新型并噻吩五元杂环酮类衍生物的合成*
2011-11-27王静,许欢
王 静, 许 欢
(1. 商丘师范学院 化学系,河南 商丘 476000;2. 兰州大学 功能有机分子化学国家重点实验室, 甘肃 兰州 730000)
有机半导体材料(如并五苯)应用在场效应晶体管中表现出很好的性质,甚至可以与非晶硅相媲美[1~3]。然而,并五苯及其衍生物在室温条件下表现出极差的稳定性。通过在分子中引入N, S, O等杂原子来调节分子的HOMO-LUMO能级位置可以提高分子的稳定性[4,5]。例如,在并苯分子中引入噻吩基团,可以增大分子的HOMO-LUMO带隙,稳定HOMO能级[6],从而达到提高稳定性的目的。目前研究人员[4,7,8]设计并合成了既具有高的迁移率,又稳定的新型半导体材料。茚并芴-6,12-二酮衍生物[9~11]由于具有较好的器件性能和使用寿命引起人们的广泛关注。
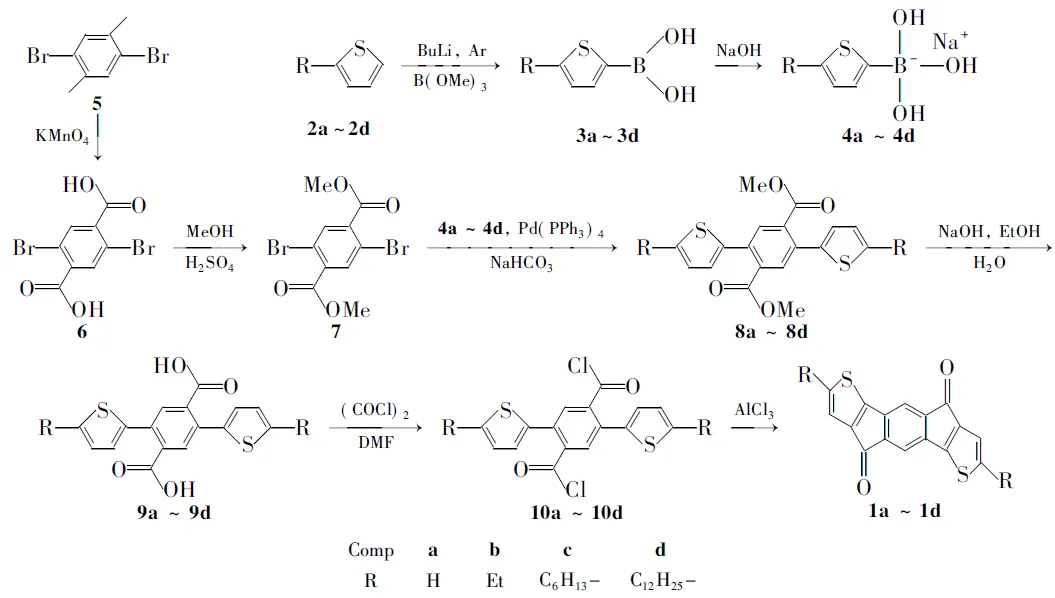
本文以茚并芴-6,12-二酮的结构为基础,设计并合成了一系列新型并噻吩五元杂环酮衍生物(1a~1d, Scheme 1), 其结构经NMR, IR, MS和元素分析表征。
1 实验部分
1.1 仪器与试剂
KER 3100-08S型精密恒温控温台(测熔点);Bruker 400 M型核磁共振仪(CDCl3为溶剂,分别以77.00或39.52定标);Bruker V70型傅立叶红外光谱仪(KBr);傅立叶变换离子回旋共振质谱仪;UBC型元素分析仪。
Scheme1
噻吩(2a), 2-乙基噻吩(2b), 2-正己基噻吩(2c),正丁基锂,Alfa Aesar公司;2,5-二溴对二甲苯(5),百灵威化学试剂公司;溴代正十二烷,硼酸三甲酯,上海国药集团化学试剂有限公司;其余所用试剂均为分析纯;THF,经钠,二甲苯酮处理,使用前现蒸;催化剂(三苯基磷)合钯[Pd(PPh3)4]按文献[12]方法制备。
1.2 合成
(1) 2-十二烷基噻吩(2d)的合成[13]
在三口瓶中加入2a1.05 g(12.50 mmol),抽真空、通氩气,加入THF 20 mL,搅拌下于-78 ℃缓慢滴加2.5 mol·L-1n-BuLi 6 mL(15. 00 mmol),滴毕,反应30 min;缓慢滴加溴代正十二烷3.72 g(15.00 mmol),滴毕,缓慢升至室温,回流反应过夜。倒入水中,分液,水层用乙醚(3×10 mL)萃取,合并有机相,用无水硫酸镁干燥,减压蒸馏,收集140 ℃~142 ℃馏分得无色液体2d1.89 g,产率60%;1H NMRδ: 7.09(dd,J=5.1 Hz, 1.2 Hz, 1H), 6.90(dd,J=5.1 Hz, 3.4 Hz, 1H), 6.77(dd,J=3.4 Hz, 1.1 Hz, 1H), 2.82(t,J=7.7 Hz, 2H), 1.67(q,J=7.7 Hz, 2H), 1.34(m, 18H), 0.89(t,J=6.6 Hz, 3H); ESI-MSm/z: 252(M+)。
(2) 4的合成(以4a为例)
在三口瓶中加入2a1.05 g(12.50 mmol),抽真空、通氩气,加入THF 20 mL,搅拌下于-78 ℃缓慢滴加2.5 mol·L-1n-BuLi 6 mL(15. 00 mmol),反应30 min;缓慢升至室温,反应1.5 h;再冷却至-78 ℃,缓慢滴加硼酸三甲酯2.60 g(25.00 mmol)的THF(15 mL)溶液(-78 ℃),滴毕,缓慢升至室温,反应过夜。加入2 mol·L-1盐酸5 mL,分液,水层用乙醚(3×10 mL)萃取,合并有机相,用无水硫酸镁干燥,减压蒸除溶剂,残余物用热甲苯溶解,搅拌下滴加饱和NaOH溶液至不再出现白色沉淀,于室温搅拌30 min,抽滤,滤饼依次用甲苯和石油醚洗涤,真空干燥得白色固体2-噻吩硼酸钠盐(4a) 1.37 g(直接用于下一步反应)。
用类似方法合成白色固体4b~4d。
4a: 产率65%, m.p.>300 ℃;1H NMR(D2O)δ: 7.32(dd,J=4.8 Hz, 1.2 Hz, 1H), 7.04(dd,J=3.6 Hz, 1.2 Hz, 1H), 7.01(dd,J=4.8 Hz, 3.6 Hz, 1H); IRν: 3 217, 1 454, 1 194, 810, 726, 645, 546 cm-1。
4b: 产率60%, m.p.>300 ℃;1H NMR(D2O)δ: 6.78(d,J=3.6 Hz, 1H), 6.65(d,J=3.6 Hz, 1H), 2.75(q,J=7.6 Hz, 2H), 1.32(t,J=7.6 Hz, 3H); IRν: 3 414, 2 958, 1 443, 1 229, 936, 530 cm-1。
4c: 产率50%, m.p.>300 ℃;1H NMR(D2O)δ: 6.78(d,J=3.6 Hz, 1H), 6.65(d,J=3.6 Hz, 1H), 2.71(t,J=7. 6 Hz, 2H), 1.58~1.63(m, 2H), 1.20~1.40(m, 6H), 0. 84(t,J=6. 8 Hz, 3H); IRν: 3 400, 2 945, 1 450, 1 230, 933, 526 cm-1。
4d: 产率70%, m.p.>300 ℃;1H NMR(D2O)δ: 6.76(d,J=3.6 Hz, 1H), 6.64(d,J=3.6 Hz, 1H), 2.70(t,J=7.6 Hz, 2H), 1.56~1.63(m, 2H), 1.20~1.32(m, 18H), 0.84(t,J=6.8 Hz, 3H); IRν: 3 371, 2 920, 1 465, 1 441, 1 237, 931, 524 cm-1。
(3) 8的合成(以8a为例)
在三口圆底烧瓶中加入4a1.68 g(10.00 mmol), 2,5-二溴对二苯甲酸甲酯(7) 880 mg(2.50 mmol)和NaHCO3840 mg(10.00 mmol)的饱和水溶液,抽真空、通氩气,加入Pd(PPh3)4115 mg(0.10 mmol)和THF 10 mL,搅拌下于70 ℃反应至终点(TLC跟踪)。冷却至室温,倾入饱和NH4Cl溶液中,分液,水层用乙酸乙酯(3×10 mL)萃取,合并有机层,用无水Na2SO4干燥,浓缩至干后经硅胶柱层析[洗脱剂:A=V(石油醚) ∶V(二氯甲烷)=1 ∶1]分离得黄绿色固体2,5-二(2-噻吩基)对二苯甲酸甲酯(8a) 358 mg。
用类似方法合成黄绿色固体8b~8d。
8a: 产率40%, m.p.170 ℃~172 ℃;1H NMRδ: 7.82(s, 2H), 7.39(dd,J=4.8 Hz, 1.2 Hz, 2H), 7.11(dd,J=3.6 Hz, 1.2 Hz, 2H), 7.08(dd,J=4.8 Hz, 3.6 Hz, 2H), 3.78(s, 6H);13C NMRδ: 168.1, 140.3, 133.6, 133.4, 131.9, 127.5, 127.0, 126.7, 52.6; IRν: 3 444, 2 952, 1 729, 1 428, 1 288, 1 247, 1 209, 1 109, 848, 718 cm-1; HR-MSm/z: Calcd for C18H14O4S2{[M+H]+} 359.040 6, found 359.041 1。
8b(洗脱剂:A=4 ∶1): 产率65%, m.p.86 ℃~90 ℃;1H NMRδ: 7.75(s, 2H), 6.91(d,J=3.6 Hz, 2H), 6.75(d,J=3.6 Hz, 2H), 3.80(s, 6H), 2.87(q,J=7.6 Hz, 4H), 1.34(t,J=7.6 Hz, 6H);13C NMRδ: 168.4, 149.1, 137.5, 133.2, 133.1, 131.5, 126.6, 123.9, 52.5, 23.5, 15.8; IRν: 3 440, 2 969, 2 848, 1 727, 1 433, 1 289, 1 238, 1 189, 1 108, 815 cm-1; HR-MSm/z: Calcd for C22H22O4S2{[M+Na]+} 437.085 2, found 437.084 6。
8c(洗脱剂:A=5 ∶1): 产率84%, m.p.88 ℃~90 ℃;1H NMRδ: 7.74(s, 2H), 6.90(d,J=3.6 Hz, 2H), 6.73(d,J=3.6 Hz, 2H), 3.79(s, 6H), 2.82(t,J=7.6 Hz, 4H), 1.66~1.73(m, 4H), 1.30~1.40(m, 12H), 0.90(t,J=6.8 Hz, 6H);13C NMRδ: 168.5, 147.7, 137.6, 133.2, 133.1, 131.4, 126.4, 124.5, 52.6, 31.6(overlapping signal), 30.2, 28.8, 22.6, 14.0; IRν: 3 440, 2 924, 2 852, 1 725, 1 433, 1 290, 1 235, 1 188, 1 110, 805 cm-1; HR-MSm/z: Calcd for C30H38O4S2{[M+H]+} 527.228 4, found 527.227 2。
8d(洗脱剂:A=7 ∶1): 产率88%, m.p.74 ℃~76 ℃;1H NMRδ: 7.74(s, 2H), 6.90(d,J=3.6 Hz, 2H), 6.73(d,J=3.6 Hz, 2H), 3.79(s, 6H), 2.82(t,J=7.6 Hz, 4H), 1.66~1.73 (m, 4H), 1.26~1.38(m, 36H), 0.88(t,J=6.8 Hz, 6H);13C NMRδ: 168.5, 147.7, 137.6, 133.2, 133.1, 131.4, 126.5, 124.6, 52.5, 31.9, 31.6, 30.2, 29.7, 29.6, 29.5, 29.4, 29.3, 29.1(overlapping signal), 22.7, 14.1; IRν: 3 440, 2 918, 2 850, 1 726, 1 434, 1 291, 1 236, 1 190, 1 111, 803 cm-1; HR-MSm/z: Calcd for C42H62O4S2{[M+H]+} 695.416 2, found 695.415 6。
(4)9的合成(以9a为例)
在圆底烧瓶中加入8a483 mg(1.35 mmol), NaOH 216 mg(5.40 mmol),乙醇27 mL和水3 mL,搅拌下回流反应过夜。蒸除约1/2溶剂,加入少量水,分液,水层加盐酸析出沉淀,过滤,滤饼干燥得黄色固体2,5-二(2-噻吩基)对二苯甲酸(9a) 290 mg。
用类似方法合成黄色固体9b~9d。
9a: 产率65%, m.p.308 ℃~310 ℃;1H NMR(DMSO-d6)δ: 13.44(b, 2H), 7.70(s, 2H), 7.67(d,J=4.8 Hz, 2H), 7.25(d,J=3.6 Hz, 2H), 7.15(dd,J=4.8 Hz, 3.6 Hz, 2H);13C NMR(DMSO-d6)δ: 169.7, 139.7, 134.5, 131.5, 130.2, 127.9, 127.5, 127.2; IRν: 2 880, 2 641, 2 530, 1 685, 1 438, 1 405, 1 285, 1 255, 1 211, 1 127, 837, 708 cm-1; HR-MSm/z: Calcd for C16H10O4S2{[M-H]-} 328.994 8, found 328.995 2。
9b: 产率85%, m.p.210 ℃~214 ℃;1H NMR(DMSO-d6)δ: 13.38(b, 2H), 7.6 (s, 2H), 7.06(d,J=3.6 Hz, 2H), 6.87(d,J=3.6 Hz, 2H), 2.85(q,J=7.6 Hz, 4H), 1.27(t,J=7.6 Hz, 6H);13C NMR(DMSO-d6)δ: 168.9, 148.3, 136.9, 134.2, 131.1, 129.7, 126.8, 124.6, 22.8, 15.7; IRν: 2 978, 2 848, 2 655, 2 564, 1 683, 1 423, 1 288, 1 244, 1 209, 1 140, 814 cm-1; HR-MSm/z: Calcd for C20H18O4S2{[M-H]-} 385.057 4, found 385.057 1。
9c: 产率96%, m.p.130 ℃~132 ℃;1H NMR(DMSO-d6)δ: 13.44(b, 2H), 7.61(s, 2H), 7.05(d,J=3.3 Hz, 2H), 6.85(d,J=3.3 Hz, 2H), 2.80(t,J=7.5 Hz, 4H), 1.58~1.65 (m, 4H), 1.20~130 (m, 12H), 0.86(t,J=6.6 Hz, 6H);13C NMR(DMSO-d6)δ: 169.1, 146.9, 137.1, 134.2, 131.2, 129.7, 126.8, 125.4, 31.2, 31.0, 29.4, 28.3, 22.1, 14.0; IRν: 2 923, 2 854, 2 634, 2 558, 1 699, 1 425, 1 289, 1 242, 1 211, 1 125, 803 cm-1; HR-MSm/z: Calcd for C28H34O4S2{[M-H]-} 497.182 6, found 497.181 6。
9d: 产率91%, m.p.318 ℃~320 ℃;1H NMR(DMSO-d6)δ: 13.44(b, 2H), 7.56(s, 2H), 7.04(d,J=3.6 Hz, 2H), 6.81(d,J=3.6 Hz, 2H), 2.78(t,J=7.5 Hz, 4H), 1.60~1.64(m, 4H), 1.15~1.41(m, 36H), 0.84(t,J=6.6 Hz, 6H) ;13C NMR(DMSO-d6)δ: 169.2, 146.5, 137.3, 134.5, 130.9, 129.5, 126.6, 125.2, 31.4, 31.2, 29.4(overlapping signals), 29.1, 29.0(overlapping signal), 28.8, 28.6, 22.2, 14.0; IRν: 2 919, 2 850, 2 639, 2 559, 1 702, 1 405, 1 288, 1 240, 1 209, 1 126, 802 cm-1; HR-MSm/z: Calcd for C40H58O4S2{[M-H]-} 665.370 4, found 665.371 5。
(5)10的合成(以10a为例)
在抽真空、通氩气的三口瓶中加入9a178 mg(0.54 mmol),干燥二氯甲烷30 mL和几滴干燥DMF,搅拌下于室温反应12 h。蒸除溶剂得10a,不经提纯,直接用于下一步反应。
用类似方法合成10b~10d。
(6)1的合成(以1a为例)
在抽真空、通氩气的三口瓶中加入10a198 mg(0.54 mmol),干燥二氯甲烷30 mL,搅拌下于0 ℃加入无水AlCl3216 mg(1.62 mmol)的干燥二氯甲烷(10 mL)悬浮液,反应20 min;于室温反应3 h。倾入盐酸冰水溶液中(出现蓝色沉淀),过滤,滤饼依次用正己烷和乙醇洗涤,干燥得蓝色粉末1a103 mg。
用类似方法合成绿色晶体1b~1d。
1a: 产率65%, m.p.350 ℃~352 ℃; IRν: 3 387, 3 107, 3 072, 1 711, 1 435, 1 320, 778, 463 cm-1; LR-MS(APCI)m/z: Calcd for [M+H]+295, found 295; Anal.calcd for C16H6O2S2: C 65.29, H 2.05, O 10.87, S 21.79; found C 65.35, H 2.08, O 10.80, S 21.77。由于1a的溶解性极差,未能测得NMR数据。
1b(柱层析分离,洗脱剂: A=3 ∶1): 产率70%, m.p.256 ℃~258 ℃;1H NMRδ: 7.11(s, 2H), 6.81(s, 2H), 2.83(q,J=7.6 Hz, 4H), 1.33(t,J=7.6 Hz, 6H);13C NMRδ: 186.5, 156.0, 153.1, 140.8, 140.0, 139.9, 117.5, 114.0, 24.0, 15.6; IRν: 3 396, 2 924, 2 854, 1 708, 1 434, 1 315, 776, 474 cm-1; LR-MS(APCI)m/z: Calcd for [M+H]+351, found 351; Anal.calcd for C20H14O2S2: C 68.54, H 4.03, O 9.13, S 18.30; found C 68.53, H 4.06, O 9.10, S 18.31。
1c(柱层析分离,洗脱剂: A=5 ∶1): 产率70%, m.p.168 ℃~170 ℃;1H NMRδ: 7.12(s, 2H), 6.80(s, 2H), 2.78(t,J=7.6 Hz, 4H), 1.64~1.70(m, 4H), 1.29~1.40(m, 12H), 0.90(t,J=6.8 Hz, 6H);13C NMRδ: 186.5, 156.1, 151.5, 140.8, 139.9, 139.8, 118.2, 114.0, 31.5, 31.3, 30.6, 28.6, 22.5, 14.0; IRν: 3 392, 2 918, 2 848, 1 729, 1 468, 1 321, 773, 480 cm-1; LR-MS(APCI)m/z: Calcd for [M+H]+463, found 463; Anal.calcd for C28H30O2S2: C 72.69, H 6.54, O 6.92, S 13.85; found C 72.75, H 6.57, O 6.91, S 13.77。
1d(柱层析分离,洗脱剂: A=5 ∶1): 产率65%, m.p.146 ℃~148 ℃;1H NMRδ: 7.11(s, 2H), 6.79(s, 2H), 2.78(t,J=7.6 Hz, 4H), 1.66~1.69(m, 4H), 1.19~1.41(m, 36H), 0.88(t,J=6.8 Hz, 6H);13C NMRδ: 186.5, 156.1, 151.6, 140.8, 140.0, 139.9, 118.2, 114.0, 31.9, 31.3, 30.6, 29.6(overlapping signal), 29.5, 29.4, 29.3, 28.9(overlapping signal), 22.7, 14.1; IRν: 3 388, 2 951, 2 850, 1 697, 1 466, 1 317, 774, 483 cm-1; LR-MS(APCI)m/z: Calcd for [M+H]+631, found 631; Anal.calcd for C40H54O2S2: C 76.14, H 8.63, O 5.07, S 10.16; found C 76.23, H 8.55, O 5.12, S 10.10。
2 结果与讨论
参照文献[13,14]方法首先以2a为起始原料合成了2d和4c。以5为原料,经高锰酸钾氧化成酸,再与甲醇发生酯化合成了7[15]。7与4a~4d发生Suzuki偶联反应,高产率地制得8a~8d;8a~8d经皂化反应生成酸(9a~9d); 再与草酰氯反应转化为酰氯(10a~10d); 活泼的酰氯不用分离提纯直接在无水AlCl3催化下发生分子内Friedel-Crafts成环反应得到目标分子1a~1d。1a为蓝色粉末,由于分子中没有助溶基团,溶解性极差,只能溶解在部分极性溶剂中。1b~1d都是具有金属光泽的绿色晶体,具有很好的溶解性,能够溶解在二氯甲烷、氯仿、THF等常用的有机溶剂中。
[1] Klauk H, Halik M, Zshieschang U,etal. High-mobility polymer gate dielectric pentacene thin film transistors[J].J Appl Phys,2002,92:5259-5263.
[2] Podzorov V, Sysoev S E, Loginova E,etal. Single-crystal organic field effect transistors with the hole mobility ~8 cm2/Vs[J].Appl Phys Lett,2003,83:3504-3506.
[3] Sundar V C, Zaumseil J, Podzorov V,etal. Elastomeric transistor stamps:Reversible probing of charge transport in organic crystals[J].Science,2004,303:1644-1646.
[4] Ebata H, Izawa T, Miyazaki E,etal. Highly soluble[1]benzothieno[3,2-b]benzothiophene(BTBT) derivatives for high-performance, solution-processed organic field-effect transistors[J].J Am Chem Soc,2007,129:15732-15733.
[5] Takimiya K, Ebata H, Sakamoto K,etal. 2,7-Diphenyl[1]benzothieno[3,2-b]benzothiophene,a new organic semiconductor for air-stable organic field-effect transistors with mobilities up to 2.0 cm2/Vs[J].J Am Chem Soc,2006,128:12604-12605.
[6] Yamamoto T, Takimiya K. Facile synthesis of highlyπ-extended heteroarenes,dinaphtho[2,3-b∶2′,3′-f]chalcogenopheno[3,2-b]chalcogenophenes,and their application to field-effect transistors[J].J Am Chem Soc,2007,129:2224-2225.
[7] Chen M C, Kim C, Chen S Y,etal. Functionalized anthradithiophenes for organic field-effect transistors[J].J Mater Chem,2008,18:1029-1036.
[8] Gao P, Beckmann D, Tsao H N,etal. Benzo[1,2-b∶4,5-b′]bis[b]benzothiophene as solution processible organic semiconductor for field-effect transistors[J].Chem Commun,2008,13:1548-1550.
[9] Usta H, Facchetti A, Marks T J. Synthesis and characterization of electron-deficient and highly soluble (bis)indenofluorene building blocks forn-type semiconducting polymers[J].Org Lett,2008,10:1385-1388.
[10] Usta H, Facchetti A, Marks T J. Air-stable,solution-processablen-channel and ambipolar semiconductors for thin-film transistors based on the indenofluorenebis(dicyanovinylene) core[J].J Am Chem Soc,2008,130:8580-8581.
[11] Usta H, Risko C, Wang Z,etal. Design,synthesis,and characterization of ladder-type molecules and polymers.Air-stable, solution-processablen-channel and ambipolar semiconductors for thin-film transistors via experiment and theory[J].J Am Chem Soc,2009,131:5586-5608.
[12] Brown H C, Bhat N G, Srebnik M. A simple general synthesis of 1-alkynyldiisop ropoxyboranes[J].Tetrahedron Letters,1988,29(22):2631-2634.
[13] Sung H, Lin H. Novel alternating fluorene-based conjugated polymers containing oxadiazole pendants with various terminal groups[J].Macromolecules,2004,37:7945-7954.
[14] Cammidge A N, Goddard V H M, Gopee H,etal. Aryl trihydroxyborates:Easily isolated discrete species convenient for direct application in coupling reactions[J].Org. Lett,2006,8:4071-4074.
[15] Hou J, Tan Z, Yan Y,etal. Synthesis and photovoltaic properties of two-dimensional conjugated polythiophenes with bi(thienylenevinylene) side chains[J].J Am Chem Soc,2006,128:4911-4916.
