DABCO催化的“一锅法”水相合成苯并色烯衍生物*
2011-11-27孙宏伟王浩宇连春霞张晓梅袁伟成
孙宏伟, 王浩宇, 连春霞, 张晓梅, 袁伟成
(1. 中国科学院 成都有机化学研究所 不对称合成与手性技术四川省重点实验室,四川 成都 610041;2. 中国科学院 研究生院,北京 100049)
苯并色烯衍生物具有广泛的药理活性和生理活性,如抗发育不全[1]、抗过敏[2]和抗癌活性[3]等。苯并色烯环上连有氰基和氨基等取代基的衍生物是合成某些具有特殊结构的天然产物的重要中间体[4,5]。文献[6~8]方法合成苯并色烯大多是通过Knoevenagel缩合和Michael加成两步反应分步完成的。
本文在前期[9]绿色合成工作的基础上,以1,4-二氮杂二环[2.2.2]辛烷(DABCO)为催化剂,水为溶剂,采用“一锅法”合成了苯并色烯衍生物(2a~2q, Scheme 1),收率26%~95%,其结构经1H NMR,13C NMR和MS确认。
该方法是一种简洁、有效的绿色合成方法。避免了使用有机溶剂造成的环境污染;“一锅法”合成目标产物可减少分步反应的产率损失。
1 实验部分
1.1 仪器与试剂
Büchi B-545型熔点测定仪;Bruker-300 MHz型核磁共振仪(DMSO-d6为溶剂,TMS为内标);Bruker BIO TOF ⅢQ型高分辨质谱仪。
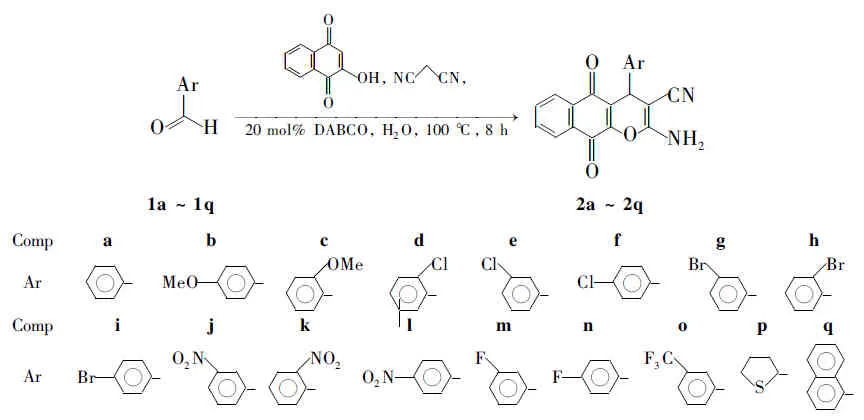
Scheme1
薄层层析硅胶,青岛海洋化工厂;其余所用试剂均为分析纯。
1.2 合成(以2a为例)
在反应管中加入苯甲醛(1a) 64 mg(0.6 mmol),丙二腈46 mg(0.7 mmol)和DABCO 11 mg(0.1 mmol)的水溶液5 mL,搅拌下于室温反应30 min;加入2-羟基-1,4-萘醌 87 mg(0.5 mmol),于100 ℃反应至终点(TLC跟踪)。旋干水后经硅胶柱层析[洗脱剂:V(二氯甲烷) ∶V(乙酸乙酯)=20 ∶1]分离得红褐色固体2a130 mg。
用类似方法合成红褐色固体2b~2q,其熔点与文献[10~12]值一致。
2a:1H NMRδ: 4.59(s, 1H), 7.20~7.31(m, 7H), 7.80~7.87(m, 3H), 8.02~8.04(m, 1H);13C NMRδ: 36.6, 57.6, 119.4, 122.1, 125.9, 126.1, 127.2, 127.8, 128.7, 130.6, 131.1, 134.2, 134.6, 143.6, 149.0, 158.4, 176.9, 182.6。
2c:1H NMRδ: 3.78(s, 3H), 4.93(s, 1H), 6.86(t,J=7.2 Hz, 1H), 6.99(d,J=8.1 Hz, 1H), 7.17~7.23(m, 4H), 7.82~7.87(m, 3H), 8.04~8.07(m, 1H);13C NMRδ: 31.2, 55.8, 56.5, 111.7, 119.4, 120.7, 121.9, 125.8, 126.0, 128.4, 129.1, 130.5, 131.0, 131.3, 134.1, 134.6, 149.5, 156.8, 158.9, 177.0, 182.5; HR-ESI-MS: Calcd for C21H14N2O4Na{[M+Na]+} 381.084 6, found 381.084 1。
2d:1H NMRδ: 5.15(s, 1H), 7.23~7.26(m, 2H), 7.34~7.44(m, 4H), 7.84~7.87(m, 3H), 8.05~8.07(m, 1H);13C NMRδ: 33.5, 56.3, 118.8, 121.3, 125.8, 126.1, 127.8, 128.7, 129.4, 130.5, 130.6, 130.9, 132.0, 134.2, 134.6, 140.9, 149.5, 158.4, 176.8, 182.4; HR-ESI-MS: Calcd for C20H11N2O3ClNa{[M+Na]+} 385.035 0, found 385.034 3。
2h:1H NMRδ: 5.15(s, 1H), 7.15~7.60(m, 6H), 7.82~7.84(m, 3H), 8.03~8.05(m, 1H);13C NMRδ: 35.9, 56.5, 118.7, 121.4, 122.7, 125.8, 126.1, 128.4, 128.9, 130.6, 130.9, 132.6, 124.2, 134.6, 142.8, 149.5, 158.3, 176.8, 182.4; HR-ESI-MS: Calcd for C20H11N2O3BrNa{[M+Na]+} 428.984 5, found 428.984 8。
2k:1H NMRδ: 5.39(s, 1H), 7.45~7.48(m, 3H), 7.58~7.67(m, 2H), 7.82~7.84(m, 3H), 7.91~7.93(m, 1H), 8.03~8.05(m, 1H);13C NMRδ: 31.1, 55.9, 118.6, 121.2, 124.0, 125.8, 126.1, 128.4, 130.6, 130.7, 131.3, 133.8, 134.2, 134.6, 137.8, 148.5, 149.0, 158.9, 176.7, 182.6; HR-ESI-MS: Calcd for C20H11N3O5Na{[M+Na]+} 396.059 1, found 396.059 9。
2m:1H NMRδ: 4.65(s, 1H), 7.02~7.07(m, 1H), 7.16~7.19(m, 2H), 7.32~7.37(m, 3H), 7.81~7.89(m, 3H), 8.03~8.06(m, 1H);13C NMRδ: 36.3, 57.1, 113.8, 114.1, 114.4, 114.7, 119.2, 121.1, 123.9, 125.8, 126.1, 130.4, 130.5, 130.8, 131.1, 134.2, 134.5, 146.5, 146.6, 149.3, 158.4, 160.7, 164.0, 176.8, 182.6; HR-ESI-MS: Calcd for C20H11N2O3FNa{[M+Na]+} 369.064 6, found 369.065 2。
2 结果与讨论
2.1 芳醛底物扩展
反应条件同1.2,扩展芳醛底物(2b~2q),实验结果见表1。由表1可见,芳环上有给电子取代基时,收率较高。芳环上有吸电子取代基时,收率相对较低。底物的空间位阻对反应的影响也很大,位阻越大,收率越低。
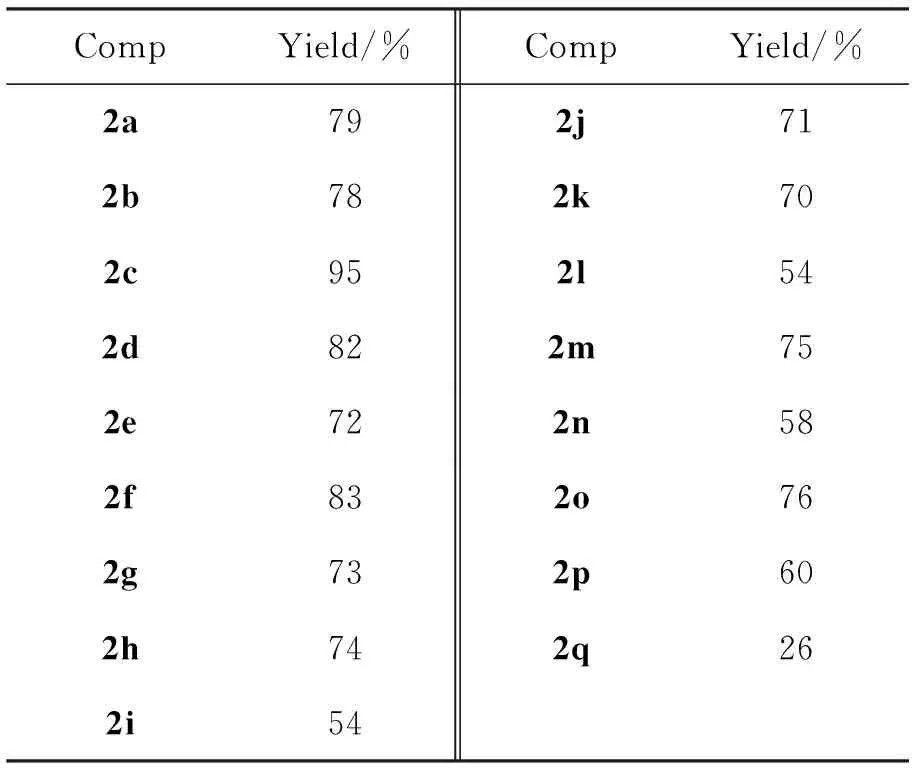
表 1 芳醛底物的扩展*
*反应条件同1.2
Scheme 2
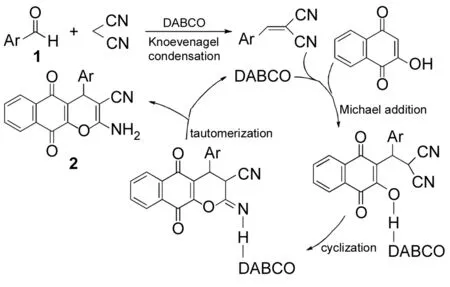
2.2 DABCO的作用及反应机理
DABCO做为碱,催化Knoevenagel缩合和Michael加成反应,经环化异构化得到产物2。DABCO在反应过程中起到重要作用,推测其反应机理如Scheme 2所示。
[1] Brooks G T, Ottridge A P, Jennings R C,etal. The effect of 2,2-dimethylchromene derivatives and some other compounds on the development of oncopeltus fasciatus(dallas) and locusta migratoria migratorioides(R&F)[J].Pestic Sci,1985,6:571-588.
[2] Witte E C, Neubert P, Roesch A. 2H-1-benzopyran-2-on derivatives, process for their preparation and medicines containing these compounds[P].DE 3 427 985,1986.
[3] Hyana T, Saimoto H. Preparation of (furanyl)pentenylchromene derivatives as anticancer agents[P].JP 62 181 276,1987.
[4] Hatakeyama S, Ochi N, Numata H,etal. A new route to substituted 3-methoxycarbonyldihydropyrans;enantioselective synthesis of (-)-methyl elenolate[J].J Chem Soc,Chem Commun,1988:1202-1204.
[5] Ocallaghan C N, Marry T B H. Synthetic reactions of methyl 2-(2-amino-3-methoxycarbonyl-4H-1-benzopyran-4-yl)-2-cyanoethanoate[J].J Chem Res,Synop,1995:214-215.
[6] Wang X S, Shi D Q, Tu S J,etal. Synthesis and crystal structure of 7,7-dimethyl-2-amino-3-cyano-4-(3,4-methylenedioxylphenyl)-5-oxo-5,6,7,8-tetrahydro-4H-benzo-[b]-pyran[J].Chin J Struct Chem,2002,21:146-148.
[7] Sayed A Z, El-Hady N A, El-Agrody A E. Condensation ofα-cyanocinnamonitriles with 6-bromo-2-naphthol: synthesis of pyrano[2,3-d]pyrimidine and pyrano[3,2-e][1,2,4]triazolo[2,3-c]pyrimidine derivatives[J].J Chem Res,Synop,2000:164-166.
[8] 周杰兴,王香善,曾兆森,等. KF-Al2O3催化下二-γ-吡喃并[2,3[a∶2′,3′-f]萘衍生物的合成[J].有机化学,2007,27:786-789.
[9] Chen W B, Liao Y H, Yuan W C,etal. Catalyst-free aldol condensation of ketones and isatins under mild reaction conditions in DMF with molecular sieves 4Å as additive[J].Green Chem,2009,11:1465-1476.
[10] Shaabani A, Ghadari R, Ghasemi S,etal. Novel one-pot three- and pseudo-five-component reactions:Synthesis of functionalized benzo[g]- and dihydropyrano[2,3-g]chromene derivatives[J].J Comb Chem,2009,11:956-959.
[11] Changsheng Y, Chenxia Y, Tuanjiea L,etal. An efficient synthesis of 4H-benzo[g]chromene-5,10-dione derivatives through triethylbenzylammonium chloride catalyzed multicomponent reaction under solvent-free conditions[J].Chin J Chem,2009,27:1989-1994.
[12] Khurana J M, Nand B, Saluja P. DBU:A highly effcient catalyst for one-pot synthesis of substituted 3,4-dihydropyrano[3,2-c]chromenes,dihydropyrano[4,3-b]pyranes,2-amino-4H-benzo[h]chromenes and 2-amino-4H-benzo[g]chromenes in aqueous medium[J].Tetrahedron,2010,66:5637-5641.
