Experimental and Modelling Studies of Biomass Pyrolysis*
2012-02-14KaLeungLamAdetoyeseOlajireOyedunandChiWaiHui
Ka Leung Lam, Adetoyese Olajire Oyedun and Chi Wai Hui**
Department of Chemical and Biomolecular Engineering, the Hong Kong University of Science and Technology,Hong Kong, China
1 INTRODUCTION
For several decades, there have been extensive researches on biomass thermal conversion. The thermal decomposition of organic materials at elevated temperatures in the absence of oxygen yields carbonaceous residues, liquid hydrocarbons and combustible gases. Depending on the process conditions and the type of feedstocks, the yield distribution among the three categories of product and their constitution can vary significantly. The understanding of how processing conditions and feedstock characteristics influence the thermal conversion outcome can greatly assist the design, operation and optimization of thermal conversion processes. Pyrolysis is one category of thermal conversion that is carried out at a relatively lower temperature range resulting in moderate to high fraction of liquid hydrocarbons.
In studying the effects of processing conditions on pyrolysis, the heating profile, essentially the heating rate and the final pyrolysis temperature, is usually being considered. The impacts being studied include product distribution, product composition, product energy content and pyrolysis temperature. The influence of heating rate on the pyrolysis performance has been studied by many [1-8]. Park et al. [3] suggested that there is a competition between gas, char, and oil yield during wood pyrolysis depending on the heating conditions. In a study by Williams & Besler on biomass [2], it shows a general trend that an increase in the heating rate slightly reduces the char yield and increases both the oil and gas yield. This change in the product distribution has also been noticed by some other works [5, 7]. These observations suggest that possibly, the pyrolysis mechanism is modified when the heating rate changes. Williams & Besler [2] also studied on the effect of final pyrolysis temperature on the yield distribution and found that a higher temperature would reduce the char yield and increase the yields of the other fractions. Other than the heating profile, Curtis and Miller [9] remarked that the increase in the mass-transfer resistance results in the decrease of condensable fraction because a longer vapour residence time in the reacting region induces more severe secondary reaction.
Mathematical models that are capable to describe pyrolysis phenomena can greatly assist the large scale development and optimization of pyrolysis processes[10]. In the field of biomass pyrolysis, there have been quite a number of works on modelling particle pyrolysis [3, 11-18]. In modelling biomass particle pyrolysis,it is common to employ a kinetic model, whether it is a simple lumped kinetic model or a complex multiple reactions one, to form the base of the model for describing pyrolysis reactions. Afterwards, the consideration of the physical processes that involve physical changes,heat transfer and mass transfer comes in place. Furthermore, additional elements such as secondary reaction, particle shrinkage, heat of pyrolysis reaction,vapour residence time and interaction with the reactor environment are usually considered and studied.
This work involves both experimental and modelling studies on three biomass materials—wood sawdust, bamboo shred and Jatropha Curcas seed cake residue. By utilizing the experimental and modelling approaches, this study aims to generally discuss on and provide insights for the considerations of feedstock size, pyrolysis energy usage, processing time and product quality in the design and operation of pyrolysis processes.
2 MATERIALS AND METHODOLOGY
2.1 Experimental study
Wood, bamboo and oil seed cake residue, were studied in this work. Wood and bamboo are in sawdust form and shred form respectively. Oil seed cake residue isJatropha Curcasseed kernel residue remaining after solvent extraction byn-hexane and is in fine powder form of less than 600 μm. TGA/DTA92 Seteram II was used to perform non-isothermal thermogravimetric analysis (TGA) and differential thermal analysis (DTA) on the three biomass feedstocks.Analyses were carried out at heating rate of 5, 10, 20,30 °C·min-1in a nitrogen-purged environment with sample masses between 2 mg and 10 mg. The analyses provide information on the pyrolysis mass loss kinetics and heat flow. Because the analyser provides a good control of pyrolysis environment, the TG data were also used to evaluate on how the pyrolysis process conditions can influence its performance. Three aspects, namely the effects of heating rate, final pyrolysis temperature and sample size, were examined.
2.2 Modelling study
A transient particle pyrolysis model has been developed in earlier work [19, 20] to simulate the pyrolysis progress of a bulk particle. This work improves on the model to include two sub-models—a particle shrinking model and a reactor model. The mathematical model, building on MATLAB®platform, utilizes the thermal analysis result from the experimental study and the physical properties or correlations from the literatures. This enables a more realistic description of the pyrolysis behaviour of selected feedstock.The model was then used for carrying out discussion on certain pyrolysis operation strategies.
3 EXPERIMENTAL
3.1 Thermal analysis result
Because of the differences in the compositions,different feedstocks have variations in the TG profiles,the major mass loss temperature ranges, the final mass loss and the pyrolysis completion temperature. Fig. 1 compares the TG profiles for the studied feedstock at heating rate of 10 °C·min-1.
In biomass pyrolysis, there exists a moisture and volatile extractives removal phase before 200 °C, which is not found in non-biomass feedstocks. About 8% of the original masses are lost during this pre-pyrolysis phase. The major pyrolysis phase involving mass loss occurs after 200 °C. For some biomass materials like wood, they mainly compose of hemicellulose, cellulose and lignin. These long chain materials are found to decompose at different temperature ranges,i.e. with hemicellulose at 220-315 °C, cellulose at 315-400 °C and lignin at 160-900 °C [21].

Figure 1 TG profiles of the studied biomass materialsat heating rate of 10 °C1—wood; 2—bamboo; 3—Jatropha Curcas seed cake
In order to account for the variations in the TG profiles among different feedstocks, a study of feedstock composition is beneficial. It is observed from Fig. 1 that the TG profiles of wood and of bamboo are quite similar. It possibly suggests that these two types of biomass have a similar constitution, but are quite different fromJatropha Curcasseed cake. This is in agreement with some wood and bamboo composition studies. Sjöström [22] studied the composition of several wood species and found the main constitutions—cellulose, hemicellulose and lignin are in the range of 39.5%-45.0%, 19.2%-30.6% and 22%-31.3% respectively. Scurlocket al. [23] determines the composition of bamboo to be 43.3%, 24.6% and 26.2% for cellulose, hemicellulose and lignin respectively.
From the composition prospective, after oil extraction,Jatropha Curcasseed cake makes up of a high portion of protein and has a relatively small amount of cellulose, hemicelluloses and lignin, which are commonly found in high proportion in the structural parts of plant species such as wood and bamboo[24, 25]. Such difference in the composition provides a possible account for the difference in the mass loss profiles observed in the pyrolysis ofJatropha Curcasseed cake and wood.
3.2 Effects of certain process conditions on pyrolysis
3.2.1Heating rates
In TGA study, it is common to observe that there is a shift of the mass loss curve to higher temperature when the heating rate increases [2, 7, 8, 26, 27], as shown in Fig. 2. This is also the case for all the analysed feedstocks in this work. The peak mass loss position shifts to higher temperature when the heating rate increases. Table 1 summarizes the residue fraction of the studied feedstocks at 800 °C for different heating rates. For the feedstock and the range of heating rate being studied, the effect of heating rate on the final mass loss is insignificant. It is suggested that theheating rate has more effect on the characteristics of primary vapours and char produced [4].
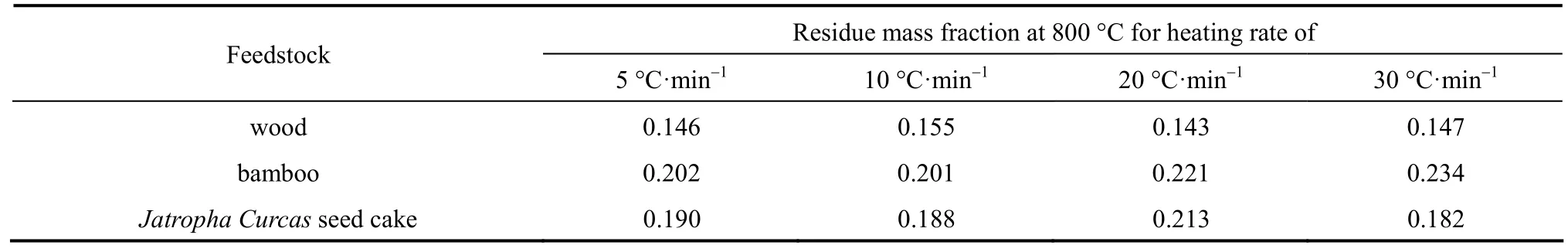
Table 1 Final residue mass fraction of different feedstock for different heating rates
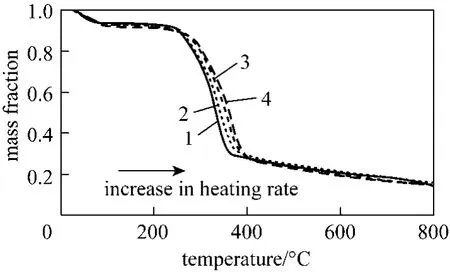
Figure 2 TG profiles of wood at different heating rates1—heating rate=5 °C·min-1; 2—heating rate=10 °C·min-1;3—heating rate=20 °C·min-1; 4—heating rate=30 °C·min-1

Figure 3 Segmented TG profiles of different feedstock at heating rate of 10 °C·min-11—wood; 2—bamboo; 3—Jatropha Curcas seed cake
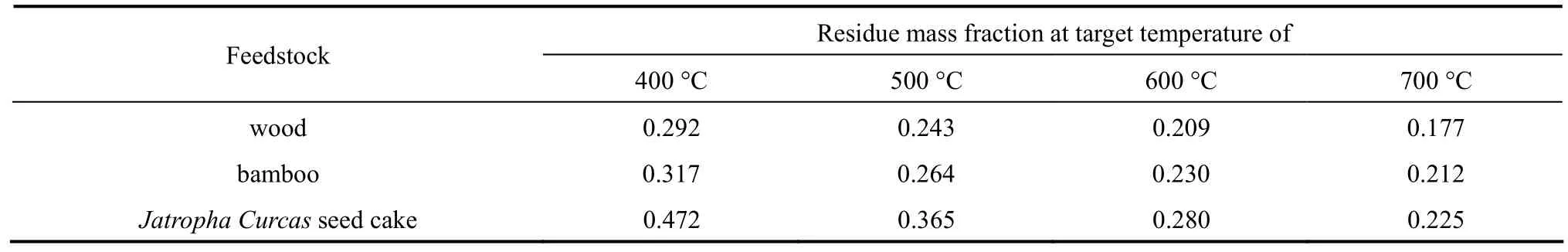
Table 2 Residue mass fraction of different feedstock for different target temperatures
3.2.2Final pyrolysis temperature
For the studied feedstocks, each of them associates with a major pyrolysis mass loss period, which is probably contributed by its main constitution. For example,wood species compose of mainly 19.2%-30.6% of hemicellulose and 39.5%-45.0% of cellulose [22],which account for the major mass loss occurring between 250 °C and 400 °C in pyrolysis [21]. After the major mass loss, devolatilization continues with the remaining residue. The main process is the ongoing thermal decomposition of lignin, which is suggested to pyrolyse with a broad temperature range from 160 °C up to 900 °C. As shown by the TG profile in Fig. 1,such devolatilization process does not complete even when thermogravimetric analyzer has reached its target temperature of 800 °C. In fact, it has a trend of continuing with devolatilization above 800 °C.
The study of the TG profiles of individual feedstocks, like Fig. 1, can help identify the major pyrolysis mass loss period and assist in choosing the desirable pyrolysis temperature. A number of works [2, 4]have shown the effect of the target temperature on pyrolysis using bench-scale pyrolysis set-ups. From the examination of the TG profiles alone, it can be noted that there exists a trade-off between the target temperature and the degree of devolatilization,i.e.residue mass fraction. It is able to obtain a residue with a lower volatile matter content,i.e. greater extend of devolatilization, in the expense of consuming more energy and possibly increasing the processing time when pyrolyzing at a higher temperature. Such dynamic will be discussed in Section 5.4 utilizing the mathematical model developed.
If the TG profiles are segmented like Fig. 3, one can estimate the possible residue mass fraction for different target temperatures, shown in Table 2, and get an idea for the choice of pyrolysis temperature.For feedstocks that concentrate in a particular constitution, the major pyrolysis temperature range is usually narrower and therefore, pyrolysis target temperature can be set slightly higher than the temperature range. The target temperature would obviously have impacts on the yield distribution, pyrolyzer design,energy consumption and product quality. More discussion will be covered in Section 5.3.
3.2.3Feedstock size
The effect of sample mass on pyrolysis at a low heating rate was studied using the three types of biomass.
Figs. 4, 5 and 6 show the TG profiles at heating rate of 5 °C·min-1for wood, bamboo andJatropha Curcasseed cake using different initial samples masses respectively. The effect of initial mass on wood and bamboo pyrolysis for the studied heating rate is insignificant. Such observation is in agreement with Mui’s work [27]. ForJatropha Curcasseed cake, the effect is prominent. All the biomass feedstocks show a general trend of increasing in the residue mass fraction when the initial sample mass increases. Possibly, it indicates that even under milligram sample size condition in TGA experiment, it is still necessary to consider mass transport phenomena. The final residue mass, essentially the char content, depends on the initial sample mass used. For wood and bamboo pyrolysis, such dependence begins to appear at around 350 °C. ForJatropha Curcasseed cake, it appears at around 250 °C.A possible account is that as the sample size increases,the mass transfer barrier becomes more significant.The volatiles products from primary reactions undergo more severe secondary reaction when there is a longer vapour residence time. The impact is more severe for the case ofJatropha Curcasseed cake because unlike wood and bamboo sample which are in shred form,the seed cake sample is in fine powder form and packed more closely together within the sample pan. The mass transport barrier appeared to be more significant and this encouraged more char-forming secondary reaction.

Figure 4 TG profiles of wood with different initial masses at heating rate of 5 °C·min-11—4.2 mg; 2—6.4 mg; 3—8.4 mg

Figure 5 TG profiles of bamboo with different initial masses at heating rate of 5 °C·min-11—3.9 mg; 2—6.1 mg; 3—8.0 mg; 4—9.5 mg
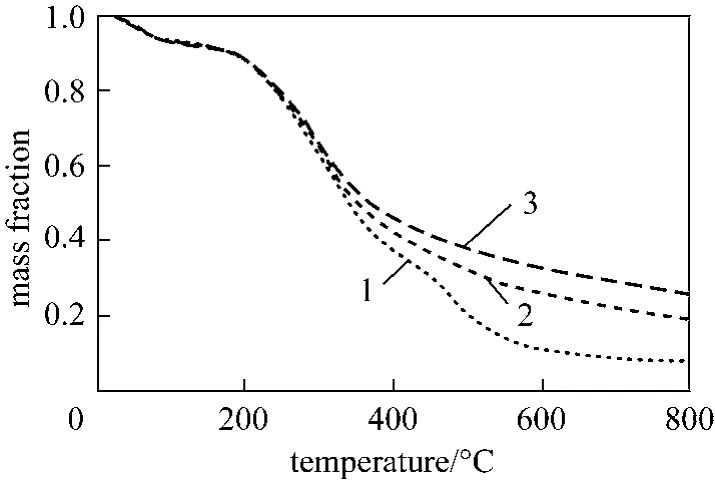
Figure 6 TG profiles of Jatropha Curcas seed cake with different initial masses at heating rate of 5 °C·min-11—2.3 mg; 2—4.1 mg; 3—5.9 mg
Koufopanoset al. [18] suggested that secondary reaction would enrich the carbon content of the final residue. When interacting with the carbonaceous solid,primary products reform into char and secondary volatile products. Ahujaet al. [28] pointed out that these secondary vapour-solid interactions may actually be the main source of char formed from biomass pyrolysis. Gomezet al. [29] observed that the pyrolysis heat demand, influenced by secondary reaction, is a strong function of the initial sample mass. In modelling biomass pyrolysis, this char-forming secondary reaction depending on the vapour residence time has been considered by many [3, 10, 12, 17, 28-30].
4 MODELLING STUDY
4.1 Overview
The developed model is a mathematical model that composes of the set of equations listed in Table 3.The model describes transiently the pyrolysis progress of multiple particles that are subjected to pyrolysis condition within a pyrolysis reactor. The pyrolyzer operates more like a batch reactor in which heating gas (either non-condensable pyrolysis gas or flue gas)flow in and contact with the feedstock particle.
The kinetics parameters and the heat flow parameters for individual feedstocks obtained from the thermal analysis and some physical properties or correlation listed in Section 4.2 serve as the model parameters. By inputting the size and the number of feedstock particle, the flow rate of the inlet heating gas, the temperature of the inlet heating gas and the heating time as model variables, the model can simulate the pyrolysis progress and output the mass loss profiles and the temperature profiles of individual particles during pyrolysis, the overall energy usage and the processing time.
In constructing the model, several assumptions were made:
(1) Feedstock particle is spherical in shape.
(2) Conduction is the only mode of heat transfer within the particle.
(3) Temperature gradients are along radial position only.
(4) Heat transfer is the only form of inter-particle interaction.

Table 3 Set of equations for modelling particle pyrolysis

Table 4 Model parameters
4.2 Numerical solution
The model was built on MATLAB®platform using finite difference method (FDM). Crank-Nicolson implicit scheme [31] was used as the numerical method to solve the energy equation, Eq. (4). Table 4 lists out the model parameters. The shrinking factor (δ)for wood was estimated from the pyrolysis of wood piece with a bench-scale pyrolysis unit.
5 PYROLYSIS OPERATION STRATEGIES
5.1 Overview
In designing and operating a pyrolysis process,the feedstock characteristic and the effect of process parameters have to be analyzed thoroughly. This section makes use of the developed model in Section 4 to have a general discussion on several design and operation considerations. Wood was selected as the studied biomass material. The feedstock characteristic of wood, including the pyrolysis behaviour from the experimental study and the physical properties from literatures, was incorporated into the model.
5.2 Case study 1: considering the feedstock size
Heat transfer, whether it is from the reactor environment to the feedstock or within the feedstock, is a key process governing the progress of pyrolysis. The thermal conductivity of biomass material is generally poor. Therefore, time required for pyrolyzing feedstock with different size can vary a lot. This case study evaluates on the impact of the feedstock size on the operation of a pyrolysis process. In this study, the scenario is that 500 kg of wood particles are to be pyrolyzed. 50% by mass of these particles is smaller with radius of 1cm and the remaining is larger with radius of 2 cm. 800 °C heating gas at a rate of 1 kg·s-1heats up the feedstock. Two cases were simulated for comparison. In one of the cases, the two types of wood particles are pyrolyzed together. In the other case, the two types of wood particles are separately pyrolyzed and each uses half the amount of the heating gas. The simulation results are summarized in Table 5. Fig. 7 shows the pyrolysis progress of the two types of wood particles.
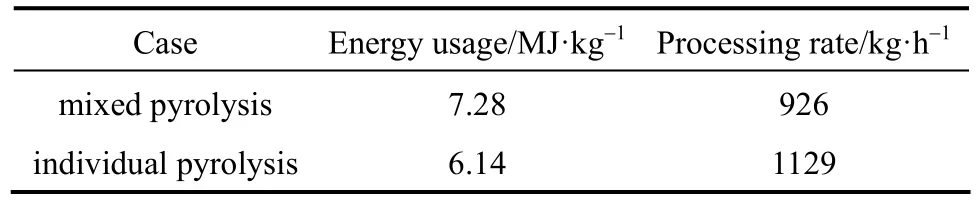
Table 5 Simulation results for case study 1

Figure 7 Mass fraction profiles of smaller particle and larger particle for case study 11—smaller particle, center; 2—smaller particle, surface; 3—larger particle, center; 4—larger particle, surface
While the case is a simplified one, it provides a basic idea for the extent to which heat transfer barrier would influence pyrolysis. For the case in which the two types of particles were separated and then pyrolyzed, it consumes less energy and has a higher processing rate because of the fact that the smaller particles finished pyrolysis much earlier than the larger particles and do not require further heating. Therefore,in operating a pyrolysis process, especially for bulk feedstocks, it is advised to separate the feedstock into different size ranges for pyrolysis instead of using a feedstock with a broad size range.
5.3 Case study 2: considering the relation between pyrolysis time and temperature
This case study aims to study the effect of the inlet gas temperature on the pyrolysis time and energy usage. 500 kg of wood particles with the size of 2 cm in radius are subjected to the heating gas at a rate of 1 kg·s-1with temperature varying from 500 °C to 1000 °C.The effects of the heating gas temperature on the energy usage and the processing time are shown in Fig. 8.Based on the simulation on wood pyrolysis, it is undesirable to have the heating gas temperature below 600 °C.When the temperature falls below that level, the processing time will rise enormously resulting in the increase in the energy usage. Apparently, the greatest the heating gas temperature, the lowest the energy usage and the shortest the processing time will be. In real scenario,because of the increasing heat loss when processing temperature increases and the constraints imposed by the material of construction, the ideal heating gas temperature would fall between 600 °C and 800 °C. In addition, the changes brought by the heating gas temperature on the energy usage and processing time are insignificant when it is beyond 600 °C. Therefore, at this point, other factors such as the product yield distribution and operation efficiency should be considered for optimizing the heating gas temperature.

Figure 8 The effect of heating gas temperature on the energy usage and the processing time for case study 21—energy usage; 2—processing time
5.4 Case study 3: considering the relation between pyrolysis time and residue mass
As mentioned in Section 3.2.2, there exists a
trade-off between the processing time and the residue mass fraction. This case study covers on this aspect.Again, 500 kg of wood particles with the size of 2 cm in radius are subjected to the heating gas of 800 °C at a rate of 1 kg·s-1. The simulation result is presented in Fig. 9. In the expense of processing time, essentially the energy usage, it is possible to obtain a low residue mass fraction, which means that both the pyrolysis oil and the non-condensable gas fraction would increase.A low residue mass fraction also indicates that the residue has low volatile matter content and high fixed-carbon content. This would be desirable for using a high fixed-carbon content residue as fuel substituent or precursor for making activated carbon.Therefore, the actual processing time chosen will depend on the quality and quantity of the target products.

Figure 9 The effect of processing time on the energy usage and residue mass fraction for case study 31—energy usage; 2—residue mass fraction
6 CONCLUSIONS
The pyrolysis behaviour of wood sawdust, bamboo shred andJatropha Curcasseed cake residue under different processing conditions including heating rate, final pyrolysis temperature and sample size were studied experimentally by thermal analysis. The effect of heating rate on the yield distribution is insignificant for the range of heating rate 5-30 °C being studied.On the other hand, the final pyrolysis temperature determines critically the extent of devolatilization, essentially the residue mass fraction. For the sample size aspect, the presence of mass transport barrier contributes to secondary char-forming reaction and influences the residue mass fraction. In the second part of this work,a mathematical model was used to simulate the pyrolysis progress of wood particles in a reactor. It forms the basis for the discussion on the considerations of and interrelation between feedstock size, pyrolysis energy usage, processing time and product quality for the design and operation of pyrolysis processes.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the support from Hong Kong RGC research grant (613808, 614307),Hong Kong PhD Fellowship Scheme for Oyedun Adetoyese Olajire (PF09-05997) and the Global Powerand Energy Company Limited.
NOMENCLATURE
Apre-exponential factor, s-1
Cpspecific heat capacity, J·kg-1·K-1
Eactivation energy, J·mol-1
Hheat of reaction, J·kg-1
hconvective heat transfer coefficient, W·m-2·K-1
krate constant, s-1
m˙mass flow rate, kg·s-1
mbulkmass of bulk environment, kg
norder of reaction
QToverall energy usage for heating the inlet gas, JRparticle radius, m
Runiversal gas constant, J·mol-1·K-1
rradius of a discrete layer, m
Δrlayer thickness, m
Ttemperature, K
Tbulkbulk environment temperature, K
TRparticle surface temperature, K
treaction time, s
tTheating time, s
Vparticle volume, m3
Vtotal particle volume, m3
V0initial particle volume, m3
initial total particle volume, m3
αiextent of reaction for mass loss reactioni
αToverall mass loss fraction
αT,ffinal total mass loss fraction
βheating rate, °C·min-1
γjextent of reaction for mass loss reactionj
δshrinking factor
λthermal conductivity, W·m-1·K-1
νvoid space fraction
ρdensity of feedstock, kg·m-3
ωimass loss contribution by mass loss reactioni
Subscripts
c char
f feedstock
g heating gas
imass loss reactioni
inlet inlet heating gas
jexothermic reactionjoutlet outlet gas
p particle
1 Font, R., Williams, P.T., “Pyrolysis of biomass with constant heating rate: Influence of the operating conditions”,Thermochim.Acta, 250,109-123 (1995).
2 Williams, P.T., Besler, S., “The influence of temperature and heating rate on the slow pyrolysis of biomass”,Renew.Energ., 7, 233-250(1996).
3 Park, W.C., Atreya, A., Baum, H.R., “Experimental and theoretical investigation of heat and mass transfer processes during wood pyrolysis”,Combust.Flame, 157, 481-494 (2010).
4 Williams, P. T., Besler, S., Taylor, D.T., “The pyrolysis of scrap automotive tyres. The influence of temperature and heating rate on product composition”,Fuel, 69, 1474-1482 (1990).
5 Wang, Z., Guo, Q., Liu, X., Cao, C., “Low temperature pyrolysis characteristics of oil sludge under various heating conditions”, Energ.Fuel, 21, 957-962 (2007).
6 Arabiourrutia, M., Lopez, G., Elordi, G., Olazar, M., Aguado, R.,Bilbao, J., “Product distribution obtained in the pyrolysis of tyres in a conical spouted bed reactor”, Chem. Eng. Sci., 62, 5271-5275 (2007).
7 González, J.F., Encinar, J.M., Canito, J.L., Rodríguez, J.J., “Pyrolysis of automobile tyre waste. Influence of operating variables and kinetics study”, J. Anal. Appl. Pyrol., 58-59, 667-683 (2001).
8 Leung, D.Y.C., Wang, C.L., “Kinetic study of scrap tyre pyrolysis and combustion”, J. Anal. Appl. Pyrol., 45, 153-169 (1998).
9 Curtis, L.J., Miller, D.J., “Transport model with radiative heat transfer for rapid cellulose pyrolysis”, Ind. Eng. Chem. Res., 27,1775-1783 (1988).
10 Di Blasi, C., “Modeling chemical and physical processes of wood and biomass pyrolysis”, Prog. Energ. Combust., 34, 47-90 (2008).
11 Di Blasi, C., “Heat, momentum and mass transport through a shrinking biomass particle exposed to thermal radiation”, Chem. Eng.Sci., 51, 1121-1132 (1996).
12 Babu, B.V., Chaurasia, A.S., “Modeling for pyrolysis of solid particle: Kinetics and heat transfer effects”, Energ. Convers. Manage., 44,2251-2275 (2003).
13 Papadikis, K., Gu, S., Bridgwater, A.V., “CFD modelling of the fast pyrolysis of biomass in fluidised bed reactors. Part B. Heat, momentum and mass transport in bubbling fluidised beds”, Chem. Eng.Sci., 64, 1036-1045 (2009).
14 Sadhukhan, A.K., Gupta, P., Saha, R.K., “Modelling of pyrolysis of large wood particles”, Bioresource Technol., 100, 3134-3139 (2009).15 Haseli, Y., Van Oijen, J.A., De Goey, L.P.H., “Modeling biomass particle pyrolysis with temperature-dependent heat of reactions”, J.Anal. Appl. Pyrol., 90, 140-154 (2011).
16 Larfeldt, J., Leckner, B., Melaaen, M. C., “Modelling and measurements of the pyrolysis of large wood particles”, Fuel, 79, 1637-1643(2000).
17 Sadhukhan, A.K., Gupta, P., Saha, R.K., “Modelling and experimental studies on pyrolysis of biomass particles”, J. Anal. Appl. Pyrol.,81, 183-192 (2008).
18 Koufopanos, C. A., Papayannakos, N., Maschio, G., Lucchesi, A.,“Modelling of the pyrolysis of biomass particles. Studies on kinetics,thermal and heat transfer effects”, Can. J. Chem. Eng., 69, 907-915(1991).
19 Cheung, K.Y., Lee, K.L., Lam, K.L., Lee, C.W., Hui, C.W., “Integrated kinetics and heat flow modelling to optimise waste tyre pyrolysis at different heating rates”, Fuel Process Technol., 92, 856-863(2011).
20 Lam, K.L., Oyedun, A.O., Cheung, K.Y., Lee, K.L., Hui, C.W.,“Modelling pyrolysis with dynamic heating”, Chem. Eng. Sci., 66,6505-6514 (2011).
21 Yang, H., Yan, R., Chen, H., Lee, D.H., Zheng, C., “Characteristics of hemicellulose, cellulose and lignin pyrolysis”, Fuel, 86,1781-1788 (2007).
22 Sjöström, E., Wood Chemistry: Fundamentals and Applications,Academic Press, San Diego (1993).
23 Scurlock, J.M.O., Dayton, D.C., Hames, B., “Bamboo: An overlooked biomass resource?”, Biomass Bioenerg., 19, 229-244 (2000).24 Staubmann, R., “Biogas production from jatropha curcas press-cake”, Appl. Biochem. Biotech., 63-65, 457-467 (1997).
25 Carrier, M., Loppinet-Serani, A., Denux, D., Lasnier, J.M.,Ham-Pichavant, F., Cansell, F., Aymonier, C., “Thermogravimetric analysis as a new method to determine the lignocellulosic composition of biomass”, Biomass Bioenerg., 35, 298-307 (2011).
26 Quek, A., Balasubramanian, R., “An algorithm for the kinetics of tire pyrolysis under different heating rates”, J. Hazard. Mater., 166,126-132 (2009).
27 Mui, E.L.K., Cheung, W.H., Lee, V.K.C., McKay, G., “Kinetic study on bamboo pyrolysis”, Ind. Eng. Chem. Res., 47, 5710-5722 (2008).
28 Ahuja, P., Kumar, S., Singh, P. C., “A model for primary and heterogeneous secondary reactions of wood pyrolysis”, Chem. Eng.Technol., 19, 272-282 (1996).
29 Gomez, C., Velo, E., Barontini, F., Cozzani, V., “Influence of secondary reactions on the heat of pyrolysis of biomass”, Ind. Eng. Chem.Rev., 48, 10222-10233 (2009).
30 Papadikis, K., Gu, S., Bridgwater, A.V., “Computational modelling of the impact of particle size to the heat transfer coefficient between biomass particles and a fluidised bed”, Fuel Process Technol., 91,68-79 (2010).
31 Crank, J., Nicolson, P., “A practical method for numerical evaluation of solutions of partial differential equations of the heat-conduction type”, Adv. Comput. Math., 6, 207-226 (1996).
32 Yang, J., Tanguy, P.A., Roy, C., “Heat transfer, mass transfer and kinetics study of the vacuum pyrolysis of a large used tire particle”,Chem. Eng. Sci., 50, 1909-1922 (1995).
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Synergistic Multilayer Adsorption for Low Concentration Dyestuffs by Biomass
- Kinetics of Photocatalytic Degradation of Gaseous Organic Compounds on Modified TiO2/AC Composite Photocatalyst*
- 3D Numerical Study on Compound Heat Transfer Enhancement of Converging-diverging Tubes Equipped with Twin Twisted Tapes*
- Techno-economic Analysis of Distributed Hydrogen Production from Natural Gas
- Error Analysis of Adsorption Isotherm Models for Acid Dyes onto Bamboo Derived Activated Carbon
- Issues in Freeze Drying of Aqueous Solutions*
