埃博拉病毒NP蛋白的单克隆抗体制备及抗原肽的定位
2012-02-09王晓杜刘阳王皓婷史子学赵凡凡魏建超邵东华马志永
王晓杜,刘阳,王皓婷,史子学,赵凡凡,,魏建超,邵东华,马志永
1 浙江农林大学林业与生物技术学院,浙江 临安 311300
2 中国农业科学院上海兽医研究所,上海 200241
Introduction
Ebola virus (EBOV) causes highly lethal hemorrhagic fever in humans and nonhuman primates and has emerged repeatedly in central Africa[1]. EBOV was first identified in equatorial Africa in 1976 and >10 pandemic outbreaks have been reported worldwide since then (http://www. who.int/mediacentre/factsheets/fs103/en/index.html). EBOV belongs to the family Filoviridae, which includes the genera Marburg virus (MARV) and EBOV. The genus EBOV is a geographically diverse group of viruses containing four species, Zaire Ebola virus (ZEBOV), Sudan Ebola virus (SEBOV), Cote-d’Ivoire Ebola virus (CIEBOV) and Reston Ebola virus (REBOV) and, tentatively, Bundibugyo Ebola virus (BEBOV)[2-5]. ZEBOV is the most virulent family member with approximately 60%–90% case fatality, followed by SEBOV (40%–60%) and BEBOV (25%)[3]. CIEBOV infects humans but no fatal case has been reported[4]. REBOV is deemed pathogenic in nonhuman primates but not in humans[5]. EBOV is thought to be a zoonotic pathogen. Humans and nonhuman primates are regarded as end hosts[6]and bats have long been thought to be potential natural reservoirs[3].
EBOV is an enveloped, non-segmented, negative-stranded RNA virus. The genome of EBOV encodes seven viral proteins, including four virion structural proteins (VP30, VP35, nucleoprotein (NP) and RNA-dependent RNA polymerase) and three membrane-associated proteins (VP40, glycoprotein and VP24)[7-9]. The viral NP is an essential component of the viral nucleocapsid and plays a central role in virus replication. The NP has 739 amino acid residues (ZEBOV species) and migrates aberrantly in sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS-PAGE), with an apparent molecular mass of 115 kDa, although its predicted molecular mass is about 85 kDa[10]. The region of the first 450 amino acid residues of NP plays a role in NP–NP interactions and viral assembly. The region between amino acid residues 451 and 600, as well as the first 450 amino acid residues required for NP–NP interaction, are required for formation of nucleocapsid-like structures and for viral genome replication[11-12]. In addition, the NP is the abundant protein in EBOV particles and a well-conserved protein among EBOV species, and has been used as a target molecule for disease diagnosis and surveillance[13].
EBOV is classified as a biosafety level 4 (BSL-4) agent. Therefore, manipulation of EBOV requires BSL-4 standard laboratories. Although it was first identified in equatorial Africa in 1976, the mechanisms underlying the viral pathogenesis and immune responses are not clearly delineated[14]and effective countermeasures against the EBOV infection have not been developed[15]. This is, in part, because of the requirement for BSL-4 standard facilities, which are not available in most research institutes and the difficulty of obtaining infected clinical samples. The heterologously expressed viral protein and monoclonal antibody (MAb) have partially circumvented such bottlenecks and facilitated our understanding of the mechanisms underlying EBOV pathogenesis and immune responses[16-18]. In this study, we generated a MAb against the NP of ZEBOV (ZEBOV-NP) via immunization of mice with prokaryotically expressed recombinant ZEBOV-NP and mapped the epitope motif required for recognition by the generated MAb.
1 Materials and methods
1.1 Gene synthesis and construction of recombinant expression plasmids
Genes encoding the full-length NPs of ZEBOV (GenBank Accession No. NC_002549), SEBOV (GenBank Accession No. EU338380), CIEBOV (GenBank Accession No. FJ217162), REBOV (GenBank Accession No. AB050936), BEBOV (GenBank Accession No. FJ217161) and MARV (GenBank Accession No. GQ433352) were chemically synthesized according to their sequence information deposited in GenBank. The synthesized genes were inserted into the prokaryotic expression vector pET-28a (Novagen, Madison, WI, USA) or the eukaryotic expression vector p3xFLAG-CMV-7.1 (Sigma, St. Louis, MO, USA) in order to express a histidine (HIS)-tagged NP in Escherichia coli or a Flag-tagged NP in Vero cells, respectively. For eukaryotic expression, the constructed plasmids expressing Flag-tagged NP were transiently transfected into Vero cells using Lipofectamine™2000 according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA) and incubated at 37 °C for 24 h.
1.2 Expression and purification of recombinant NP
The recombinant proteins HIS-tagged NP are usually expressed in the E. coli cells strain BL21 (DE3) transfected with pET-28(a)-ZEBOV-NP plasmid. The resulting transformations were grown aerobically at 37 °C until mid-log phase (A600=0.7–0.8). Expression was induced by treatment with 1 mmol/L isopropyl-b-D-thiogalactopyranoside (IPTG) at 37 °C for 4 h. After induction, the bacterial cells were harvested by centrifugation and assessed by 10% SDS-PAGE followed by staining with Coomassie brilliant blue R250. For purification of the HIS-tagged NP of ZEBOV (HIS-ZEBOV-rNP), the IPTG-induced bacterial cells were harvested by centrifugation and sonication in an ice-water bath. The HIS-ZEBOV-rNP, which was expressed as insoluble inclusion bodies in E. coli, was separated by centrifugation and the insoluble pellet was then resuspended in 6 mol/L urea buffer (6 mol/L urea, 500 mmol/L NaCl, 20 mmol/L Tris-HCl, 5 mmol/L imidazole, pH 7.9). The urea-soluble HIS-ZEBOV-rNP was purified under denaturing conditions using a His Band kit (Novagen), according to the manufacturer’s instructions. The purity of the preparation was assessed by SDS-PAGE and the protein content was determined with a BCA protein assay kit (Pierce Biotechnology, Rockford, IL, USA).
1.3 Generation of MAb
BALB/c mice were immunized with 50 mg of the purified HIS-ZEBOV-rNP emulsified in Freund’s complete adjuvant (Sigma) via hypodermic injection. Subsequent booster immunizations of 50 mg of the purified HIS-ZEBOV-rNP emulsified in Freund’s incomplete adjuvant (Sigma) were given three times at 14-day intervals. The mice were further injected intraperitoneally (i.p.) with 50 mg of the purified His-ZEBOV-rNP in phosphate-buffered saline three days pre-fusion. Hybridomas were produced routinely by fusing myeloma Sp2/0 cells with spleen cells from the immunized BALB/c mice that were euthanized according to the Guidelines on the Humane Treatment of Laboratory Animals (Ministry of Science and Technology of the People’s Republic of China, Policy No. 2006 398). Hybridomas were screened by an indirect enzyme-linked immunosorbent assay (ELISA) using the purified HIS-ZEBOV-rNP as a coated antigen. The positive clones were injected into the peritoneal cavity of mice for production of ascitic fluids containing MAb.
1.4 Western blotting
Western blotting analysis was done as described[19]. The commercial antibodies employed in Western blotting were an anti-Flag MAb (M2, Sigma), an anti-HIS MAb (HIS-1, Sigma), an anti-b-actin MAb (AC-15, Sigma) and an anti-glutathione-S-transferase (GST) polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
1.5 Epitope mapping
ZEBOV-NP was artificially divided into three truncated fragments (amino acid residues 1–267, 268–533 and 534–739) according to the potential epitopes predicted with software (data not shown). The three truncated fragments of ZEBOV-NP were expressed in E. coli as HIS-tagged proteins and their reactivity with the MAb generated in this study was analyzed by Western blotting. The fragment between amino acid residues 534–739, which showed positive to the MAb, was further divided into five shorter fragments (amino acid residues 675–739, 567–660, 575–597, 575–590 and 582–597). The five shorter fragments were expressed in E. coli as glutathione-S-transferase (GST)-tagged proteins and their reactivity with the MAb was determined by Western blotting analysis. The gene segments encoding the truncated NP mutants mentioned above were prepared by polymerase chain reaction (PCR) using ZEBOV-NP as template and inserted into prokaryotic expression vector pET-28a or pGEX-6p-1 (Amersham Pharmacia Biotech, Tokyo, Japan) for heterologous expression as His- or GST-tagged proteins, respectively. To map the minimal motif of epitope recognized by the MAb, five of the 8-mer NP peptides (amino acid residues 580–587, 581–588, 582–589, 583–590 and 584–591) were expressed in E. coli as GST-tagged proteins and incubated with the MAb for determination of their reactivity in Western blotting analysis. The synthetic DNA fragments of five 8-mer NP peptides were annealed in pairs and subcloned into expression vector pGEX-6p-1 as described[20-21]. Each gene segment inserted into the expression vectors was sequenced to make sure that it was identical with the original sequence. The primers used for amplification of the gene segments of ZEBOV-NP were given in Table 1.
Table 1 Sequences of primers used for generation of truncated NP mutants
2 Results
2.1 Expression and purification of recombinant NP of ZEBOV (HIS-ZEBOV-rNP)
To obtain HIS-ZEBOV-rNP, plasmid expressing the full-length ZEBOV-NP was transformed into E. coli and the expression was induced by treatment with IPTG. A protein band of about 120 kDa molecular mass corresponding to HIS-ZEBOV-rNP was detected in the lysate from IPTG-treated transformants (Fig. 1, lane 2). The HIS-ZEBOV-rNP that was expressed as insoluble inclusion bodies (Fig. 1, lane 4) was purified under denaturing conditions (6 mol/L urea) by Ni2+affinity chromatography using His Bind Resin. Peak A280fractions eluted from the column were pooled and showed a predominant single band of HIS-ZEBOV-rNP (Fig. 1, lane 5). Because we did not have an antibody specific to ZEBOV-NP, the purified HIS-ZEBOV-rNP was determined by MALDI-TOF-TOF analysis. The results indicated that the amino acid sequence of the purified HIS-ZEBOV-rNP corresponded to that of ZEBOV-NP (GenBank Accession No. NP_066243) (data not shown).
Fig. 1 Analysis of the expression and purification of recombinant NP of ZEBOV (HIS-ZEBOV-rNP). Bacterial lysates and purified HIS-ZEBOV-rNP were separated by electrophoresis in SDS-PAGE gel and stained with Coomassie brilliant blue R250. M: protein marker; 1: uninduced bacterial cells; 2: bacterial cells induced with IPTG; 3: supernatant from the sonicated IPTG-induced bacterial cells; 4: pellet from the sonicated IPTG-induced bacterial cells; 5: HIS-ZEBOV-rNP eluted from the His Bind Resin column.
2.2 Generation of MAb against ZEBOV-NP
To generate a MAb specific to ZEBOV-NP, BALB/c mice were immunized with the purified HIS-ZEBOV-rNP and the hybridomas generated by fusing myeloma cells with spleen cells from the immunized BALB/c mice were screened by ELISA. A positive clone of hybridomas was selected for production of mouse ascitic fluids. The MAb produced as mouse ascitic fluids was temporarily designated anti-NP antibody and used for subsequent experiments. To test its antigen-antibody recognition specificity, the anti-NP antibody was incubated with the HIS-ZEBOV-rNP and a HIS-tagged NS3 fragment (HIS-NS3c) of Japanese encephalitis virus[22], both of which were expressed and purified under the same condition. Western blotting analysis revealed the specific reactivity with HIS-ZEBOV-rNP, and no reactivity with HIS-NS3c (Fig. 2A), indicating that the anti-NP antibody reacts with the ZEBOV-NP rather than the HIS tag. To test whether the anti-NP antibody recognizes ZEBOV-NP expressed in eukaryotic cells, Vero cells were transfected with plasmid for expression of Flag-tagged ZEBOV-NP (Flag-ZEBOV-NP). A plasmid expressing Flag-tagged NS3 of Japanese encephalitis virus (Flag-NS3)[22]was transfected as a parallel control. The transfectants were then subjected to western blot analysis using the anti-NP antibody as a probe. Western blotting analysis revealed that the anti-NP antibody reacted specifically with the Flag-ZEBOV-NP expressed in Vero cells (Fig. 2B). Taken together, these results indicated that the anti-NP antibody is highly specific and can recognize the ZEBOV-NP expressed both in prokaryotic and eukaryotic cells.
2.3 Cross-reactivity of the anti-NP antibody with the members of the family Filoviridae
Fig. 2 Western blotting analysis of the specificity of the anti-NP antibody. (A) The purified HIS-tagged ZEBOV-rNP (HIS-ZEBOV-rNP) and NS3 fragment of Japanese encephalitis virus (HIS-NS3c) were probed with the anti-NP antibody (left-hand panel) and then re-probed with anti-HIS antibody (right-hand panel). (B) Cell lysates (20 μg) from Vero cells transfected with plasmid expressing Flag-tagged ZEBOV-NP (Flag-ZEBOV) or NS3 protein of Japanese encephalitis virus (Flag-NS3) were probed with the anti-NP antibody (left-hand panel) and then re-probed with anti-Flag antibody (right-hand panel).
The family Filoviridae contains ZEBOV, SEBOV, CIEBOV, REBOV, BEBOV and MARV (23). To test the cross-reactivity of the anti-NP antibody specific to ZEBOV-NP, the NP genes of SEBOV (SEBOV-NP), CIEBOV (CIEBOV-NP), REBOV (REBOV-NP), BEBOV (BEBOV-NP) and MARV (MARV-NP) were chemically synthesized and inserted into expression vectors for prokaryotic and eukaryotic expression. As shown in Fig. 3A, the prokaryotic expression of the HIS-tagged NP of EBOV and MARV was determined by SDS-PAGE (upper panel) and by Western blotting analysis using anti-HIS antibody as a probe (middle panel). In addition to HIS-ZEBOV-rNP, the anti-NP antibody reacted with HIS-CIEBOV-rNP, HIS-REBOV-rNP and HIS-BEBOV-rNP but not with HIS-SEBOV-rNP or HIS-MARV-rNP (lower panel). As an independent test to analyze the cross-reactivity of the anti-NP antibody, Flag-tagged NPs of EBOV (Flag-ZEBOV-NP, Flag-REBOV-NP, Flag-CIEBOV-NP, Flag-SEBOV-NP and Flag-BEBOV-NP) and MARV (Flag-MARV-NP) were expressed in Vero cells, as confirmed by Western blotting analysis using anti-Flag antibody (Fig. 3B, middle panel). The anti-NP antibody recognized Flag-ZEBOV-NP, Flag-REBOV-NP, Flag-CIEBOV-NP and Flag-BEBOV-NP but not Flag-SEBOV-NP or Flag-MARV-NP (Fig. 3B, upper panel). Taken together, these results indicated that the anti-NP antibody specific to ZEBOV-NP cross-reacts with the NPs of REBOV, CIEBOV and BEBOV but not SEBOV or MARV.
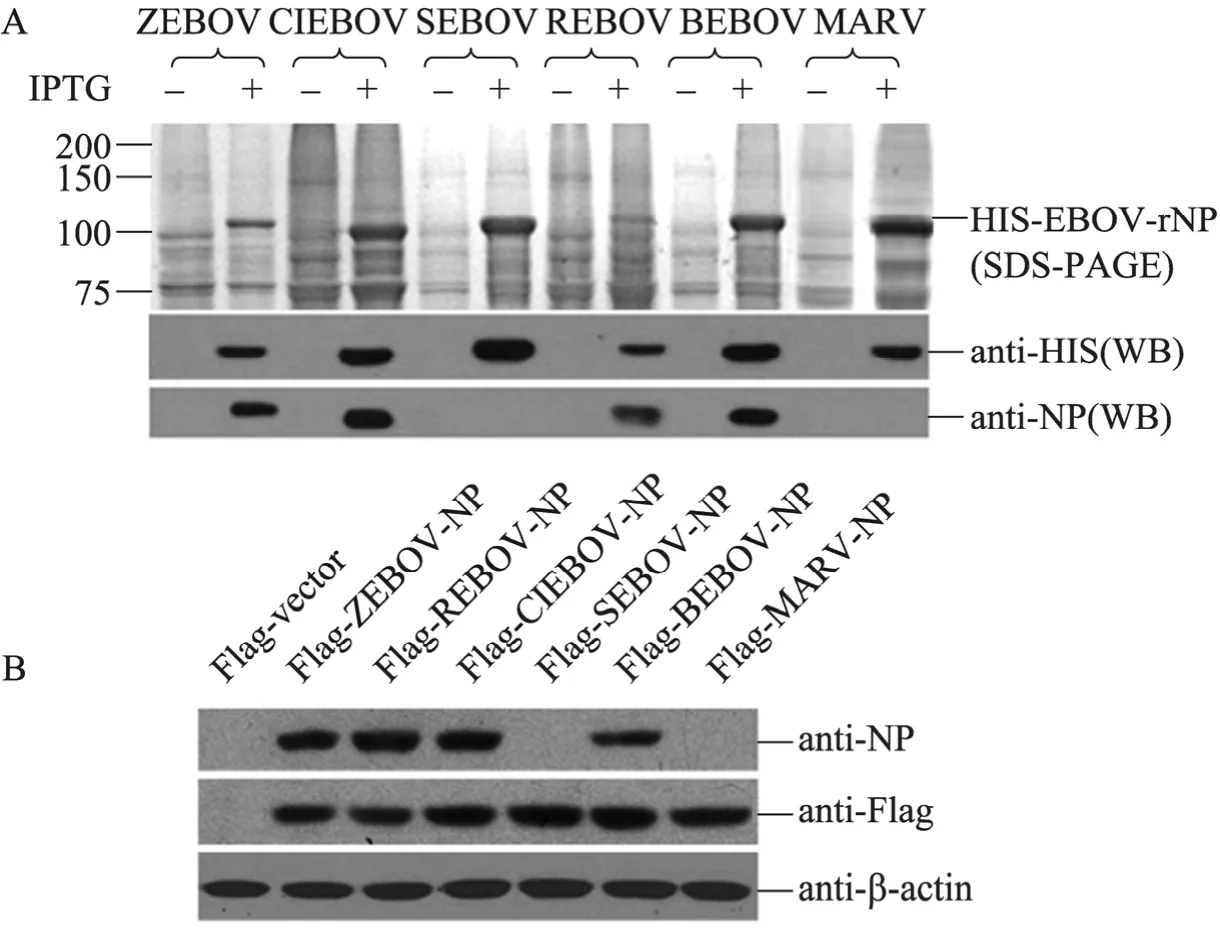
Fig. 3 Western blotting analysis of the cross-reactivity of the anti-NP antibody with the members of the family Filoviridae. (A) The lysates from bacteria expressing the HIS-tagged NP of Zaire Ebola virus (ZEBOV), Cote-d’Ivoire Ebola virus (CIEBOV), Sudan Ebola virus (SEBOV), Reston Ebola virus (REBOV), Bundibugyo Ebola virus (BEBOV) or Marburg virus (MARV) were separated by electrophoresis in SDS-PAGE gel and stained with Coomassie brilliant blue R250 (upper panel). The lysates were probed with anti-HIS antibody (middle panel) and then re-probed with the anti-NP antibody (lower panel). (B) The lysates from Vero cells transfected with plasmids encoding the Flag-tagged NP of ZEBOV (Flag-ZEBOV-NP), REBOV (Flag-REBOV-NP), CIEBOV (Flag-CIEBOV-NP), SEBOV (Flag-SEBOV-NP), BEBOV (Flag-BEBOV-NP), MARV (Flag-MARV-NP) or Flag-vector were probed with the anti-NP antibody (upper panel) and then re-probed with anti-Flag antibody (middle panel). β-Actin was stained with anti-β-actin antibody as an internal control (lower panel).
2.4 Epitope mapping of the anti-NP antibody
The data mentioned above indicated that the anti-NP antibody specific to ZEBOV-NP cross-reacted with REBOV-NP, CIEBOV-NP and BEBOV-NP. It was interesting to identify the conserved epitope recognized by the anti-NP antibody. Antigenic index analysis with webserver ABCpred (www.imtech.res.in/raghava/abcpred/) suggested that there is a multitude of potential epitopes in ZEBOV-NP (data not shown). To map the epitope recognized by the anti-NP antibody, ZEBOV-NP was prokaryotically expressed in E. coli as truncated proteins with different lengths according to the predicted potential epitopes (Fig. 4A) and their reactivity with the anti-NP antibody was determined by Western blotting analysis (image data not shown). The recognition site of the anti-NP antibody was finally narrowed down to the region from amino acid residues 582–597 (Fig. 4A). To map the minimal motif of the epitope, five of the 8-mer NP peptides with an overlap of six amino acid residues (Fig. 4B) were expressed in E. coli as GST-tagged proteins (GST-rNP peptides) (Fig. 4C, upper and lower panels) and their reactivity with the anti-NP antibody was determined by Western blotting analysis. Among the five GST-rNP peptides, those corresponding to the regions between amino acid residues 581 and 588 (rNP 581–588), 582 and 589 (rNP 582–589) and 583 and 590 (rNP 583–590) were reacted with the anti-NP antibody (Fig. 4C, middle panel), suggesting that the motif PPLESD located between amino acid residues 583 and 588 is the minimal sequence of the epitope required for recognition by the anti-NP antibody (Fig. 4B).
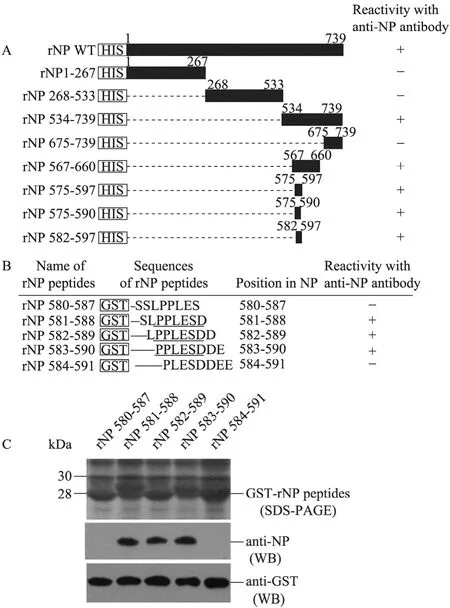
Fig. 4 Mapping of the epitope recognized by the anti-NP antibody. (A) Schematic representation of HIS-tagged wild type NS (rNP WT) and HIS/GST-tagged deletion mutants (rNP). The numbers indicate amino acid positions. (B) Representation of amino acid sequences of 8-mer NP peptides fused with GST for mapping of the epitope recognized by the anti-NP antibody. The epitope motif PPLESD required for recognition by the anti-NP antibody is underlined. (C) The lysates from bacteria expressing GST-tagged 8-mer NP peptides were separated on a SDS-PAGE gel and stained with Coomassie brilliant blue R250 (upper panel). The lysates were probed with the anti-NP antibody (middle panel) and then re-probed with the anti-GST antibody (lower panel). (+), Recombinant protein reacts with the anti-NP antibody in Western blotting analysis; (–), recombinant protein does not react with the anti-NP antibody in Western blotting analysis.
2.5 Alignment of the epitope motif recognized by anti-NP antibody among the members of the family Filoviridae
The anti-NP antibody reacted with ZEBOV-NP, REBOV-NP, CIEBOV-NP and BEBOV-NP but not SEBOV-NP or MARV-NP (Fig. 3). To interpret this observation, we used a multiple alignment of the amino acid sequences of ZEBOV-NP, SEBOV-NP, CIEBOV-NP, REBOV-NP, BEBOV-NP and MARV-NP and found that the epitope motif PPLESD is well conserved among ZEBOV-NP, CIEBOV-NP, REBOV-NP, BEBOV-NP but not SEBOV-NP or MARV-NP (Fig. 5). These data supported the results described above (Fig. 3) and allowed us to interpret the different cross-reactivity of the anti-NP antibody with the members of the family Filoviridae. We additionally blasted the epitope motif PPLESD against all NP sequences of Ebola viruses deposited in GenBank. The epitope motif was found to be conserved in ZEBOV (16 strains), CIEBOV (one strain) and BEBOV (one strain); however, four of the five strains of REBOV contain the conserved epitope motif. No SEBOV strain was found to harbor the conserved epitope motif among the four strains deposited in GenBank (data not shown).
Fig. 5 Alignment of the epitope motif recognized by anti-NP antibody among the members of the family Filoviridae. The partial amino acid sequence (residues 570–600) of NP of ZEBOV (ZEBOV-NP, GenBank Accession No. NP_066243) was aligned with that of CIEBOV (CIEBOV-NP, GenBank Accession No. ACI28629), REBOV (REBOV-NP, GenBank Accession No. BAB69003), BEBOV (BEBOV-NP, GenBank Accession No. ACI28620), SEBOV (SEBOV-NP, GenBank Accession No. ABY75321) and MARV (MARV-NP, GenBank Accession No. ADM72988). The epitope motif PPLESD required for recognition by the anti-NP antibody is boxed. Asterisks indicate residues identical with those of ZEBOV-NP. The numbers indicate amino acid positions. +: NP reacts with the anti-NP antibody in western blot analysis; –: NP does not react with the anti-NP antibody in Western blotting analysis.
3 Discussion
EBOV causes hemorrhagic fevers in humans and nonhuman primates with high fatality rates and thus it is a serious threat to public health[24]. The magnitude of international trade and travel is continuously increasing and there is a significant risk of introduction of EBOV to a virus-free country or to a new host[3]. Therefore, new tools, such as monoclonal antibodies specific to EBOV, are needed for understanding EBOV pathogenesis and immune responses in order to develop effective countermeasures against transmission and infection.
The NP of EBOV, an essential component of the viral nucleocapsid, plays a central role in virus replication[11]and serves as a target molecule for disease diagnosis and surveillance[14]. A MAb specific to EBOV-NP would be a useful tool for studies of the biological functions of EBOV-NP and for development of diagnostic methods. The manipulation of EBOV requires BSL-4 standard laboratories, which are not yet available in China. To obviate the need for BSL-4 standard laboratories, we took advantage of the sequence information deposited in GenBank to synthesize EBOV-NP genes for production of the recombinant protein as well as the subsequent MAb.
The gene encoding full-length ZEBOV-NP was synthesized and expressed prokaryotically in E. coli as a HIS-tagged protein (Fig. 1). Because of the lack of a ZEBOV-NP-specific antibody, we sequenced the purified HIS-ZEBOV-rNP to avoid any mutational artifact (data not shown). The confirmed HIS-ZEBOV-rNP was used for generation of a MAb (anti-NP antibody) specific to ZEBOV-NP and able to recognize ZEBOV-NP expressed in prokaryotic and eukaryotic cells (Fig. 2). The anti-NP antibody cross-reacted with REBOV-NP, CIEBOV-NP and BEBOV-NP but not with SEBOV-NP or MARV-NP (Fig. 3). The minimal epitope sequence required for recognition by the anti-NP antibody was the motif PPLESD, which is located between amino acid residues 583 and 588 at the C-terminus of ZEBOV-NP (Fig. 4). The epitope motif PPLESD is well conserved among all strains of ZEBOV (16 strains), CIEBOV (one strain) and BEBOV (one strain), whose sequence information is available in GenBank. In REBOV, however, the epitope motif is conserved in four out of five strains.
EBOV-NP is 739 amino acids long (ZEBOV species) and can be divided into a hydrophobic N-terminal half (about 350 amino acids) and a hydrophilic C-terminal half. The N-terminal half of NP is well conserved with 86%–92% of amino acid identity, whereas the C-terminal half of NP shows only 46%–67% of amino acid identity, among the five EBOV species. We immunized the mice with the full-length recombinant NP intending to generate a MAb that recognizes an epitope located within the well-conserved N-terminal half and cross-reacts with five species of EBOV. However, the generated anti-NP antibody cross-reacted with four species of EBOV (ZEBOV, REBOV, CIEBOV and BEBOV) but not with the SEBOV species (Fig. 3). Epitope mapping showed that the epitope motif PPLESD recognized by the generated anti-NP antibody was located between amino acid residues 583 and 588 in the C-terminal half of NP (Fig. 4). These results implied that the antigenicity of the C-terminal half is greater than that of the N-terminal half. Our data further support the suggestion that the antigenic region of NP is located in the C-terminal half as described[23]. The epiptope motif PPLESD is strongly acidic antigens(PI:3.367), the mouse IgG reacted strongly to acidic antigens[25].
The NP, an abundant and well-conserved protein, has been used as a target molecule for diagnosis and surveillance of EBOV infection[14]. Although the anti-NP antibody does not react with SEBOV species, it still could be used as a tool for detection of EBOV infection as well as for differential diagnosis of SEBOV infection. In addition, the epitope motif (amino acid residues 583–588) recognized by the anti-NP antibody is located in a region (amino acid residues 451–600) required for the formation of nucleocapsid-like structures of EBOV[11,26-27]. Thus, the anti-NP antibody would be useful for studies of the function of NP in viral replication.
REFERENCES
[1] Taylor DJ, Leach RW, Bruenn J. Filoviruses are ancient and integrated into mammalian genomes. BMC Evol Biol, 2010, 10(1): 193.
[2] Suzuki Y, Gojobori T. The origin and evolution of Ebola and Marburg viruses. Mol Biol Evol, 1997, 14(8): 800−806.
[3] Feldmann H, Geisbert TW. Ebola haemorrhagic fever. The Lancet, 2011, 377(9768): 849−862.
[4] Formenty P, Boesch C, Wyers M, et al. Ebola virus outbreak among wild chimpanzees living in a rain forest of Côte d'Ivoire. J Infect Dis, 1999, 179(Suppl 1): S120−S126.
[5] Barrette RW, Metwally SA, Rowland JM, et al. Discovery of swine as a host for the Reston ebolavirus. Science, 2009, 325(5937): 204−206.
[6] Groseth A, Feldmann H, Strong JE. The ecology of Ebola virus. Trends Microbiol, 2007, 15(9): 408−416.
[7] Han ZY, Boshra H, Sunyer JO, et al. Biochemical and functional characterization of the Ebola virus VP24 protein: implications for a role in virus assembly and budding. J Virol, 2003, 77(3): 1793−1800.
[8] Kingston RL, Baase WA, Gay LS. Characterization of nucleocapsid binding by the measles virus and mumps virus phosphoproteins. J Virol, 2004, 78(16): 8630−8640.
[9] Sanchez A, Khan AS, Zaki SR, et al. Filoviridae: Marburg and Ebola viruses//Knipe DM, Howley PM, eds. Fields Virology. Philadelphia, PA: Lippincott, Williams &Wilkins, 2001: 1279−1304.
[10] Shi W, Huang Y, Sutton-Smith M, et al. A filovirus-unique region of Ebola virus nucleoprotein confers aberrant migration and mediates its incorporation into virions. J Virol, 2008, 82(13): 6190−6199.
[11] Watanabe S, Watanabe T, Noda T, et al. Production of novel Ebola virus-like particles from cDNAs: an alternative to Ebola virus generation by reverse genetics. J Virol, 2004, 78(2): 999−1005.
[12] Watanabe S, Noda T, Kawaoka Y. Functional mapping of the nucleoprotein of Ebola virus. J Virol, 2006, 80(8): 3743−3751.
[13] Sullivan N, Yang ZY, Nabel GJ. Ebola virus pathogenesis: implications for vaccines and therapies. J Virol, 2003, 77(18): 9733−9737.
[14] Saijo M, Niikura M, Ikegami T, et al. Laboratory diagnostic systems for Ebola and Marburg hemorrhagic fevers developed with recombinant proteins. Clin Vaccine Immunol, 2006, 13(4): 444−451.
[15] Sullivan NJ, Martin JE, Graham BS, et al. Correlates of protective immunity for Ebola vaccines: implications for regulatory approval by the animal rule. Nat Rev Microbiol, 2009, 7(5): 393−400.
[16] Wilson JA, Hevey M, Bakken R, et al. Epitopes involved in antibody-mediated protection from Ebola virus. Science, 2000, 287(5458): 1664−1666.
[17] Niikura M, Ikegami T, Saijo M, et al. Analysis of linear B-cell epitopes of the nucleoprotein of Ebola virus that distinguish Ebola virus subtypes. Clin Diagn Lab Immunol, 2003, 10(1): 83−87.
[18] Ikegami T, Niikura M, Saijo M, et al. Antigen capture enzyme-linked immunosorbent assay for specific detection of Reston Ebola virus nucleoprotein. Clin Diagn Lab Immunol, 2003, 10(4): 552−557.
[19] Qiu YF, Shen Y, Li XD, et al. Polyclonal antibody to porcine p53 protein: a new tool for studying the p53 pathway in a porcine model. Biochem Biophys Res Commun, 2008, 377(1): 151−155.
[20] Li XD, Qiu YF, Shen Y, et al. Splicing together different regions of a gene by modified polymerase chain reaction-based site-directed mutagenesis. Anal Biochem, 2008, 373(2): 398−400.
[21] Xu WX, He YP, Tang HP, et al. Minimal motif mapping of a known epitope on human zona pellucida protein-4 using a peptide biosynthesis strategy. J Reprod Immunol, 2009, 81(1): 9−16.
[22] Deng XF, Shi ZX, Li SQ, et al. Characterization of nonstructural protein 3 of a neurovirulent Japanese encephalitis virus strain isolated from a pig. Virol J, 2011, 8(1): 209.
[23] Saijo M, Niikura M, Morikawa S, et al. Enzyme-linked immunosorbent assays for detection of antibodies to Ebola and Marburg viruses using recombinant nucleoproteins. J Clin Microbiol, 2001, 39(1): 1−7.
[24] Geisbert TW, Jahrling PB. Exotic emerging viral diseases: progress and challenges. Nat Med, 2004, 10: S110−S121.
[25] Júnior ÁF, Santiago FM, Silva MV, et al. Production, characterization and applications for Toxoplasma gondii-specific polyclonal chicken egg yolk immunoglobulins. PLoS ONE, 2012, 7(7): e40391.
[26] Halfmann P, Kim JH, Ebihara H, et al. Generation of biologically contained Ebola viruses. Proc Natl Acad Sci USA, 2008, 105(4): 1129−1132.
[27] Watanabe S, Watanabe T, Noda T, et al. Production of novel Ebola virus-like particles from cDNAs: an alternative to Ebola virus generation by reverse genetics. J Virol, 2004, 78(2): 999−1005.