紫珠的松香烷型二萜成分研究
2011-10-09芦毅,华燕
芦 毅 ,华 燕
西南林学院,昆明 650224
紫珠的松香烷型二萜成分研究
芦 毅 ,华 燕*
西南林学院,昆明 650224
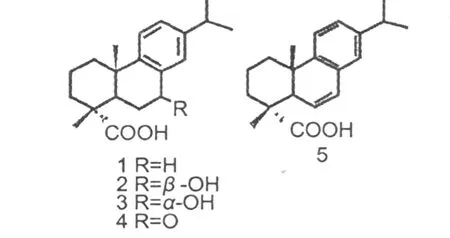
从紫殊全草的乙醇提取物的氯仿部分中分得 5个松香烷型二萜,经光谱数据分别鉴定为脱氢枞酸 (1)、7β-羟基 -脱氢枞酸(2)、7α-羟基 -脱氢枞酸 (3)、7-羰基 -脱氢枞酸 (4)和 18-羧基 -6,8,11,13-松香烷酸 。以上化台物均为首次从该植物中分得。
紫珠;脱氢枞酸;松香烷二萜
Abstract:Five abietane diterpeneswere isolated from CHCl3-solved portion of E tOH extraction fromCallicarpa pedunculataR.Brown.On the basis of spectroscopic analysis,these compoundswere identified as dehydroabietic acid(1),7βhydroxydehydroabietic acid(2),7α-hydroxydehydroabietic acid(3),7-oxodehydroabietic acid(4),and 6,8,11,13-abietatrien-18-oic acid(5).These compoundswere isolated from this plant for the first time.
Key words:Callicarpa pedunculataR.Brown;dehydroabietic acid;abietane diterpene
紫珠 (Callicarpa pedunculataR.Brown)为马鞭草科 (Verbenaceae)紫珠属植物,落叶灌木,高 1~3 m。生于海拔 1590 m以下的山坡林边、溪边,主要分布在我国南部地区,根、茎、叶及种子均可入药,有通经活血之功效[1]。该属植物在中医临床上主要用于各种出血症,有抗菌作用。紫珠首载于《本草拾遗》,别名止血草,性凉、味苦涩、具有收敛止血、清热解毒的功效,可用于各种内外出血症,对肺胃之出血尤为多用,也可治疗烧烫伤及热毒疮癌。该植物在民间主要用于治疗偏头风痛,吐血,跌打肿痛,外伤出血等。经文献查阅,国内外对紫珠属植物化学成分研究的报道较少,报道的化合物主要为黄酮、二萜和三萜类成分[2-9]。我们从中分离并鉴定了 5个化合物,均为首次从该植物中分得。
1 实验部分
1.1 仪器与材料
E IMS和 FAB-MS由 VG Auto Spec-3000质谱仪测定,其中 E IMS在 70eV下测定;1H,13C NMR和2D NMR谱在 Bruker AM-400或 DRX-500核磁共振仪上测定 (T MS为内标);柱层析用硅胶 G(200~300目)或硅胶 H(10~40μm)及薄层层析板均为青岛海洋华工厂产品。薄层层析通过 5%硫酸-乙醇溶液加热观察其斑点。SaphadexLH-20为 Pharmcia公司产品。反向材料 RP-18及 RP-18薄层板为Merck公司产品。所用试剂均为工业重蒸。紫珠全草采集于贵州遵义地区,经西南林学院杜凡教授鉴定,凭证标本存放于西南林学院植物标本室。
1.2 提取与分离
紫珠粗粉 10 kg,用 95%乙醇加热回流提取三次 (每次为 8 h),合并提取液,减压回收溶剂得浸膏500 g。而后将浸膏用氯仿萃取,减压回收氯仿得氯仿萃取物。取氯仿萃取物 500 g硅胶拌样进行硅胶中压柱层析 (1.5 kg硅胶 H填柱),分别用石油醚/乙酸乙酯 (1∶0,10∶1,5∶1,4∶1,3∶1,1∶1,0∶1),得到7个部分,Fr.3反复硅胶柱层析,石油醚 /乙酸乙酯(15∶1)洗脱得化合物 1(110 mg)、5(15 mg),Fr.4反复硅胶柱层析,石油醚 /乙酸乙酯 (10∶1)洗脱得化合物 4(11 mg)。Fr.5反复硅胶柱层析,石油醚 /乙酸乙酯 (5∶1)洗脱得化合物 2(6 mg)、3(25 mg)。
2 结构鉴定
脱氢枞酸 (dehydroabietic acid,1) 无色针晶(石油醚 /乙酸乙酯);C20H28O2;E IMSm/z(%):300[M]+(47),285(97),267(4),239(100),225(9),197(49),159(13),141(40),129(17),117(14),105(4),91(8),81(4),77(3),55(2)。1H NMR(CDCl3,400 MHz)δ:1.22(6H,d,J=6.68 Hz,CH3-16,CH3-17),1.25(3H,s,CH3-20),1.28(3H,s,CH3-19),2.82(1H,m,H-15),6.88(1H,brs,H-14),7.00(1H,d,J=8.08 Hz,H-12),7.16(1H,d,J=8.08 Hz,H-11)。13C NMR(CDCl3,100.6 MHz)δ:37.9(C-1,t),18.5(C-2,t),36.7(C-3,t),47.4(C-4,s),44.6(C-5,d),21.7(C-6,t),30.0(C-7,t),134.7(C-8,s),146.7(C-9,s),36.6(C-10,s),124.1(C-11,d),123.9(C-12,d),145.7(C-13,s),126.9(C-14,d),33.5(C-15,d),24.0(C-16,C-17,q),184.1(C-18,s),16.3(C-19,q),25.1(C-20,q)。1H,13C NMR数据与文献报道[10]一致。
7β-羟 基 -脱 氢 枞 酸(7β-hydroxydehydroabietic acid,2) 无色针晶 (石油醚 /乙酸乙酯);C20H28O3;E IMSm/z(%):316[M]+(42),298[M-H2O]+(39),283(22),273(22),237(100),197(33),195(89),162(58),141(20),115(10),105(7),77(4),55(5)。1H NMR(CDCl3,400MHz)δ:1.15(3H,s,H-20),1.28(6H,d,J=6.9 Hz,H-16/17),1.54(3H,s,H-19),2.47(1H,dd,J=2.7,12.9,H-5),2.88(1H,q,J=6.8 Hz,H-15),4.89(1H,dd,J=1.96,7.45 Hz,H-7),7.09(1H,brs,H-12),7.20(1H,brs,H-11),7.36(1H,brs,H-14)。13C NMR(CDCl3,100.6 MHz)δ:38.0(C-1,t),18.5(C-2,t),36.3(C-3,),47.1(C-4,s),43.4(C-5,d),32.8(C-6,t),70.7(C-7,d),137.5(C-8,s),146.6(C-9,s),37.5(C-10,s),124.1(C-11,d),125.2(C-12,d),147.8(C-13,s),125.8(C-14,d),33.7(C-15,d),23.8(C-16,q),24.0(C-17,q),183.7(C-18,s),16.2(C-19,q),25.4(C-20,q)。1H,13C NMR数据与文献报道[11]一致。
7α-羟 基 -脱 氢 枞 酸 (7α-hydroxydehydroabietic acid,3) 无色针晶 (石油醚 /乙酸乙酯);C20H28O3;E IMSm/z(%):316[M]+(42),298[M-H2O]+(39),283(22),273(22),237(100),197(33),195(89),162(58),141(20),115(10),105(7),77(4),55(5)。1H NMR(CDCl3,500 MHz)δ:1.15(3H,s,H-20),1.24(6H,d,J=6.9 Hz,H-16/17),1.25(3H,s,H-19),2.47(1H,dd,J=2.7,12.9,H-5),2.87(1H,q,J=6.8 Hz,H-15),4.81(1H,brs,H-7),7.11(1H,brs,H-12),7.13(1H,brs,H-11),7.19(1H,brs,H-14)。13C NMR(CDCl3,125.8 MHz)δ:37.7(C-1,t),18.5(C-2,t),36.0(C-3,t),47.0(C-4,s),39.7(C-5,d),30.7(C-6,t),68.2(C-7,d),135.4(C-8,s),146.5(C-9,s),37.3(C-10,s),124.1(C-11,d),126.6(C-12,d),147.7(C-13,s),128.2(C-14,d),33.5(C-15,d),23.8(C-16,q),24.0(C-17,q),182.4(C-18,s),16.2(C-19,q),24.1(C-20,q)。1H,13C NMR数据与文献报道[11]一致。
7-羰基-脱氢枞酸 (7-oxodehydroabietic acid,4)
无色油状物;C20H26O3;E IMSm/z(%):314[M]+(20),300(18),285(15),267(25),253(100),245(38),225(13),211(29),199(17),187(50),147(48),107(31),91(34),81(44),77(22),55(33)。1H NMR(CDCl3,400MHz)δ:0.92(6H,d,J=6.16 Hz,Me-16,Me-17),0.96(3H,s,Me-20),1.01(3H,s,Me-19),2.63(1H,m,6α-H),2.67(1H,m,6β-H),7.20(1H,d,J=8.18 Hz,H-11),7.32(1H,dd,J=1.83,8.18 Hz,H-12),7.78(1H,d,J=1.83 Hz,H-14)。13C NMR(CDCl3,100.6MHz)δ:37.3(C-1,t),17.9(C-2,t),37.5(C-3,t),46.1(C-4,s),43.3(C-5,d),36.3(C-6,t),198.7(C-7,s),130.3(C-8,s),152.8(C-9,s),37.0(C-10,s),123.5(C-11,d),132.5(C-12,d),146.6(C-13,s),124.8(C-14,d),33.3(C-15,d),23.7(C-16,C-17,q),182.4(C-18,s),16.1(C-19,q),23.4(C-20,q)。1H,13C NMR数据与文献报道[11]一致。
18-羧基-6,8,11,13-松香烷酸 (6,8,11,13-abi
etatrien-18-oic acid,5) 无色油状物;C20H26O2;1H NMR(CDCl3,400 MHz)δ:1.16(3H,s,H-20),1.31(3H,d,J=10.5 Hz,H-16,H-17),1.48(3H,s,H-19),5.87(1H,dd,J=1.84,7.63 Hz,H-6),5.60(1H,dd,J=1.84,7.63 Hz,H-7),7.00(1H,brs,H-14),7.13(1H,m,H-12),7.16(1H,m,H-11)。13CNMR(CDCl3,100.6 MHz)δ:35.7(C-1,t),18.3(C-2,t),35.3(C-3,t),46.1(C-4,s),46.5(C-5,d),129.7(C-6,d),125.8(C-7,d),132.5(C-8,s),146.4(C-9,s),124.7(C-11,d),121.6(C-12,d),145.0(C-13,s),128.4(C-15,d),33.6(C-15,d),24.0(C-16,C-17,q),184.2(C-18,s),17.6(C-19,q),20.8(C-20,q)。1H,13C NMR数据与文献报道[12]一致。
1 Jang Su new medical college(江苏新医学院),Dictionary of Traditional Chinese Medicine(中药大辞典),Shanghai Scientific and Technical Publishers(上海科学技术出版社),1990,p2346-2348.
2 Kawazu K,Inaba M,Mitsui T.Studies on fish-killing compounds ofCallicarpa candicaus.Agric B iol Chem,1967,31,498-506.
3 Kawazu K,Nishino C,Mccridle R,et al.The stereochemistry ofmaingayic acid.Agric B iol Chem,1972,36,1245-1246.
4 Xu J,Harrison JL,Vittal JJ,et al.Four new clerodane diterpenoids fromCallicarpa pentandra.J Nat Prod.2000,63,1062-1065.
5 Hosozawa S,Kato N,Munakata K.5,6,7-Trimethoxy flavone fromCallicarpa japonica.Phytochem istry,1972,11,2362-2362.
6 Hosozawa S,Kato N,Munakata K.Antifeeding active substances for insect in plant.Agric B iol Chem.1974,38,1045-1058.
7 Ahmad SA,Zaman A.Diterpenoid constituents ofCalliparpa macrophyllaVahl.Tetrahedron Lett.,1973,3,2179-2182.
8 Hu YY(胡益明),Gu QX(顾琼仙),Studies on Constituents ofCallicarpa pedunculataR.Brown,Chin Tedit Herb D rugs(中草药),2001,32:1063-1065.
9 Liu HY,He HP,Gao S,et al,Two New Diterpenoids from Callicarpa pedunculata.Helv Chim Acta.2006,89:1017-1022.
10 Ulubelen A,MiskiM.A new diterpene acid fromSalvia tomentosa.J Nat Prod,1981,44,119-124.
11 Cheung HTA,Miyase T,Lenguyen PM,et al.Further acidic constituents and neutral compounds ofPinus massoniana Resin.Tetrahedron,1993,49,7903-7915.
12 Marchand-Geneste N,CarpyA.Theoretical study of the thermal degradation pathways of abietane skeleton diterpenoids:aromatization to retene.J Mol Strut(Theochem).2003,635,55-82.
Abietane diterpenes from Callicarpa pedunculata
LU Yi,HUA Yan*
Southwest Forestry University,Kunm ing 650224,China
Q946.91;R284.2
A
1001-6880(2011)01-0066-03
2008-12-15 接受日期:2009-03-20
*通讯作者 Tel:86-871-3863840;E-mail:huayan@mail.kib.ac.cn
