well as nanomaterials. In pharmaceutical and biological industries, water in a dilute aqueous solution is usually removed by freeze drying, leaving the dried products to be packaged or further processed [38].Freeze drying of pharmaceutical proteins is often a greater challenge to the scientists than that of more traditional, and low molecular weight compounds.
A detailed and comprehensive review on the basic physics, chemistry and material science of freezing and freeze drying is available elsewhere with particular reference to protein postprocessing [2]. The article involved a variety of aspects concentrating on frozen materials formulation and freeze-drying process development seeking ways what they called “work smarter, not harder”. In the former part, they emphasized that it is wise to keep the formulation as simple as possible, and the concentration should be kept to a minimum if an additional agent has to be used during freezing no matter what kinds of buffers, salts and excipients. The latter part involved enhancement of heat and mass transfer during freeze-drying. They recommended elimination of product trays and changes in vials to improve the thermal efficiency.Meanwhile, they reaffirmed that the controlling resistance to mass transfer is almost always located at the partially dried layer, and concluded that very high concentrations of solute may not be appropriate for optimum freeze-drying due to lower inherent porosity.
A brief review article on freeze drying of pharmaceutical products was published by Sadikoglu et al.[39], involving choosing of excipients in appropriate quantities and effects of glass transition temperature in the formulation period and freezing, primary and secondary stages drying, and optimal control and remote monitoring from viewpoint of mathematical modeling.The sublimation kinetics during freeze drying of pharmaceutical protein formulation was investigated by Aurelie et al [40]. More recently, the effect of lyophilizate collapse on pharmaceutically relevant proteins was examined by Schersch et al [41]. The freezing step in lyophilization and its impact on quality attributes of biopharmaceuticals and process performance were reviewed by Kasper and Friess [42].
In the food industry, freeze drying is used to dry a wide range of products including dairy products,meats, fruits, vegetables and sea foods. Quality inspections of a dried food product are usually conducted in terms of texture, flavor and aroma, color and appearance, nutritive value, and sensory evaluation. A review article on drying of vegetables, fruits, and aquatic products with and/or without microwave heating was presented by Zhang et al [43]. Various drying methods were compared, among which the freeze drying is the best method for the highest quality compared to other methods of food drying. Materials involved are strawberries, tropical fruits, cabbage, potato, carrot,banana and sea foods, etc. It has to be pointed out,however, that shelf food processed with freeze drying method may have a market bearing problem, i.e. the high price. The better solution to the problem is freeze drying combined with dielectric heating, minimizing drying time without a measurable adverse effect on product qualities. Woo and Mujumdar [44] provided a review on application of electric and magnetic field to the freezing and the freeze drying process that is anticipated in commercial application.
Freeze drying can not only remove the solvent but also offer various advantages desired for different applications of the nanomaterials. Not surprisingly,freeze drying can be found in this area for removing solvent from electrochemical, environmental, engineered materials. Chen and Wang [45] presented a review on role of freeze drying in nanotechnology highlighting the opportunities and challenges of freeze drying in processing of such nanoparticles as pharmaceuticals, catalysts, ceramics, aerogels and electrochemical materials. They concluded that primary reason for choosing freeze drying is that the nanoparticles can be easily formed in solution where the homogenous properties can be retained. The relatively cheap operation cost compared to supercritical fluid extraction is another reason. Qian and Zhang [46] gave another review involving in discussion of the theory of freezing, basics of freeze drying, and the dependence of pore size, pore volume and pore morphology on variables such as freeze temperature, solution concentration, nature of solvent and solute, and the control of the freeze-direction during freeze drying. Recently,multifarious nanoparticles were experimentally prepared with the freeze drying technique including polymeric nanoparticles [47], silver nanopowder [48],Co-doped ZnO nanomaterials [49] as well as W-Cu nano-composite powder [50].
Improving the process economy by reducing freeze drying time is well accepted to be an ultimate goal in R&D of freeze drying. The authors’ research team has been engaged in looking for ways to increase energy utilization efficiency of the process since 2000.Dielectrically assisted heating offers a prospective to settle this problem. Because ice hardly absorbs electric and magnetic wave, microwave energy absorbed by solid matrix in frozen materials from dilute aqueous solution is not enough to enhance the process. Therefore, Wang and Chen [33] proposed a novel idea, i.e.dielectric material assisted microwave freeze drying,for the first time in an attempt to improve the process heat transfer. The results of theoretical investigations demonstrated that the dielectric material can significantly enhance the microwave freeze-drying of aqueous solutions [35, 51, 52]. Experimental verifications were later on conducted, which indicates that dielectric material assisted microwave freeze drying is workable, and about 20% of drying time can be saved compared with conventional freeze drying under the operating conditions tested [53].
Wang and Chen also found that excessive microwave input might cause materials overheating and even crack due to sublimated vapor accumulation within the materials. This suggests that the idea only solves heat transfer problem. As a consequence, Wang and Chen [54] proposed an alternative new idea, i.e.freeze drying of initially porous frozen material, to enhance the process mass transfer. Perhaps, combination of the two ideas is the optimal destination in settling the issue, i.e. simultaneous enhancement of mass and heat transfer, using porous materials with a certain initial porosity to enhance mass transfer, at the same time using microwave heating with the assistance of dielectric material to enhance heat transfer.
Mathematical modeling of solids drying remains an important technique in understanding the drying mechanism and in guiding the dryers design. Wang et al. [55] published a review on this topic analyzed various aspects including solid material as a porous medium, contributions of Luikov and Whitaker, transport mechanisms, equilibrium relationship, volumetric heat source, drying induced deformation, basic assumptions, and current research state. The relationship among accuracy, generality and complexity/simplicity was discussed. The accuracy and generality have similar significance when evaluating an acquired model.At the same time, they pointed out some fundamental data are still lacking due to either lack of understanding of the process or limitation of measurements.
More recently, a brief review was published by summarizing the modeling works of Wang et al [56].The article presented how the mathematical model for various drying purposes evolves from a general mathematical model based on the analysis of coupled heat and mass transport mechanisms. The specific applications studied include the low intensity convective drying, the fixed bed drying, the fluidized bed drying of porous particles, the freeze drying of various high value materials with (or without) microwave heating.
7 SUMMARY
From the viewpoint of energy-saving, freeze drying should be carried out at the highest temperature possible, which is limited by a “maximum allowable temperature”. This temperature indicates the eutectic temperature for a solute that crystallizes during freezing, or the glass transition temperature for a solution that remains amorphous. The freezing stage is of paramount importance in affecting the extent of freeze concentration, size of ice crystals, and consequently primary and secondary drying rates. The use of excipients improves the solid matrix structure against collapse during freeze drying. However, an unconventional excipient may cause market acceptability concerns; those that have a long history are more favorable.In the primary drying stage, the major rate-limiting factor is the movement of water vapor in the partially dried product, or mass transfer. The main factor affecting the desorption rate in the secondary drying stage is the heat transfer from the surrounding to the material. Freeze drying rate may be enhanced by introducing volumetric heating source. Mass transfer rate can be promoted if a porous structure was formed in the freezing stage. Although there is a significant body of literature with a number of reviews available,the research on freeze drying is still in demand with some fundamental physical data not yet available for the materials interacted with water molecules. Innovative ideas in this field are encouraged for a high product quality and low cost process.
1 Brulls, M., Rasmuson, A., “Heat transfer in vial lyophilization”, International Journal of Pharmaceutics, 246 (1/2), 1-16 (2002).
2 Nail, S.L., Jiang, S., Chongprasert, S., Knopp, S.A., “Fundamentals of freeze-drying”, In: Pharmaceutical Biotechnology 14, Nail, S.L.,Akers, M.J., eds., Kluwer Academic/Plenum Publishers, New York,281-360 (2002),.
3 Pikal, M.J., Roy, M.L., Shah, S., “Heat and mass transfer in vial freeze-drying of pharmaceuticals: role of the vial”, Journal of Pharmaceutical Science, 73 (9), 1224-1237 (1984).
4 Liapis, A.I., Bruttini, R., “Freeze drying”, In: Handbook of Industrial Drying, 2nd edition., Mujumdar, A.S., eds., Marcel Dekker, New York, 305-343 (1995).
5 Franks, F., “Improved freeze-drying: an analysis of the basic scientific principles”, Process Biochemistry, 24 (1), Supplement: ProBio-Tech, iii-vii (1989).
6 Searles, J.A., Carpenter, J.F., Randolph, T.W., “The ice nucleation temperature determines the primary drying rate of lyophilization for samples frozen on a temperature-controlled shelf”, Journal of Pharmaceutical Sciences, 90 (7), 860-871 (2001).
7 Nail, S., Gatlin, L., “Freeze drying: principle and practice”, In:Pharmaceutical Dosage Forms, Avis, A., Liebermann, A., Lachmann,L., eds., Marcel Dekker, New York, 163-333 (1993).
8 Franks, F., “Freeze-drying of bioproducts: Putting principles into practice”, European Journal of Pharmaceutics and Biopharmaceutics, 45 (3), 221-229 (1998).
9 Hatley, R.H.M., Franks, F., Brown, S., Sandhu, G., Gray, M., “Stabilization of a pharmaceutical drug substance by freeze-drying: A case study”, Drug Stability, 1, 73-85 (1996).
10 Franks, F., “Freeze-drying: from empiricism to predictability”,Cryoletters, 11 (2), 93-110 (1990).
11 Pikal, M.J., “Freeze drying”, In Encyclopedia of Pharmaceutical Technology, Marcel Dekker, New York, 1299-1326 (2002).
12 Pikal, M.J., Shah, S., “The collapse temperature in freeze drying:dependence on measurement methodology and rate of water removal from the glassy phase”, International Journal of Pharmaceutics, 62(2/3), 165-186 (1990).
13 Hartel, R.W., “Ice crystallization during the manufacture of ice cream”, Trends in Food Science and Technology, 7 (10), 315-321(1996).
14 Russel, A.B., Cheney, P.E., Wantling, S.D., “Influence of the freezing conditions on ice crystallization in ice cream”, Journal of Food Engineering, 39, 179-191 (1999).
15 Millman, M.J., Liapis, A.I., Marchello, J.M., “An analysis of the lyophilization process using a sorption-sublimatin model and various operational policies”, AIChE J., 31 (10), 1594-1604 (1985).
16 Petropoulos, J.H., Petrou, J.K., Liapis, A.I., “Network model investigation of gas-transport in bidisperse porous adsorbents”, Ind. Eng.Chem. Res., 30 (6), 1281-1289 (1991).
17 Pikal, M.J., Shah, S., Senior, D., Lang, J.E., “Physical chemistry of freeze-drying: measurement of sublimation rates for frozen aqueous solutions by a microbalance technique”, Journal of Pharmaceutical Sciences, 72 (6), 635-650 (1983).
18 Willemer, H., “Measurements of temperatures, ice evaporation rates and residual moisture contents in freeze-drying”, Developments in Biological Standardization, 74, 123-134 (1992).
19 Searles, J.A., Carpenter, J.F., Randolph, T.W., “Annealing to optimize the primary drying rate, reduce freezing-induced drying rate heterogeneity, and determine Tg′ pharmaceutical lyophilization”,Journal of Pharmaceutical Sciences, 90 (7), 872-887 (2001).
20 Suzuki, Y., Sumi, Y., Mizuta, T., “Studies on development of manufacturing processes for pharmaceutical freeze-dried products”, In:Freeze-drying/Lyophilization of Pharmaceutical and Biological Products, Rey, L., May, J.C., eds., Marcel Dekker, New York,433-463 (1999).
21 Hellawell, A., “Local convective flows in partly solidified alloys”, In:Structure and Dynamics of Partially Solidified Systems, Loper, D.E.,eds., Martinus Nijhoff Publishers, Boston, 5-23 (1986).
22 Shackelford, J.F., Introduction to Materials Science for Engineers,3rd edition, Prentice-Hall International, London, 404 (1992).
23 Craig, D.Q.M., Royall, P.G., Kett, V.L., Hopton, M.L., “The relevance of the amorphous state to pharmaceutical dosage forms:Glassy drugs and freeze dried systems”, International Journal of Pharmaceutics, 179 (2), 179-207 (1999).
24 Snowman, J.W., “Formulation and cycle development for lyophilization: first steps”, Pharmaceutical Engineering, 13, 26-34 (1993).
25 Hsu, C.C., Walsh, A.J., Nguyen, H.M., Overcashier, D.E.,Koning-Bastiaan, H., Bailey, R.C., Nail, S.L., “Design and application of a low-temperature Peltier-cooling microscope stage”, Journal of Pharmaceutical Sciences, 85 (1), 70-74 (1996).
26 Tang, X., Pikal, M.J., “Design of freeze-drying processes for pharmaceuticals: practical advice”, Pharmaceutical Research, 21 (2),191-200 (2004).
27 Kim, A.I., Akers, M.J., Nail, S.L., “The physical state of mannitol after freeze-drying: effects of mannitol concentration, freezing rate,and a noncrystallizing cosolute”, Journal of Pharmaceutical Sciences, 87 (8), 931-935 (1998).
28 Wang, W., “Lyophilization and development of solid protein pharmaceuticals”, International Journal of Pharmaceutics, 203 (1/2),1-60 (2000).
29 Hatley, R.H.M., Franks, F., “Application of DSC in the development of improved freeze-drying process for labile biologicals”, Journal of Thermal Analysis, 37 (8), 1905-1914 (1991).
30 Hatley, R.H.M., Blair, J.A., “Stabilisation and delivery of labile materials by amorphous carbohydrates and their derivatives”, Journal of Molecular Catalysis—B Enzymatic, 7 (1-4), 11-19 (1999).
31 Livesey, R.G., Rowe, T.W.G., “A discussion of the effect of chamber pressure on heat and mass transfer in freeze-drying”, Journal of Parenteral Science and Technology, 41 (5), 169-171 (1987).
32 Wolff, E., Gibert, H., Rodolphe, F., “Vacuum freeze-drying kinetics and modeling of a liquid in a vial”, Chem. Eng. Process., 25 (3),153-158 (1989).
33 Wang, W., Chen, G.H., “Numerical investigation on dielectric material assisted microwave freeze-drying of aqueous mannitol solution”,Drying Technology, 21 (6), 995-1017 (2003).
34 Chen, G.H., Wang, W., Mujumdar, A.S., “Theoretical study of microwave heating patterns on batch fluidized bed drying of porous material”, Chem. Eng. Sci., 56 (24), 6823-6835 (2001).
35 Wang, W., Chen, G.H., “Heat and mass transfer in dielectric-material-assisted microwave freeze-drying of skim milk with hygroscopic effect”, Chem. Eng. Sci., 60 (23), 6542-6550 (2005).
36 Fakes, M.G., Dali, M.V., Haby, T.A., Morris, K.R., Varia, S.A.,Serajuddin, A.T.M., “Moisture sorption behavior of selected bulking used in lyophilized products”, PDA Journal of Pharmaceutical Science and Technology, 54 (2), 144-149 (2000).
37 Oetjen, G.W., Haseley, P., Freeze Drying, 2nd edition, Wiley-VCH,Weinheim, 345-355 (2004).
38 Dolan, J.P., Scott, E.P., “Microwave freeze-drying of aqueous solutions”, In: Advances in Heat and Mass Transfer in Biological Systems ASME, HTD-Vol. 288, Hayea, L.J., Roemer, R.B., eds., United Engineering, New York, 91-98 (1994).
39 Sadikoglu, H., Ozdemir, M., Seker, M., “Freeze-drying of pharmaceutical products: research and development needs”, Drying Technology, 24 (7), 849-861 (2006).
40 Aurelie, H., Julien, A., Severine, V., “Sublimation kinetics during freeze-drying of pharmaceutical protein formulation”, Drying Technology, 25 (4-6), 753-758 (2007).
41 Schersch, K., Betz, O., Garidel, P., “Systematic investigation of the effect of lyophilizate collapse on pharmaceutically relevant proteins I: Stability after freeze-drying”, Journal of Pharmaceutical Sciences,99 (5), 2256-2278 (2010).
42 Kasper, J.C., Friess, W., “The freezing step in lyophilization: Physico-chemical fundamentals, freezing methods and consequences on process performance and quality attributes of biopharmaceuticals”,European Journal of Pharmaceutics and Biopharmaceutics, 78,248-263 (2011).
43 Zhang, M., Jiang, H., Lim, R.X., “Recent developments in microwave-assisted drying of vegetables, fruits, and aquatic products:drying kinetics and quality considerations”, Drying Technology, 28(11), 1307-1316 (2010).
44 Woo, M.W., Mujumdar, A.S., “Effects of electric and magnetic field on freezing and possible relevance in freeze drying”, Drying Technology, 28 (4), 433-443 (2010).
45 Chen, G.H., Wang, W., “Role of freeze drying in nanotechnology”,Drying Technology, 25 (1-3), 29-35 (2007).
46 Qian, L., Zhang, H.F., “Controlled freezing and freeze drying: a versatile route for porous and icro-/nano-structured materials”,Journal of Chemical Technology & Biotechnology, 86, 172-184(2011).
47 Patil, V.V., Dandekar, P.P., Patravale, V.B., “Freeze drying: potential for powdered nanoparticulate product”, Drying Technology, 28 (5),624-635 (2010).
48 Shi, W.Z., Liu, J., Cheng H.X., “Preparation of silver nanopowder by freeze-drying procedure”, Drying Technology, 27 (4), 529-533(2009).
49 Yang, H.M., Nie, S., “Preparation and characterization of Co-doped ZnO nanomaterials”, Materials Chemistry and Physics, 114 (1),279-282 (2009).
50 Xi, X.L., Xu, X.Y., Nie, Z.R., He, S., Wang, W., Yi, J., Zuo, T.Y.,“Preparation of W-Cu nano-composite powder using a freeze-drying technique”, International Journal of Refractory Metals and Hard Materials, 28 (2), 301-304 (2010).
51 Wang, W., Chen, G.H., Gao, F.R., “Effect of dielectric material on microwave freeze drying of skim milk”, Drying Technology, 23 (1/2),317-340 (2005).
52 Wang, W., Chen, G.H., “Theoretical study on microwave freeze-drying of an aqueous pharmaceutical excipient with the aid of dielectric material”, Drying Technology, 23 (9-11), 2147-2168 (2005).
53 Wang, W., Chen, G.H., “Freeze drying with dielectric-material-assisted microwave heating”, AIChE J., 53 (12), 3077-3088 (2007).
54 Wang, W., Chen, G.H., “Numerical investigation on freeze-drying of initially porous frozen material from aqueous solution”, In: Proceedings of the 16th International Drying Symposium, Hyderabad,India, 508-513 (2008).
55 Wang, W., Chen, G.H., Mujumdar, A.S., “Physical interpretation of solids drying: An overview on mathematical modeling research”,Drying Technology, 25, 659-668 (2007).
56 Wang, W., Ma, H.X., Chen, G.H., “A model for drying of porous materials: from generality to specific applications”, Drying Technology, 29 (13), 1542-1555 (2011).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Experimental and Modelling Studies of Biomass Pyrolysis*
- Synergistic Multilayer Adsorption for Low Concentration Dyestuffs by Biomass
- Kinetics of Photocatalytic Degradation of Gaseous Organic Compounds on Modified TiO2/AC Composite Photocatalyst*
- 3D Numerical Study on Compound Heat Transfer Enhancement of Converging-diverging Tubes Equipped with Twin Twisted Tapes*
- Techno-economic Analysis of Distributed Hydrogen Production from Natural Gas
- Error Analysis of Adsorption Isotherm Models for Acid Dyes onto Bamboo Derived Activated Carbon
