血霉酚酸水平监测在肾移植临床的应用现状及进展
2011-09-19张桓熙刘岩峰王长希
张桓熙,刘岩峰,王长希
(中山大学附属第一医院器官移植中心,广东广州510080)
作为抗代谢免疫抑制剂,霉酚酸(Mycophenolic Acid,MPA)以其低毒副作用的优点已经在实体器官移植中得到广泛应用[1,2]。目前,常规的免疫抑制方案组成包括:皮质类固醇,钙调神经磷酸酶抑制剂(Calcineurin inhibitors,CNIs)和 MPA。但前两者的长期使用会导致较多甚至严重的副作用[3]。因此,近年来,以MPA为主的无肾毒性的免疫抑制方案日益受到重视,特别在移植稳定期采用低剂量或者撤除CNIs或皮质类固醇的方案中,MPA更是起着核心作用。目前临床应用的霉酚酸药物主要有霉酚酸酯(Mycophenolatemofetil,MMF,骁悉,Cellcept)和霉酚酸钠肠溶片(Enteric-coated mycophenolate sodium,EC-MPS,米芙,Myfortic)。研究表明,MPA的代谢存在明显的个体内和个体间差异并受诸多因素影响,如:移植肾功能、白蛋白水平、钙调磷酸酶抑制剂的配伍等[4,5]。监测MPA血药浓度,可使临床医生针对不同情况的患者均能取得最好疗效并且减少不良反应的发生。本综述通过总结近几年霉酚酸水平监测的研究进展,为进一步合理使用MPA并探究如何优化国人抗排斥方案提供线索。
1 MPA 检测方法
MPA的血浓度曲线下面积(area under the plasma concentration time curve,AUC)是监测MPA暴露的最重要的动力学参数。目前,MPA水平测定方法(见表1)主要有:高效液相色谱法(High-performance liquid chromatography,HPLC)和酶免疫分析法(Enzyme immunoassay,EIA)。前者可根据检测器的不同,分为紫外检测法[6~11]、荧光检测法[12~14]、联用串联质谱法[15~19];后者可分为酶放大免疫分析法(Enzyme multiplied immunoassay technique,EMIT)[12,20~22]、克隆酶供体免疫分析法(Cloned enzyme donor immunoassay,CEDIA)[23,24]、基于 IMPDH的酶抑制分析法(IMPDH-based enzyme inhibition assay)[25,26]和最新研究但还没进入市场的颗粒增强比浊抑制免疫分析法(Particle-enhanced turbidimetric inhibition immunoassay,PETINIA)[27]。
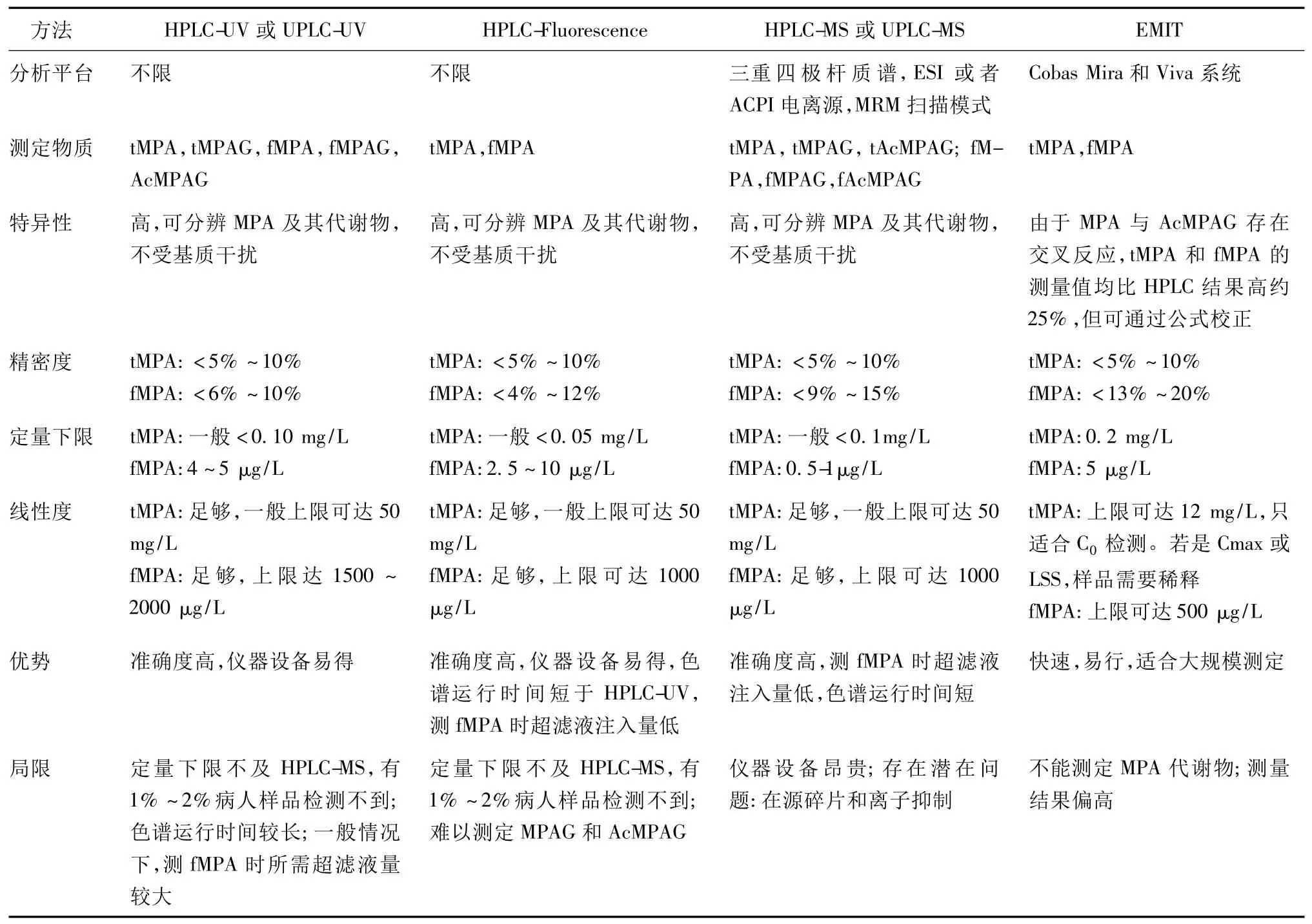
表1 MPA检测方法归纳
MPA游离浓度测定方法,包括上面提到的HPLC(紫外[6~10]、荧光[12~14]、串联质谱[15~19])和 EMIT[12,20]。其优缺点及各参数见表 1。测定血游离MPA(free MPA,fMPA)时,样品需要经过前处理,即把游离的以及与血浆蛋白结合的MPA分离,常用的方法是超滤法[8,28]。此外还有平衡透析[28]和超高速离心法[29],但由于操作繁琐,分离效果不理想,已极少使用。检测方法的选用,主要取决于实验室或医院的仪器设备和实验目的。酶免疫分析法由于快速易行和标准化的需要,多应用于临床监测,而高效液相色谱法多用于研究。近几年的大规模临床试验,两者均有使用[30~32]。
2 治疗药物检测
2.1 药代动力学 MPA药代动力学过程已十分明确,简述如下。MMF口服后迅速在胃肠吸收,并通过肠壁、肝脏和其他组织的酯酶水解为MPA,达峰时间为1h,其中97-99%与血浆白蛋白结合。MPA在肝脏中通过二磷酸尿苷葡萄糖醛酸转移酶(Uri-dine diphosphate gluconosyltransferases,UGTs)代谢为无药理活性的7-O-葡萄糖苷酸霉酚酸(7-O-MPA-glucuronide,MPAG),及少量的酰基葡萄糖苷酸(Acyl glucuronide,AcMPAG),苯基葡萄糖苷(Phenolic glucoside,MPAG1s)[33]和 6-O-去甲基霉酚酸(6-ODM-MPA)。MPAG主要通过肾小管分泌经尿液排出。部分通过肝脏分泌入胆汁,在肠道细菌葡萄糖苷酶的作用下去糖脂化,生成MPA,在结肠被重吸收入血,此为肠肝循环[34]。体外实验发现,游离MPA发挥药物活性,能抑制次黄嘌呤核苷脱氢酶(IMPDH),阻断T和B淋巴细胞增殖过程中鸟嘌呤核苷酸的从头合成步骤,妨碍其进行有丝分裂[34]。EC-MPS药动学与MMF存在差异,口服后经小肠吸收,达峰时间为 1.5 ~2.75 h[5]。
MPA的代谢存在明显的个体内和个体间差异并受诸多因素影响。在服用固定剂量MMF的肾移植患者中,发现MPA-AUC可达10倍的个体间差异,而对同一个体,在移植后几周内tMPA AUC值至少比之后(移植后1-6个月内)的值低30-50%,MPA游离分数也存在2-5倍的差异[35]。造成如此差异的因素可归纳如下:①种族:美裔非洲人的MPA药动学参数与白种人没有统计学意义上的差异[36],而在相同剂量下,中国肾移植患者的tMPA AUC值比前述两种人高[37,38]。②性别:据骁悉 药物说明书,合并几项研究数据发现,男(n=79)女(n=41)性间tMPA AUC0-12没有统计学意义上的差异[4]。③血浆白蛋白浓度:血浆白蛋白浓度降低,导致MPA和MPAG可结合位点减少,游离MPA增多,其排出也增加,最终导致 tMPA AUC减少。[39]白蛋白浓度低于31 g/L,游离MPA分数大大增加[40]。④移植肾功能:当患者GFR <25 mL/min时,血浆MPAG浓度增加3~6倍[41]。肾功能受损导致的 MPAG增多,酸中毒或尿毒症,都会减少MPA与白蛋白的结合,导致游离MPA增多,MPA糖脂化和排出相应增加,结果是tMPA AUC增加,而fMPA AUC基本不变或增加[42]。⑤进食:据吗替麦考酚酯胶囊药物说明书,若患者服用1.5 bid MPA,则进食(27 g脂肪,650卡)不会影响 MPA AUC,但会减少约 40%的Cmax[4]。⑥药物配伍:相比起他克莫司或西罗莫司,配伍环孢霉素会降低20% ~50%的 tMPA AUC[43]。类固醇的使用也可能降低 tMPA AUC[44]。
2.2 MPA暴露量与疗效、不良反应的关系 进行治疗药物监测之前,必须明确MPA暴露量与疗效、不良反应的关系,确定合适的MPA目标值。衡量MPA暴露量常用以下指标:tMPA(或fMPA)的AUC或谷浓度C0。MPA AUC的计算方法现有两种:一是有限取样法(Limited sampling strategy,LSS)近似计算(拟合方法为多重线性回归(MLR)[45]或贝叶斯估计[46,47]),二是全取样法。全点 AUC 与临床结果的相关性最强,但其测定需要患者在服药后12小时内住院,且需10个以上血液样本,在临床中难以实施。而只需单个样品测定的谷浓度与临床结果相关性不强。因此,通过有限取样法估计全点AUC逐渐成为监测的必要手段。2008年,Miura等建立了适合日本肾移植患者的MLR拟合模型,在服药后第2、4、9 h 取样,近似 AUC0-12 最接近全点 AUC[45]。2004年Le Guellec等和2005年Premaud等均建立了贝叶斯估计模型,只需在第20 min、1 h和3 h取样[46,47]。两种LSS比较,贝叶斯估计需要复杂的数学运算,但可弹性选择取样时间点,并对其偏离进行校正;两者均需预先对特定的患者群体建立回归方程。研究表明,低MPA AUC或谷浓度值会增加急性排斥的风险,高MPA AUC或谷浓度值会增加发生不良反应的风险[34]。有文献指出,监测时应控制MPA AUC0-12在 30 ~60 mg/(h·L)[48]。但 2008 年Kuypers等研究表明,控制患者MPA AUC不超过60 mg/(h·L)只有助于减少白细胞减少症和贫血现象的发生,但不会减少肠胃不适和感染的发生[49]。
血游离MPA暴露量与疗效的关系在肾移植患者中没有得到充分的研究,但对预测发生不良反应的风险比tMPA 更有意义[50~53]。1998年 Kaplan等报道,在肾功能损伤的胰腺移植患者中,出现白细胞减少的患者具有较高MPA游离分数和fMPA AUC值[50]。2002年Weber等报道,高fMPA AUC值会增加发生白细胞减少症和感染的风险[51]。2004年,Mudge等报道,在tMPA AUC较低的情况下,患者仍出现胃肠不适或血细胞减少等不良反应,经检测,这些患者的fMPA AUC值和MPA游离分数较高[52]。2005年,Atcheson等报道,出现血小板较少、白细胞减少或者感染的患者,fMPA AUC值会偏高[53]。后面三份报告均为肾移植。然而至今没有文献指出监测fMPA的目标值,其原因可能是检测技术不足和成本高。
2.3 治疗药物监测的现状 从2007年起,有三篇大规模临床试验报告和一份系统评价讨论MPA监测的意义。2007年Le Meur等报道,APOMYGRE试验比较了固定剂量(FD)方案(n=70)和MPA总浓度监控(CC)方案(n=67)的疗效,其中,FD组给MMF 2g/d,CC组控制MPA AUC0-12值达到40 mg/(h·L),通过 LSS和贝叶斯估计计算 MPA AUC值。结果是,采用CC组患者急性排斥率较低。CC组患者在第14天,第1月,第3月调整剂量均高于2 g/d[30]。
2008年,van Gelder等报道,FDCC试验比较了固定剂量方案(n=452)和MPA总浓度监控方案(n=449)的疗效,FD组每人2 g/d MMF(儿童每人每天1.2 g/m2),CC组控制MPA AUC0~12值范围30~60 mg/(h·L),通过有限取样法和多重线性回归近似计算AUC0-12。结果是,两方案活检证实急排发生率相当[31]。但本试验实际操作上存在不完善之处,使其结果说服力下降。该文献承认,若剂量调整准确,MPA监测会有积极意义[31]。
2010年Premaud分析了APOMYGRE证明监测有效而FDCC得出相反结论的原因。第一,APOMYGRE中72-85%病例严格按照剂量调整指南执行,而FDCC只有48%。第二,APOMYGRE采用贝叶斯估计的LSS,取样时间窗口更宽,估计AUC0-12更加接近全点AUC,而FDCC采用MLR LSS,取样时间窗口窄(±5 min),取样时间点偏差会导致AUC0-12估计不准确,且在低范围值时估计值往往会偏高[54]。
2009年,Gaston等报道,Opticept试验比较了固定剂量方案和MPA总浓度监控方案的疗效(n=565),并且研究监控方案能否有助于减少CNIs的使用。FD组为每人2 g/d(儿童每人每天1.2 mg/m2)MMF,CC组为控制MPA谷浓度>1.3 mg/L(环孢霉素)或者>1.9 mg/L(他克莫司)。结果是,至第12个月实验结束为止,各组活检证实急排发生率相当。此研究证明了,MPA总浓度监控方案联合减低剂量的CN I抗排斥效果不会差于其他方案,且可能有助于提高移植物远期效果,但监控方案没有明显优势[32]。MPA谷浓度与临床结果相关性不强,作为监测指标效果不佳。
2008年,一篇系统评价归纳了2007年及之前有关实体器官移植的文献,得出结论:MPA监测不能使患者受益。但其结论需辩证看待。首先,大部分研究并非完全随机和盲性设计;此外,相当一部分研究没有控制干扰因素,如配伍的药物[55]。
3 发展方向
尽管欧美国家对于MPA药物及其监测已有较深入的研究,但其研究结论尚不统一,其临床研究数据及经验也不能简单照搬到中国。例如,临床应用发现,若按照欧美剂量,国人会出现较多不良反应。然而我们可以借鉴国外的监测经验及机理,观察中国不同人群、不同情况下的MPA药代动力学特点,建立以MPA为基础的激素及CNIs组成的免疫抑制维持方案,了解能否在减少药物不良反应而又不增加排斥反应、肾炎复发及尿蛋白发生率的情况下,提高肾移植长期存活率的目的。另外,由于EC-MPS与MMF在人体内的代谢过程不同,且可用于监测MMF的有限取样法不能用于EC-MPS的监测[56],因此对于EC-MPS的用药研究。因此,需要建立其他的方法去监测服用EC-MPS患者的MPA暴露量,从而做到个体化用药。
[1]Shaw LM.Using established immunosuppressant therapy effectively:lessons from the measurement ofmycophenolic acid plasma concentrations[J].Ther Drug Monit,2004,26(4):347-351.
[2]Land W,Vincenti F.Toxicity-sparing protocols using mycophenolate mofetil in renal transplantation[J].Transplantation,2005,80(2 Suppl):S221-234.
[3] Liptak P,Ivanyi B.Primer:Histopathology of calcineurin-inhibitor toxicity in renal allografts[J].Nat Clin Pract Nephrol,2006,2(7):398-404.
[4] Hoffman-La Roche Ltd.CellCept Prescribing information.2010-02-23.http://www.gene.com/gene/products/information/cellcept/pdf/pi.pdf Accessed.
[5]Novartis Pharmaceuticals Corporation,Myfortic prescribing information.2009-10-07.http://www.pharma.us.novartis.com/product/pi/pdf/myfortic.pdf Accessed.
[6]Zeng L.HPLC-UV assay formonitoring total and unbound mycophenolic acid concentrations in children[J].Biomed Chromatogr,2009,23(1):92-100.
[7] Yau WP.Simple reversed-phase liquid chromatographic assay for simultaneous quantification of free mycophenolic acid and its glucuronide metabolite in human plasma[J].JChromatogr B Analyt Technol Biomed Life Sci,,2007,846(1-2):313-318.
[8] Cussonneau X.Relationship between MPA free fraction and free MPAG concentrations in heart transplant recipients based on simultaneous HPLC quantification of the target compounds in human plasma[J].JChromatogr B Analyt Technol Biomed Life Sci,2007,852(1-2):674-678.
[9]Aresta A.Simultaneous determination of freemycophenolic acid and its glucuronide in serum of patients under mycophenolate mophetil therapy by ion-pair reversed-phase liquid chromatography with diode array UV detection[J].JChromatogr B Analyt Technol Biomed Life Sci,2004,810(2):197-202.
[10]Mandla R.Automated determination of free mycophenolic acid and its glucuronide in plasma from renal allograft recipients[J].Ther Drug Monit,2003,25(3):407-414.
[11]Shipkova M.Simultaneous determination of mycophenolic acid and its glucuronide in human plasma using a simple high-performance liquid chromatography procedure[J].Clin Chem,1998,44(7):1481-1488.
[12]Chen B.Establishment of high-performance liquid chromatography and enzymemultiplied immunoassay technology Methods for determination of freemycophenolic acid and its application in Chinese liver transplant recipients[J].Ther Drug Monit,2010,32(5):653-660.
[13]Jiao Z.Totaland freemycophenolic acid and its7-O-glucuronidemetabolite in Chinese adult renal transplant patients:pharmacokinetics and application of limited sampling strategies[J].Eur JClin Pharmacol,2007,63(1):27-37.
[14]Shen J.Quantification of total and freemycophenolic acid in human plasma by liquid chromatography with fluorescence detection[J].J Chromatogr B Analyt Technol Biomed Life Sci,2005,817(2):207-213.
[15]FigurskiMJ.High-performance liquid chromatography-mass spectroscopy/mass spectroscopymethod for simultaneous quantification of total or free fraction ofmycophenolic acid and its glucuronidemetabolites[J].Ther Drug Monit,2009,31(6):717-726.
[16]Shen B.Determination of total,free and saliva mycophenolic acid with a LC-MS/MSmethod:application to pharmacokinetic study in healthy volunteers and renal transplant patients[J].JPharm Biomed Anal,2009,50(3):515-521.
[17]Atcheson B.Quantification of freemycophenolic acid and its glucuronidemetabolite in human plasma by liquid-chromatography using mass spectrometric and ultraviolet absorbance detection[J].JChromatogr B Analyt Technol Biomed Life Sci,2004,799(1):157-163.
[18]Streit F.Validation of a rapid and sensitive liquid chromatographytandem mass spectrometry method for free and total mycophenolic acid[J].Clin Chem,2004,50(1):152-159.
[19]Willis C.Quantification of free mycophenolic acid by high-performance liquid chromatography-atmospheric pressure chemical ionisation tandem mass spectrometry[J].J Chromatogr B Biomed Sci Appl,2000,748(1):151-156.
[20]Rebollo N.Modification of the EMIT immunoassay for the measurement of unbound mycophenolic acid in plasma[J].Clin Biochem,2011,44(2-3):260-263.
[21]Premaud A.Determination ofmycophenolic acid plasma levels in renal transplant recipients co-administered sirolimus:comparison of an enzymemultiplied immunoassay technique(EMIT)and liquid chromatography-tandem mass spectrometry[J].Ther Drug Monit,2006,28(2):274-277.
[22]Beal JL.Evaluation of an immunoassay(EMIT)for mycophenolic acid in plasma from renal transplant recipients compared with a highperformance liquid chromatography assay[J].Ther Drug Monit,1998,20(6):685-690.
[23]Shipkova M.Investigation of the crossreactivity ofmycophenolic acid glucuronidemetabolites and of mycophenolate mofetil in the Cedia MPA assay[J].Ther Drug Monit,2010,32(1):79-85.
[24]Westley IS,Ray JE,Morris RG.CEDIA mycophenolic acid assay compared with HPLC-UV in specimens from transplant recipients[J].Ther Drug Monit,2006,28(5):632-636.
[25]van Gelder T.Clinical utility of a new enzymatic assay for determination ofmycophenolic acid in comparison with an optimized LC-MS/MSmethod[J].Ther Drug Monit,2009,31(2):218-223.
[26]Brandhorst G.Multicenter evaluation of a new inosine monophosphate dehydrogenase inhibition assay for quantification of totalmycophenolic acid in plasma[J].Ther Drug Monit,2008,30(4):428-433.
[27]Goss SP.Performance of themycophenolic acid method on the Dade Behring Dimension(R)clinical chemistry system[J].CLINICAL CHEMISTRY,2006,52S(6):A63-A63.
[28]Nowak I,Shaw LM.Mycophenolic acid binding to human serum albumin:characterization and relation to pharmacodynamics[J].Clin Chem,1995,41(7):1011-1017.
[29]Legg B,Rowland M.Cyclosporin:measurement of fraction unbound in plasma[J].JPharm Pharmacol,1987,39(8):599-603.
[30]Le Meur Y.Individualized mycophenolate mofetil dosing based on drug exposure significantly improves patient outcomes after renal transplantation[J].Am JTransplant,2007,7(11):2496-2503.
[31]van Gelder T.Comparingmycophenolatemofetil regimens for de novo renal transplant recipients:the fixed-dose concentration-controlled trial[J].Transplantation,2008,86(8):1043-1051.
[32]Gaston RS.Fixed-or controlled-dose mycophenolate mofetil with standard-or reduced-dose calcineurin inhibitors:the Opticept trial[J].Am JTransplant,2009,9(7):p.1607-1619.
[33]Shipkova M.Glucuronide and glucoside conjugation ofmycophenolic acid by human liver,kidney and intestinal microsomes[J].Br J Pharmacol,2001,132(5):1027-1034.
[34]Staatz CE,Tett SE.Clinical pharmacokineticsand pharmacodynamics ofmycophenolate in solid organ transplant recipients[J].Clin Pharmacokinet,2007,46(1):13-58.
[35]Shaw LM.Mycophenolic acid pharmacodynamics and pharmacokinetics provide a basis for rationalmonitoring strategies[J].Am JTransplant,2003,3(5):534-542.
[36]Pescovitz MD.Equivalent pharmacokinetics ofmycophenolatemofetil in African-American and Caucasianmale and female stable renal allograft recipients[J].Am JTransplant,2003,3(12):1581-1586.
[37]Zicheng Y.Investigation on pharmacokinetics ofmycophenolic acid in Chinese adult renal transplant patients[J].Br JClin Pharmacol,2006,62(4):446-452.
[38]Zhou PJ.Pharmacokinetics of mycophenolic acid and estimation of exposure usingmultiple linear regression equations in Chinese renal allograft recipients[J].Clin Pharmacokinet,2007,46(5):389-401.
[39]deWinter BC.Pharmacokinetic role of protein binding ofmycophenolic acid and its glucuronidemetabolite in renal transplant recipients[J].JPharmacokinet Pharmacodyn,2009,36(6):541-564.
[40]Atcheson BA.Freemycophenolic acid should bemonitored in renal transplant recipients with hypoalbuminemia[J].Ther Drug Monit,2004,26(3):284-286.
[41]Johnson HJ.The pharmacokinetics of a single oral dose ofmycophenolatemofetil in patientswith varying degrees of renal function[J].Clin Pharmacol Ther,1998,63(5):512-518.
[42]van Hest RM.Explaining variability in mycophenolic acid exposure to optimizemycophenolatemofetil dosing:a population pharmacokinetic meta-analysis ofmycophenolic acid in renal transplant recipients[J].JAm Soc Nephrol,2006,17(3):871-80.
[43]Grinyo JM.The pharmacokinetics ofmycophenolate mofetil in renal transplant recipients receiving standard-dose or low-dose cyclosporine,low-dose tacrolimus or low-dose sirolimus:the Symphony pharmacokinetic substudy[J].Nephrol Dial Transplant,2009,24(7):2269-2276.
[44]Cattaneo D.Glucocorticoids interfere withmycophenolatemofetil bioavailability in kidney transplantation[J].Kidney Int,2002,62(3):1060-1067.
[45]Miura M.Limited sampling strategy for simultaneousestimation of the area under the concentration-time curve of tacrolimus and mycophenolic acid in adult renal transplant recipients[J].Ther Drug Monit,2008,30(1):52-59.
[46]Le Guellec C.Population pharmacokinetics and Bayesian estimation ofmycophenolic acid concentrations in stable renal transplant patients[J].Clin Pharmacokinet,2004,43(4):253-266.
[47]Premaud A.Maximum a posteriori bayesian estimation ofmycophenolic acid pharmacokinetics in renal transplant recipients at different postgrafting periods[J].Ther Drug Monit,2005,27(3):354-361.
[48]van Gelder T.Therapeutic drugmonitoring ofmycophenolatemofetil in transplantation[J].Ther Drug Monit,2006,28(2):145-154.
[49]Kuypers DR.Current target ranges of mycophenolic acid exposure and drug-related adverse events:a 5-year,open-label,prospective,clinical follow-up study in renal allograft recipients[J].Clin Ther,2008,30(4):673-683.
[50]Kaplan B.Decreased protein binding ofmycophenolic acid associated with leukopenia in a pancreas transplant recipientwith renal failure[J].Transplantation,1998,65(8):1127-1129.
[51]Weber LT.The pharmacokinetic-pharmacodynamic relationship for total and freemycophenolic Acid in pediatric renal transplant recipients:a report of the german study group on mycophenolate mofetil therapy[J].JAm Soc Nephrol,2002,13(3):759-768.
[52]Mudge DW.Severe toxicity associated with amarkedly elevated mycophenolic acid free fraction in a renal transplant recipient[J].Ther Drug Monit,2004,26(4):453-455.
[53]Atcheson BA.Mycophenolic acid pharmacokinetics and related outcomes early after renal transplant[J].Br JClin Pharmacol,2005,59(3):271-280.
[54]Premaud A.Feasibility of,and critical paths formycophenolatemofetil Bayesian dose adjustment:pharmacological re-appraisal of a concentration-controlled versus fixed-dose trial in renal transplant recipients[J].Pharmacol Res,2010,61(2):167-174.
[55]Oremus M.Utility of monitoring mycophenolic acid in solid organ transplant patients[J].Evid Rep Technol Assess(Full Rep),2008,164:1-131.
[56]Tett SE.Mycophenolate,clinical pharmacokinetics,formulations,and Methods for assessing drug exposure[J].Transplant Rev(Orlando),2011,25(2):47-57.
