Liver transplantation for hepatocellular carcinoma:an update
2011-12-14AliZarrinparFadyKaldasandRonaldBusuttil
Ali Zarrinpar, Fady Kaldas and Ronald W Busuttil
Los Angeles, USA
Liver transplantation for hepatocellular carcinoma:an update
Ali Zarrinpar, Fady Kaldas and Ronald W Busuttil
Los Angeles, USA
(Hepatobiliary Pancreat Dis Int 2011; 10: 234-242)
liver transplantation;hepatocellular carcinoma;liver neoplasm;downstaging;immunosuppression;locoregional therapy
Introduction
Hepatocellular carcinoma (HCC) is a heterogeneous malignancy with multiple etiologies and a mean survival of six to twenty months.[1]It arises almost exclusively from a background of cirrhosis of any cause, though most commonly chronic hepatitis B or C infection, and hereditary hemochromatosis. It continues to be the sixth most common neoplasm worldwide and due to its high mortality the third most common cause of cancer mortality, causing 9.2% of all cancer deaths.[2]The overall incidence of HCC has been estimated at 10.8 per 100 000 with the geographical distribution of mortality re fl ecting that of the incidence. This demonstrates that how little progress has been made in treating the disease even in the developed world. In 2008, almost 750 000 new HCC cases were reported worldwide, with 13 300 HCC cases in North America and 57 900 in Europe, all increased from 2002.[3]Most of the burden is in developing countries, where almost 85% of the cases occur. Nevertheless, Western countries have also seen rising numbers of cases. The economic burden of HCC in the United States alone is estimated to be $455 million each year, and it will increase, given that the incidence of HCC continues to grow.[4]While worldwide hepatitis B (HBV) is the primary cause of HCC,[5]in Western countries hepatitis C (HCV) causes the largest number of HCC. Of the three to four million people in the United States who are chronically infected with HCV, 20% will develop cirrhosis and up to 5%will die of HCC, and the incidence is rising.[6,7]HCC in the United States has more than doubled over the last 20 years, and more than 10 000 cases occur annually.[8]The incidence of hepatitis C related HCC is expected to double yet again over the next 20 years.
Liver transplantation for HCC: rationale
The standard surgical management for patients with HCC consists of locoregional ablation, surgical resection,or liver transplantation, depending on the state of the liver. Eighty percent of patients initially presenting with HCC are unresectable,[9]either due to the extent of tumor or the level of underlying hepatic dysfunction.While in patients with no evidence of cirrhosis and good hepatic function, resection has been the treatment of choice, it is contraindicated in patients with moderate to severe cirrhosis (Child class B or C), leaving these patients with liver transplantation as the only option.Moreover, transplantation is the optimal treatment even for small, otherwise resectable HCC. This is a re fl ection of a number of factors. Liver transplantation is the most bene fi cial oncologic treatment, which most likely results in a resection with microscopically negative margins.Most HCCs are multifocal, arising from a " fi eld defect"in the liver; though pre-neoplastic lesions may not be visible at time of operation, they are likely to continue to evolve into new primary HCCs. Furthermore,transplantation also eliminates cirrhosis and restores normal hepatic function. However, limited organ availability mandates the restriction of transplantation to only those patients with early stage tumors.
Organ Procurement Transplant Network (OPTN)data con fi rm the evolution of liver transplantation from futile to the fi rst choice therapy for selected patients.[10]Not only has the number of HCC patients listed for liver transplantation increased from 1998 to 2006, but those listed have had more advanced HCC. The importance of patient selection was emphasized by Mazzaferro and colleagues in 1996. Limiting the eligibility to patients with early stage tumors through the use of these Milan criteria[11]is usually credited with establishing liver transplantation as the best curative treatment. However,there is increasing evidence that these criteria may be conservative, and that many patients with larger or more numerous tumors can have also acceptable outcomes.
Orthotopic liver transplantation for HCC outside Milan criteria
Mazzaferro et al[11]in their small but very in fl uential study established the Milan criteria (1 lesion ≤5 cm,or 2 to 3 lesions ≤3 cm) in an attempt to parse out the patient population that would respond well to liver transplantation as the treatment modality for their HCC. More than 85% of these patients had survived to four years and only 8% had recurred. These criteria became widely accepted and were corroborated by other centers, showing that patients with HCC meeting these criteria could exceed a 5-year survival rate of 70%. Since then, this boundary has been challenged, with many suggesting that these criteria are too restrictive and that expansion or modi fi cation of tumor size and number requirements could achieve comparable survival rates.
Critical studies from UCSF and UCLA show that indeed the criteria can be expanded. Yao et al examined a modest expansion of the criteria for orthotopic liver transplantation in 2001. They looked at patients with solitary tumors ≤6.5 cm, or three or fewer nodules with the largest one ≤4.5 cm and total tumor diameter ≤8 cm.These patients were found to have survival rates of 90%and 75%, at 1 and 5 years,[12]thus establishing the UCSF criteria. This study was corroborated and even expanded at different centers (Table), including a study at UCLA by Duffy et al,[13]which also demonstrated the applicability of preoperative imaging, as opposed to explant pathology, in the new expanded criteria. The UCLAseries retrospectively evaluated 536 patients transplanted for HCC over 25 years, with a mean follow-up of 7 years.Hepatitis C was the most common underlying liver disease, followed by hepatitis B and alcoholic cirrhosis.Seventy-eight percent of the tumors were diagnosed preoperatively, while 22% were found incidentally on examination of the explanted liver. The factors diminishing post-transplant survival on multivariate analysis included multifocality, lymphovascular invasion and poor differentiation. Recurrence free survival based on preoperative imaging or explant pathology was similar for the Milan and UCSF criteria, but signi fi cantly worse beyond the UCSF criteria. Data from UCLA and other centers show consistent achievement of 5-year posttransplant survival rate near or above 50% for tumors beyond the Milan criteria based on explant pathology.
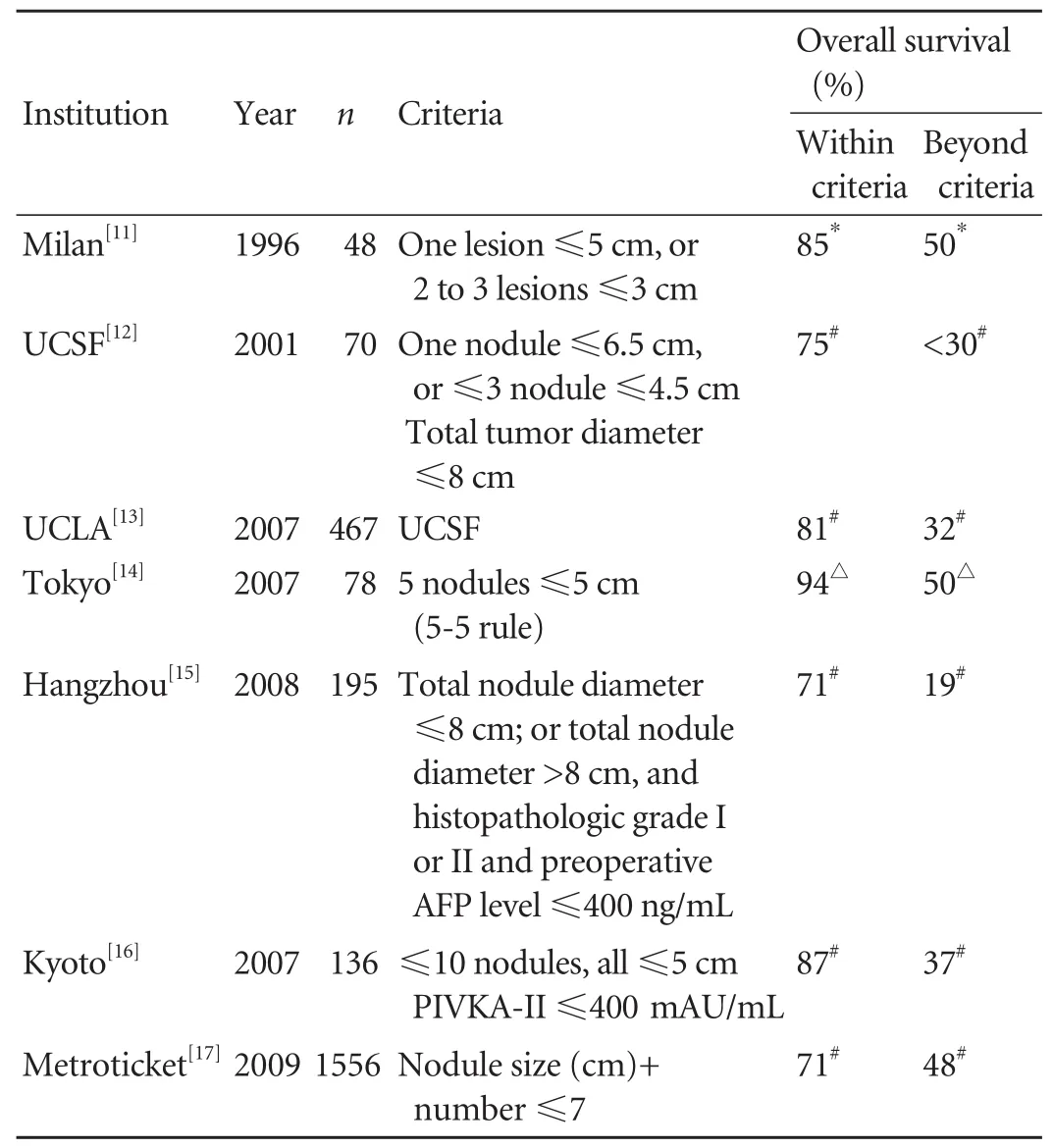
Table. Overview of orthotopic liver transplantation criteria for HCC
Adopting the model for end-stage liver disease(MELD) allocation system in 2002 in the united states offered priority for patients with HCC within the Milan criteria. Since that time, it has led to a 6-fold increase in the proportion of patients with HCC who were transplanted.
Locoregional therapy/downstaging
Selected patients with HCC con fi ned to the liver,who are otherwise not eligible for resection or transplantation, can undergo preoperative treatment aimed at decreasing tumor burden to ful fi ll listing criteria for HCC and to improve survival. This locoregional treatment includes percutaneous injection of ethanol (PEI) or acetic acid (PAI), radiofrequency ablation (RFA), transarterial embolization (TAE) or chemoembolization (TACE), radioactive microspheres,and stereotactic radiation. While only resection can cure HCC, locoregional therapy does destroy tumor tissue while preserving the remaining liver parenchyma and may serve to downstage the tumor and bridge many patients to liver transplantation.[18-21]Furthermore, there are data suggesting that the response to locoregional treatment predicts post-transplant outcome and that this response may be used for patient selection. A number of groups[22,23]have found that, while not statistically signi fi cant, patients who had complete or partial response tended towards better survival.
Percutaneous ablation under ultrasound guidance is best applied to early-stage HCC not amenable to surgical resection or transplantation. This can involve injection with 95% ethanol (PEI) or 50% acetic acid(PAI) or the application of microwaves via an electrode inserted into a tumor (RFA). The injection of tumors with ethanol or acetic acid is inexpensive, simple, and well tolerated. It results in complete necrosis of almost all tumors ≤2 cm and a complete response in 70% to 80%of solitary tumors ≤3 cm.[24]However, when the tumor diameter is 3-5 cm the response rate drops to 50% even when combining percutaneous ablation with arterial embolization.[25]Thus, in large HCCs, percutaneous ablation compares poorly with surgical resection. This is likely due to the limitations of diffusion, vascular washout,and heterogeneity. The best candidates are Child-Pugh class A patients with small tumors who can achieve a 50%5-year survival rate without operative intervention.[26-30]
RFA also produces areas of coagulative necrosis,this time by producing a zone of thermal desiccation to ablate the tumor and can be delivered percutaneously or through laparoscopy or laparotomy. The ef fi cacy is limited to tumors <3 cm and away from blood vessels,with larger ones requiring multiple applications.[30-32]While available data show an ef fi cacy similar if not better than percutaneous injection,[33]RFA can have more severe side effects, including tumor dissemination in subcapsular HCC.[34]Therefore, PEI therapy is reserved for tumors that are unsafe or dif fi cult to approach with RFA, like those in close proximity to intestinal loops, in the hepatic dome, in the subcapsular area, or surrounded by large vessels. Review of the literature also reveals mixed results in comparing the curative ef fi cacy of RFA to surgery. However, the comparison may not be pertinent since those eligible for either resection or transplant should undergo an operation. Therefore,RFA is reserved for the treatment of small HCC lesions in patients with unresectable tumors, either to prevent progression prior to transplant and to downsize tumors to meet criteria or as palliation.
For larger tumors TACE is the preferred locoregional therapy, delivering both chemotherapy and vascular embolization particles through the hepatic artery, to stop the arterial fl ow for both ischemic injury and direct cytotoxicity. Its ef fi cacy relies on the idea that HCC tumors larger than 2 cm receive most of their blood supply from the arterial circulation. The chemotherapy agent is either delivered prior to embolization or simultaneously, and incorporated into gelatin particles that also act to embolize the artery.[35]
Like RFA, there appears to be some advantage in TACE compared with the supportive care, but this has not been consistent.[36,37]The suggested use for TACE again is in patients with tumors not amenable to surgery or RFA. However, its use is limited by its higher morbidity rate especially in patients with tumors larger than 10 cm, or those with decompensated liver failure,with complications ranging from fevers and abdominal pain to hepatic necrosis and liver failure.[38]
A newer locoregional treatment against HCC is transarterial radioembolization (TARE) with yttrium-90. Similar to TACE, intraarterial injection of glass microspheres loaded with 90Y delivers brachytherapy into the affected region, with minimal toxicity to the surrounding benign liver tissue. Safety and ef fi cacy of TARE in the treatment of advanced HCC have been demonstrated in various studies[39-41]and recently tumor response rates of 49%-58%have been reported after TARE.[42,43]Retrospective experiences show longer time-to-progression following radioembolization than chemoembolization, but there has not been consistent evidence in favor of a difference in median survival times.[43]
Other regional therapy includes infusion of chemotherapy without embolization, embolization without chemotherapy, infusion of drug-eluting beads,high-intensity focused ultrasound,[44]and radiation. The evidence for any of these approaches is not yet robust enough to elevate one to the forefront.
The question of how well locoregional therapy works to treat or shrink lesions, and more importantly to improve long-term survival, has been examined by various groups. There is good evidence that pretransplantation therapy downstages the primary tumors and improves survival of patients. As one would expect,complete tumor necrosis with locoregional therapy is associated with better recurrence-free survival.[22]A more recent study, looking speci fi cally at patients with stage III and IV disease receiving TACE found that,while only a small portion of patients with stage III/IV HCC could be downstaged to the Milan criteria with TACE, those who were downstaged had survival rates similar to stage II HCC.[45]
Having established the ef fi cacy of downstaging, the methodology for assessing the downstaging becomes of utmost importance in preoperative patient selection.Speci fi cally it matters how best to assess the tumor burden, whether by loss of enhancement or by decrease in size. Furthermore, preoperative imaging tends to understage tumor burden. In the UCLA series[13]preoperative imaging showed tumors in 173 patients to be within the Milan criteria, 185 beyond the Milan criteria but within the UCSF criteria, and 109 beyond the UCSF criteria. By explant pathology, these numbers were 126, 208, and 133, respectively.
Immunosuppression
Much has changed in immunosuppression since the advent of the more selective and less toxic immunosuppression regimen of cyclosporine (CsA)transformed liver transplantation.[46]While results of liver transplantation including survival and rates of rejection were dramatically improved in cyclosporine treated patients compared with "historical controls," a high incidence of neoplasm and its aggressive phenotype were found to be due to CsA and its activation of transforming growth factor-beta (TGF-β). Hojo et al[47]showed that cyclosporine actually induced invasiveness in non-transformed cells and further altered the morphology, motility, and invasive growth of previously transformed adenocarcinoma cells. This fi nding was con fi rmed via a retrospective analysis of 70 patients with HCC who received cyclosporine after liver transplantation. HCC recurrence in 7 (10.0%) of these patients was statistically associated on multivariate analysis with CsA levels, but not steroids or azathioprine use.[48]
Tacrolimus, another calcineurin inhibitor was also found to promote cell cycle progression by an increase in cdk4 kinase activity and thus was linked to increased tumor recurrence.[49]On the other hand, the calcineurin-independent immunosuppressive agent sirolimus (otherwise known as rapamycin), a binder of mTOR, inhibits tumor growth in cell lines,[50,51]and it inhibits primary and metastatic tumor growth in vivo.[52]In a study looking speci fi cally at HCC, sirolimus induced cell cycle arrest and blocked proliferation of an HCC cell line. In the same study in a mouse model of human HCC, sirolimus prevented tumor growth and metastatic progression by down-regulating the mRNA expression of VEGF and HIF-1α.[53]Encouraging results from a retrospective comparison of HCC patients treated with sirolimus to patients treated with calcineurin inhibitors showed a post-OLT survival bene fi t without any differences in the incidence of major complications.[54,55]
A number of other mTOR inhibitors, sirolimus analogues with better pharmacokinetic pro fi les, have been developed as anti-cancer agents, owing to their ability to inhibit cell growth, proliferation, angiogenesis,and metabolism.[56]One of these agents showing promise in the treatment of HCC is everolimus. Everolimus suppresses the growth of subcutaneous patient-derived HCC xenografts in mice.[57]Clinical data are only beginning to accrue on its application in the treatment of refractory rejection and the prevention of recurrence of large or invasive tumors.[58]
Evidence that steroids play a role in the recurrence of HCC after liver transplantation came early.[59]Therefore,some groups attempted to withdraw steroids early after liver transplantation in patients with advancedstage HCC. Fortunately, this was shown not only to be safe, but, in one study, when steroids were withdrawn 3 months after transplantation, tumor recurrence rates and adverse effects decreased signi fi cantly, possibly leading to an increase in long-term survival.[60]Though a later study did not fi nd a similar decrease in HCC recurrence, acute rejection episodes and HBV recurrence did occur less frequently.[61]
Adjuvant chemotherapy
HCC tumors have proven themselves over the years to be clinically chemotherapy-resistant. The value of adjuvant therapy in HCC has not yet been established. As in any carcinoma, there is a theoretical bene fi t to adjuvant therapy, especially since the manipulation required in total hepatectomy can result in intra-operative dissemination of tumor cells. A number of studies showed promising preliminary results for adjuvant chemotherapy. An early study of 20 patients looking at doxorubicin after orthotopic liver transplantation found 1-, 2-, and 3-year survival rates of 74%, 61%, and 54%.[62]A later study with 25 patients undergoing a sixmonth protocol including doxorubicin, fl uorouracil, and cisplastin included 11 patients (44%) with stage IV HCC.The overall 3-year survival rate was 46% compared to 5.8% in historic controls, with an overall recurrence rate of 20%.[63]These results are in contrast to a more recent study, which included neoadjuvant treatment.Sixty-two patients were randomized to two groups,34 on the protocol and 28 in the control group. The protocol included preoperative biweekly doxorubicin,an intraoperative dose, and post-operative therapy from day 10 for a total dose of 300 mg/m2. While there was a trend toward improved results, overall and disease-free survival differences were not statistically signi fi cant.[64]The advent of orally available, small molecule,kinase inhibitors seems to have changed the landscape,however. One such drug, sorafenib, an inhibitor of the serine-threonine kinases Raf-1 and B-Raf and the receptor tyrosine kinases vascular endothelial growth factor receptors (VEGFRs) 1, 2, and 3 and plateletderived growth factor receptor β (PDGFR-β), appears to function by blocking tumor-cell proliferation and angiogenesis and increasing the rate of apoptosis. The SHARP Trial demonstrated, in patients with advanced HCC and Child-Pugh class A cirrhosis, a 3-month increase of overall survival from a median 34.4 weeks(7.9 months) in the placebo group to 46.3 weeks (10.7 months) in the sorafenib group with mild side-effects.[65]A matched control study of sorafenib after liver transplantation for HCC at UCLA[66]further investigated the safety, tolerability, and ef fi cacy of sorafenib. In this study of 16 patients, 8 in each arm, recurrence was 50%versus 12.5%, and disease free 1-year survival rate was 57% versus 86%, favoring treatment.
With the demonstration of the promise of this active systemic agent in advanced HCC, it remains to be seen what role sorafenib will play after resection and after orthotopic liver transplantation. Further long-term studies remain to be conducted to address the questions of safety and ef fi cacy. Even more exciting is that the adjuvant setting allows for prospective tissue collection and the development of biomarkers.
Imaging
The major limitations of the treatment of HCC stem from the preoperative diagnosis and staging of the tumor. Early diagnosis of HCC is of paramount importance in effective treatment. Currently this relies heavily on the accuracy of imaging. Unfortunately, this process is very fl awed with up to a third of patients being understaged.[11]Another more disheartening study found preoperative imaging to diagnose the number of tumors incorrectly 66% of the time and the size of the tumor 71% of the time.[67]Advanced imaging techniques for HCC are being developed to improve recipient selection. Quantitative perfusion MRI, which measures blood fl ow in tumor relative to the liver, is proposed to distinguish among early HCC (visualizing a tumor supplied by the hepatic artery and the portal vein),advanced HCC (tumor supplied only by the artery),and colon cancer (lower artery and venous fl ow than HCC).[68]There is also contrast enhanced ultrasound,wherein the micro fl ow vascular image predicts HCC differentiation,[69]C11-acetate-PET activity with[70]or without[71,72]FDG-PET activity, and complete necrosis on imaging after TACE.[73]
Tumor biology
Thus far the principal determinants of outcome have repeatedly been found to be lymphovascular invasion and poor differentiation. However, this has been dif fi cult to assess with certainty preoperatively. The question has yet to be answered whether tumor size or number, the criteria currently used to select patients for transplantation, re fl ect invasion or differentiation.Biomarkers have been proposed to be better than preoperative imaging in predicting post-transplantation HCC recurrence. In one study of 83 patients immunohistochemistry of explants was performed looking at adhesion molecules: E-cadherin, β-catenin,the MIB-1 proliferative index, cyclin-dependent kinase inhibitor p27, vascular invasion, and tumor grade. At multivariate analysis, only high MIB-1 index, low or equal E-cadherin levels (compared to nonneoplastic surrounding tissue), and the presence of nuclear β-catenin were found to be independent predictors of recurrence. In fact, any one of these parameters was associated with a greater than 88% risk of HCC recurrence and up to 99% for the presence of all three parameters.[74]The limitation here is that it relies on the use of biopsies of tumors, a process that is not without its drawbacks, especially since the risk of biopsy tract seed is upward of 3%.[75]However, this issue will need to be readdressed, especially in the light of new results indicating that the biology trumps size, namely that poor differentiation is a better predictor of recurrence than being within or outside the Milan criteria.[76]
There have been a number of studies using molecular approaches to look for predictors of poor prognosis in HCC. AFP is the most widely used marker. It can be a marker for the presence of tumor with good sensitivity(39%-65%) and speci fi city (76%-94%), though it is best if combined with ultrasound.[77]There is also evidence that AFP can be a predictor of recurrence, but 30%-40%of HCC patients have normal levels of serum AFP and more than 30% of patients with high AFP levels do not have HCC.[78]The Lens culinaris agglutinin-reactive fraction of AFP (AFP-L3) performs better as a marker of recurrence, with a sensitivity of 41.1% at 24 months,compared to 18%, using AFP levels >20.[79]Protein induced by vitamin K absence (PIVKA), otherwise known as des-gamma-carboxyprothrombin (DCP or DGC), has also been proposed as a marker, progressing to the point of being included in the Kyoto criteria for selection (size ≤5 cm, number of tumors ≤10 and DCP≤400 mAU/mL).[80]Other proposed biomarkers include innumerable other molecular markers using proteins ranging from those involved in cell proliferation, cell adhesion and extracellular matrix, angiogenesis, cell surface markers, and transcription factors among others.
Remaining challenges for HCC
Much can be improved in the diagnosis and treatment of HCC. A great challenge will be to improve patient selection beyond "crude" clinical parameters and rather to select patients based on tumor biology. One way to develop a means of selecting patients based on tumor biology would be to use a tumor explant library and to correlate the molecular characterization and the clinical database. Microarray technology has already revolutionized the understanding of the molecular basis of colon cancer, among others.[81]Similar high throughput analyses of genes or proteins can be performed to obtain comprehensive studies in HCC to identify molecular pro fi les to improve cancer staging, prediction of recurrence, prognosis, and treatment selection. Emerging data provide insight into distinct genetic and molecular differences across the spectrum of HCC.[82,83]An elegant study looking at gene expression in patients with HCC showed that differences between tumors themselves had a much poorer power in predicting recurrence than gene expression in nontumoral tissues of the same patients.[84]Proteomics,the differential expression of proteins, may also prove to be powerful tool in assessing tumor progression,invasion, metastasis, response to treatment, recurrence,and prognosis. This methodology also has the advantage of applicability to tissue, serum, urine, saliva, among other samples. Such studies can help fi nd surrogate markers which will then allow for better surveillance and patient selection.
Another challenge will be to incorporate active systemic agents post-operatively in patients at high risk for recurrence, paying close attention to ef fi cacy and safety. The future direction of the effort in treating HCC will be to stimulate prospective trials, to develop molecular imaging of lymphovascular invasion, to improve recipient selection, and to investigate biomarkers of tumor biology.
Funding: None.
Ethical approval: Not needed.
Contributors: ZA wrote the main body of the article under the supervision of BRW. KF provided advice on medical aspects. BRW is the guarantor.
Competing interest: No bene fi ts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Bialecki ES, Di Bisceglie AM. Diagnosis of hepatocellular carcinoma. HPB (Oxford) 2005;7:26-34.
2 Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008:GLOBOCAN 2008. Int J Cancer 2010;127:2893-2917.
3 Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics,2002. CA Cancer J Clin 2005;55:74-108.
4 Hepatocellular carcinoma-United States, 2001-2006. Centers for Disease Control and Prevention (CDC). MMWR Morb Mortal Wkly Rep 2010;59:517-520.
5 Kim WR. Epidemiology of hepatitis B in the United States.Hepatology 2009;49:S28-34.
6 El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med 1999;340:745-750.
7 Chiaramonte M, Stroffolini T, Vian A, Stazi MA, Floreani A, Lorenzoni U, et al. Rate of incidence of hepatocellular carcinoma in patients with compensated viral cirrhosis. Cancer 1999;85:2132-2137.
8 Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 2009;27:1485-1491.
9 Thomas MB, Jaffe D, Choti MM, Belghiti J, Curley S, Fong Y,et al. Hepatocellular carcinoma: consensus recommendations of the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol 2010;28:3994-4005.
10 Pelletier SJ, Fu S, Thyagarajan V, Romero-Marrero C, Batheja MJ, Punch JD, et al. An intention-to-treat analysis of liver transplantation for hepatocellular carcinoma using organ procurement transplant network data. Liver Transpl 2009;15:859-868.
11 Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A,Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-699.
12 Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma:expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-1403.
13 Duffy JP, Vardanian A, Benjamin E, Watson M, Farmer DG, Ghobrial RM, et al. Liver transplantation criteria for hepatocellular carcinoma should be expanded: a 22-year experience with 467 patients at UCLA. Ann Surg 2007;246:502-511.
14 Sugawara Y, Tamura S, Makuuchi M. Living donor liver transplantation for hepatocellular carcinoma: Tokyo University series. Dig Dis 2007;25:310-312.
15 Zheng SS, Xu X, Wu J, Chen J, Wang WL, Zhang M, et al. Liver transplantation for hepatocellular carcinoma:Hangzhou experiences. Transplantation 2008;85:1726-1732.
16 Takada Y, Ito T, Ueda M, Sakamoto S, Haga H, Maetani Y, et al. Living donor liver transplantation for patients with HCC exceeding the Milan criteria: a proposal of expanded criteria.Dig Dis 2007;25:299-302.
17 Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M,Mariani L, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009;10:35-43.
18 Heckman JT, Devera MB, Marsh JW, Fontes P, Amesur NB, Holloway SE, et al. Bridging locoregional therapy for hepatocellular carcinoma prior to liver transplantation. Ann Surg Oncol 2008;15:3169-3177.
19 Belghiti J, Carr BI, Greig PD, Lencioni R, Poon RT. Treatment before liver transplantation for HCC. Ann Surg Oncol 2008;15:993-1000.
20 Pompili M, Francica G, Rapaccini GL. Bridge treatments of hepatocellular carcinoma in cirrhotic patients submitted to liver transplantation. Dig Dis Sci 2008;53:2830-2831.
21 Zimmerman MA, Ghobrial RM, Tong MJ, Hiatt JR, Cameron AM, Hong J, et al. Recurrence of hepatocellular carcinoma following liver transplantation: a review of preoperative and postoperative prognostic indicators. Arch Surg 2008;143:182-188.
22 Bharat A, Brown DB, Crippin JS, Gould JE, Lowell JA, Shenoy S, et al. Pre-liver transplantation locoregional adjuvant therapy for hepatocellular carcinoma as a strategy to improve longterm survival. J Am Coll Surg 2006;203:411-420.
23 Millonig G, Graziadei IW, Freund MC, Jaschke W,Stadlmann S, Ladurner R, et al. Response to preoperative chemoembolization correlates with outcome after liver transplantation in patients with hepatocellular carcinoma.Liver Transpl 2007;13:272-279.
24 Vilana R, Bruix J, Bru C, Ayuso C, Solé M, Rodés J. Tumor size determines the ef fi cacy of percutaneous ethanol injection for the treatment of small hepatocellular carcinoma.Hepatology 1992;16:353-357.
25 Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T,Solbiati L, et al. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology 2000;214:761-768.
26 Arii S, Yamaoka Y, Futagawa S, Inoue K, Kobayashi K, Kojiro M, et al. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology 2000;32:1224-1229.
27 Livraghi T, Giorgio A, Marin G, Salmi A, de Sio I, Bolondi L, et al. Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection.Radiology 1995;197:101-108.
28 Llovet JM, Mas X, Aponte JJ, Fuster J, Navasa M, Christensen E,et al. Cost effectiveness of adjuvant therapy for hepatocellular carcinoma during the waiting list for liver transplantation. Gut 2002;50:123-128.
29 Grasso A, Watkinson AF, Tibballs JM, Burroughs AK.Radiofrequency ablation in the treatment of hepatocellular carcinoma--a clinical viewpoint. J Hepatol 2000;33:667-672.
30 Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L,Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology 1999;210:655-661.
31 Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut 2005;54:1151-1156.
32 Hong SN, Lee SY, Choi MS, Lee JH, Koh KC, Paik SW, et al. Comparing the outcomes of radiofrequency ablation and surgery in patients with a single small hepatocellular carcinoma and well-preserved hepatic function. J Clin Gastroenterol 2005;39:247-252.
33 Germani G, Pleguezuelo M, Gurusamy K, Meyer T, Isgrò G, Burroughs AK. Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocelullar carcinoma: a meta-analysis. J Hepatol 2010;52:380-388.
34 Llovet JM, Vilana R, Brú C, Bianchi L, Salmeron JM, Boix L, et al. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma.Hepatology 2001;33:1124-1129.
35 Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology 2004;127:S179-188.
36 No authors listed. A comparison of lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. Groupe d'Etude et de Traitement du Carcinome Hépatocellulaire. N Engl J Med 1995;332:1256-1261.
37 Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma.Hepatology 2002;35:1164-1171.
38 Thornton RH, Covey A, Petre EN, Riedel ER, Maluccio MA, Sofocleous CT, et al. A comparison of outcomes from treating hepatocellular carcinoma by hepatic artery embolization in patients younger or older than 70 years.Cancer 2009;115:5000-5006.
39 Kulik LM, Carr BI, Mulcahy MF, Lewandowski RJ, Atassi B, Ryu RK, et al. Safety and ef fi cacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology 2008;47:71-81.
40 Geschwind JF, Salem R, Carr BI, Soulen MC, Thurston KG,Goin KA, et al. Yttrium-90 microspheres for the treatment of hepatocellular carcinoma. Gastroenterology 2004;127:S194-205.
41 Hilgard P, Hamami M, Fouly AE, Scherag A, Müller S, Ertle J,et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology 2010;52:1741-1749.
42 Lewandowski RJ, Kulik LM, Riaz A, Senthilnathan S,Mulcahy MF, Ryu RK, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma:chemoembolization versus radioembolization. Am J Transplant 2009;9:1920-1928.
43 Salem R, Lewandowski RJ, Kulik L, Wang E, Riaz A, Ryu RK,et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2011;140:497-507.
44 McWilliams JP, Yamamoto S, Raman SS, Loh CT, Lee EW,Liu DM, et al. Percutaneous ablation of hepatocellular carcinoma: current status. J Vasc Interv Radiol 2010;21:S204-213.
45 Chapman WC, Majella Doyle MB, Stuart JE, Vachharajani N, Crippin JS, Anderson CD, et al. Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg 2008;248:617-625.
46 Cohen DJ, Loertscher R, Rubin MF, Tilney NL, Carpenter CB,Strom TB. Cyclosporine: a new immunosuppressive agent for organ transplantation. Ann Intern Med 1984;101:667-682.
47 Hojo M, Morimoto T, Maluccio M, Asano T, Morimoto K,Lagman M, et al. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature 1999;397:530-534.
48 Vivarelli M, Cucchetti A, Piscaglia F, La Barba G, Bolondi L, Cavallari A, et al. Analysis of risk factors for tumor recurrence after liver transplantation for hepatocellular carcinoma: key role of immunosuppression. Liver Transpl 2005;11:497-503.
49 Baksh S, DeCaprio JA, Burakoff SJ. Calcineurin regulation of the mammalian G0/G1 checkpoint element, cyclin dependent kinase 4. Oncogene 2000;19:2820-2827.
50 Schumacher G, Oidtmann M, Rosewicz S, Langrehr J, Jonas S, Mueller AR, et al. Sirolimus inhibits growth of human hepatoma cells in contrast to tacrolimus which promotes cell growth. Transplant Proc 2002;34:1392-1393.
51 Schumacher G, Oidtmann M, Rueggeberg A, Jacob D, Jonas S, Langrehr JM, et al. Sirolimus inhibits growth of human hepatoma cells alone or combined with tacrolimus, while tacrolimus promotes cell growth. World J Gastroenterol 2005;11:1420-1425.
52 Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med 2002;8:128-135.
53 Wang Z, Zhou J, Fan J, Tan CJ, Qiu SJ, Yu Y, et al. Sirolimus inhibits the growth and metastatic progression of hepatocellular carcinoma. J Cancer Res Clin Oncol 2009;135:715-722.
54 Zimmerman MA, Trotter JF, Wachs M, Bak T, Campsen J, Skibba A, et al. Sirolimus-based immunosuppression following liver transplantation for hepatocellular carcinoma.Liver Transpl 2008;14:633-638.
55 Toso C, Merani S, Bigam DL, Shapiro AM, Kneteman NM.Sirolimus-based immunosuppression is associated with increased survival after liver transplantation for hepatocellular carcinoma. Hepatology 2010;51:1237-1243.
56 Yuan R, Kay A, Berg WJ, Lebwohl D. Targeting tumorigenesis: development and use of mTOR inhibitors in cancer therapy. J Hematol Oncol 2009;2:45.
57 Huynh H, Chow KH, Soo KC, Toh HC, Choo SP, Foo KF,et al. RAD001 (everolimus) inhibits tumour growth in xenograft models of human hepatocellular carcinoma. J Cell Mol Med 2009;13:1371-1380.
58 Bilbao I, Sapisochin G, Dopazo C, Lazaro JL, Pou L, Castells L,et al. Indications and management of everolimus after liver transplantation. Transplant Proc 2009;41:2172-2176.
59 Mazzaferro V, Rondinara GF, Rossi G, Regalia E, De Carlis L, Caccamo L, et al. Milan multicenter experience in liver transplantation for hepatocellular carcinoma. Transplant Proc 1994;26:3557-3560.
60 Chen ZS, He F, Zeng FJ, Jiang JP, Du DF, Liu B. Early steroid withdrawal after liver transplantation for hepatocellular carcinoma. World J Gastroenterol 2007;13:5273-5276.
61 Kim JM, Joh JW, Kim SJ, Kwon CH, Song S, Shin M, et al.Steroid withdrawal in adult liver transplantation: occurrence at a single center. Transplant Proc 2010;42:4132-4136.
62 Stone MJ, Klintmalm GB, Polter D, Husberg BS, Mennel RG, Ramsay MA, et al. Neoadjuvant chemotherapy and liver transplantation for hepatocellular carcinoma: a pilot study in 20 patients. Gastroenterology 1993;104:196-202.
63 Olthoff KM, Rosove MH, Shackleton CR, Imagawa DK,Farmer DG, Northcross P, et al. Adjuvant chemotherapy improves survival after liver transplantation for hepatocellular carcinoma. Ann Surg 1995;221:734-743.
64 Pokorny H, Gnant M, Rasoul-Rockenschaub S, Gollackner B, Steiner B, Steger G, et al. Does additional doxorubicin chemotherapy improve outcome in patients with hepatocellular carcinoma treated by liver transplantation? Am J Transplant 2005;5:788-794.
65 Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-390.
66 Saab S, McTigue M, Finn RS, Busuttil RW. Sorafenib as adjuvant therapy for high-risk hepatocellular carcinoma in liver transplant recipients: feasibility and ef fi cacy. Exp Clin Transplant 2010;8:307-313.
67 Sotiropoulos GC, Malagó M, Molmenti E, Paul A, Nadalin S, Brokalaki E, et al. Liver transplantation for hepatocellular carcinoma in cirrhosis: is clinical tumor classi fi cation before transplantation realistic? Transplantation 2005;79:483-487.
68 Abdullah SS, Pialat JB, Wiart M, Duboeuf F, Mabrut JY,Bancel B, et al. Characterization of hepatocellular carcinoma and colorectal liver metastasis by means of perfusion MRI. J Magn Reson Imaging 2008;28:390-395.
69 Sugimoto K, Moriyasu F, Kamiyama N, Metoki R, Yamada M, Imai Y, et al. Analysis of morphological vascular changes of hepatocellular carcinoma by micro fl ow imaging using contrast-enhanced sonography. Hepatol Res 2008;38:790-799.
70 Yun M, Bang SH, Kim JW, Park JY, Kim KS, Lee JD. The importance of acetyl coenzyme A synthetase for 11C-acetate uptake and cell survival in hepatocellular carcinoma. J Nucl Med 2009;50:1222-1228.
71 Kornberg A, Küpper B, Thrum K, Katenkamp K, Steenbeck J,Sappler A, et al. Increased 18F-FDG uptake of hepatocellular carcinoma on positron emission tomography independently predicts tumor recurrence in liver transplant patients.Transplant Proc 2009;41:2561-2563.
72 Lee JW, Paeng JC, Kang KW, Kwon HW, Suh KS, Chung JK,et al. Prediction of tumor recurrence by 18F-FDG PET in liver transplantation for hepatocellular carcinoma. J Nucl Med 2009;50:682-687.
73 Shim JH, Kim KM, Lee YJ, Ko GY, Yoon HK, Sung KB, et al. Complete necrosis after transarterial chemoembolization could predict prolonged survival in patients with recurrent intrahepatic hepatocellular carcinoma after curative resection.Ann Surg Oncol 2010;17:869-877.
74 Fiorentino M, Altimari A, Ravaioli M, Gruppioni E, Gabusi E, Corti B, et al. Predictive value of biological markers for hepatocellular carcinoma patients treated with orthotopic liver transplantation. Clin Cancer Res 2004;10:1789-1795.
75 Silva MA, Hegab B, Hyde C, Guo B, Buckels JA, Mirza DF.Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Gut 2008;57:1592-1596.
76 DuBay D, Sandroussi C, Sandhu L, Cleary S, Guba M, Cattral MS, et al. Liver transplantation for advanced hepatocellular carcinoma using poor tumor differentiation on biopsy as an exclusion criterion. Ann Surg 2011;253:166-172.
77 Daniele B, Bencivenga A, Megna AS, Tinessa V. Alphafetoprotein and ultrasonography screening for hepatocellular carcinoma. Gastroenterology 2004;127:S108-112.
78 Tateishi R, Yoshida H, Matsuyama Y, Mine N, Kondo Y, Omata M. Diagnostic accuracy of tumor markers for hepatocellular carcinoma: a systematic review. Hepatol Int 2008;2:17-30.
79 Sterling RK, Jeffers L, Gordon F, Sherman M, Venook AP,Reddy KR, et al. Clinical utility of AFP-L3% measurement in North American patients with HCV-related cirrhosis. Am J Gastroenterol 2007;102:2196-2205.
80 Fujiki M, Takada Y, Ogura Y, Oike F, Kaido T, Teramukai S,et al. Signi fi cance of des-gamma-carboxy prothrombin in selection criteria for living donor liver transplantation for hepatocellular carcinoma. Am J Transplant 2009;9:2362-2371.81 Nannini M, Pantaleo MA, Maleddu A, Astol fi A, Formica S, Biasco G. Gene expression pro fi ling in colorectal cancer using microarray technologies: results and perspectives.Cancer Treat Rev 2009;35:201-209.
82 Jonas S, Al-Abadi H, Benckert C, Thelen A, Hippler-Benscheid M, Saribeyoglu K, et al. Prognostic signi fi cance of the DNA-index in liver transplantation for hepatocellular carcinoma in cirrhosis. Ann Surg 2009;250:1008-1013.
83 Wang W, Peng JX, Yang JQ, Yang LY. Identi fi cation of gene expression pro fi ling in hepatocellular carcinoma using cDNA microarrays. Dig Dis Sci 2009;54:2729-2735.
84 Tsuchiya M, Parker JS, Kono H, Matsuda M, Fujii H, Rusyn I.Gene expression in nontumoral liver tissue and recurrencefree survival in hepatitis C virus-positive hepatocellular carcinoma. Mol Cancer 2010;9:74.
BACKGROUND: Hepatocellular carcinoma (HCC) is a heterogeneous malignancy with multiple etiologies, high incidence, and high mortality. The standard surgical management for patients with HCC consists of locoregional ablation, surgical resection, or liver transplantation,depending on the background state of the liver. Eighty percent of patients initially presenting with HCC are unresectable,either due to the extent of tumor or the level of underlying hepatic dysfunction. While in patients with no evidence of cirrhosis and good hepatic function resection has been the surgical treatment of choice, it is contraindicated in patients with moderate to severe cirrhosis. Liver transplantation is the optimal surgical treatment.
DATA SOURCES: PubMed search of recent articles (from January 2000 to March 2011) was performed looking for relevant articles about hepatocellular carcinoma and its treatment.Additional articles were identi fi ed by evaluating references from selected articles.
RESULTS: Here we review criteria for transplantation, the types, indications, and role of locoregional therapy in treating the cancer and in downstaging for possible later transplantation. We also summarize the contribution of immunosuppression and adjuvant chemotherapy in the management and prevention of HCC recurrence. Finally we discuss recent advances in imaging, tumor biology, and genomics as we delineate the remaining challenges for the diagnosis and treatment of this disease.
CONCLUSIONS: Much can be improved in the diagnosis and treatment of HCC. A great challenge will be to improve patient selection to criteria based on tumor biology. Another will be to incorporate systemic agents post-operatively in patients at high risk for recurrence, paying close attention to ef fi cacy and safety. The future direction of the effort in treating HCC will be to stimulate prospective trials, develop molecular imaging of lymphovascular invasion, to improve recipient selection,and to investigate biomarkers of tumor biology.
Author Af fi liations: Dumont-UCLA Transplant Center, Division of Liver and Pancreas Transplantation, Department of Surgery, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA (Zarrinpar A, Kaldas F and Busuttil RW)
Ronald W Busuttil, MD, PhD, Ronald Reagan-UCLA Medical Center, 757 Westwood Plaza, Suite 8236, Los Angeles, CA 90095, USA (Tel: 310-267-8054; Fax: 310-267-3668; Email: rbusuttil@mednet.ucla.edu)
© 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
March 1, 2011
Accepted after revision May 8, 2011
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Dose requirement and complications of diluted and undiluted propofol for deep sedation in endoscopic retrograde cholangiopancreatography
- Clinical features and treatment of sump syndrome following hepaticojejunostomy
- Borderline resectable pancreatic tumors: Is there a need for further re fi nement of this stage?
- Current surgical management of pancreatic endocrine tumor liver metastases
- Predictive value and main determinants of abnormal features of intraoperative cholangiography during cholecystectomy
- Emergency re-routing of anterior sector venous outflow for right lobe living donor liver transplantation including the middle hepatic vein
