Follicular dendritic cell sarcoma of the liver: unusual presentation of a rare tumor and literature review
2011-07-05PauloMartinsSanjayReddyAnnBrittMartinsandMarceloFacciuto
Paulo N Martins, Sanjay Reddy, Ann-Britt Martins and Marcelo Facciuto
New York, USA
Follicular dendritic cell sarcoma of the liver: unusual presentation of a rare tumor and literature review
Paulo N Martins, Sanjay Reddy, Ann-Britt Martins and Marcelo Facciuto
New York, USA
BACKGROUND:Hepatic follicular dendritic cell (FDC) sarcoma is an extremely rare neoplasm. Most commonly, FDC sarcoma presents as a solitary mass in lymph nodes, however, several extra-nodal locations have been identified.
METHODS:We report a case of a 53-year-old female who presented with symptoms of abdominal pain, fever, anemia, and jaundice. After an extensive review of the literature, we have found only 12 cases of hepatic FDC sarcoma.
RESULTS:The tumor was 11.5 cm in diameter and composed of spindle and epithelioid cells with ovoid nuclei and associated with mixed inflammatory infiltrate. Immunohistochemical stains were positive for CD35 and CD21. The patient underwent a left hepatic lobectomy.
CONCLUSIONS:Liver follicular dendritic cell sarcoma is a very rare tumor. Most cases present with abdominal pain and weight loss, and most of them can be managed by hepatic resection with excellent short-term outcomes.
(Hepatobiliary Pancreat Dis Int 2011; 10: 443-445)
follicular dendritic cell tumor; liver neoplasms; sarcoma; jaundice
Introduction
Follicular dendritic cells (FDCs) are located primarily in the germinal centers of secondary lymphoid organs and their function is to present antigens to specialized lymphocytes. FDC sarcoma of the liver is an extremely rare neoplasm with only 12 previous cases reported in the literature (Table).
Case report
A 53-year-old Hispanic woman initially complained of jaundice associated with abdominal pain, anemia, and fever. Alpha-fetoprotein, carcinoembryonic antigen, and CA19-9 levels were within normal limits. Laboratory values obtained during the initial work-up were: total protein 79 g/L, albumin 48 g/L, total bilirubin 87.21 μmol/L, direct bilirubin 8.55 μmol/L, AST 15 U/L, ALT 14 U/L, alkaline phosphatase 153 U/L, GGT 99 U/L, WBC 9.4×109/L (differential: lymphocytes 26.5%, neut 59.1%, eos 7.9%, mono 6.6%, baso 7.2%), hemoglobin 5.4 g/dL, hematocrit 24.6%, platelets 341×109/L, and alphafetoprotein 2.0 ng/mL. A hepatitis profile showed hepatitis B core, surface antibody, antigen, and hepatitis C antibody all non-reactive. Hepatitis A antibody was reactive. Viral hepatitis serologic test and antimitochondrial antibody levels were negative. MRI of the abdomen showed an 11.5 cm lobulated mass in the left hepatic lobe, with central calcifications. The left portal vein and left hepatic vein were not visualized (Fig. 1A). The bone marrow specimen showed no malignancy. The patient underwent a left lobe liver resection. Intra-operatively, the abdomen was carefully inspected, and no lesions were noted within the mesentery, small bowel, or spleen. Frozen sections of lymph nodes were all negative for tumor. The left lobe of the liver was notably atrophied, likely secondary to thrombosis of the left portal vein, which was identified on previous imaging. The patient tolerated the procedure well, the post-operative course was unremarkable, and she was discharged on postoperative day 5. Six months to the present after the operation, she is asymptomatic with no evidence of recurrence. The anemia and jaundice have also improved without any specific treatment.

Table. Characteristics of patients with hepatic FDC sarcoma (Adapted from Bai 2006)[2]
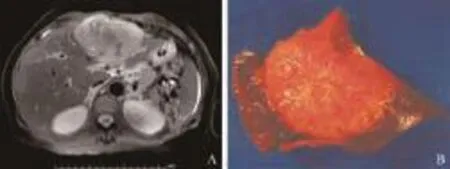
Fig. 1. A: MRI of the abdomen showing an 11.5 cm lesion on the left hepatic lobe. No evidence of other abdominal lesions. B: The tumor measured 11.5×10.5×8.5 cm. It was tan pink, homogeneous with whitish calcified areas and had a firm calcified center. The peripheral rim of the lesion was light brown and soft and the surgical margins were free of tumor.
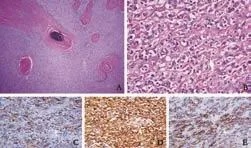
Fig. 2. Pathological analysis revealed a tumor most consistent with FDC sarcoma. A and B: HE staining (original magnification ×4 and ×40) shows foci of calcification, spindle cells with oval nuclei, violaceous nuclear membrane, and small nucleolus. Immunohistochemical staining was positive for vimentin, CD56, CD35 (C, original magnification ×40), clusterin (D, original magnification ×40), and CD21 (E, original magnification ×40).
Histopathologic analysis of the mass (Fig. 1B) revealed a tumor composed of spindle and epithelioid cells (Fig. 2A, B). The tumor had sclerotic, dense fibrous septa with areas of calcification and fibrous stroma. The nuclei were ovoid to polygonal with prominent eosinophilic nucleoli and minimal mitotic activity. Immunohistochemical staining was positive for CD35, clusterin, CD21 (Fig. 3C-E), vimentin, CD56, S-100, CD3, CD20, CD68, CD138, and CD117 (C-Kit), and negative for CD1a, CD31, CD23, CD34, and Epstein-Barr virus (EBV).
Discussion
FDC sarcoma is a very rare nonlymphoid malignant tumor. It was first described in 1986 by Monda, and there are less than 80 cases reported in the English literature.[1-4]The differential diagnosis includes Hodgkin's disease, sarcoma, leiomyosarcoma, gastrointestinal stromal tumor, and inflammatory pseudotumor.[5,6]FDC sarcomas can be recognized histologically by their oval nuclei, violaceous nuclear membrane, small nucleolus, and empty nucleoplasm.[2,7]They have very distinct immunohistochemical features and the diagnosis is made by reactivity for FDC markers. CD21 (C3d receptor) (positive in 93% of cases) and CD35 (C3b receptor) (positive in 89% of cases) are the most commonly used FDC tumor markers. Other markers used are: R4/23 (63%), Ki-M4, CAN.42, CD68, Ki-67 (5%-50%), EMA (41%), desmoplakin, vimentin (61%), HLA-DR (57%), CD45 (21%), S-100 protein (31%), and clusterin.[2,3]FDC tumors are stained negative for CD1a (marker for Langerhans dendritic and interstitial dendritic cells), cytokeratin, and vascular markers.[3,4,7,8]
Primary sarcomas of the liver are very rare, representing less than 0.1% of all primary hepatic tumors.[9]A primary FDC tumor of the liver is even rarer with only 12 cases reported in the literature (Table). Shek et al[10]reported the first case in 1996. The most common initial presentation of the patient was abdominal pain and weight loss. Anemia and fever were also part of the initial presentation in some cases. In three cases, patients were completely asymptomatic and the tumor was found incidentally. Their age at initial presentation ranged from 19 to 82 years (mean 46.7), the mean tumor diameter was 12.1 cm, and the mean survival was more than 2 years (follow-up ranging from 3 months to 5 years).
Our case was the first to present with marked hemolytic anemia leading to jaundice. In addition, this is the second report of liver FDC tumor that is negative for EBV.[11]Different from other FDC tumors, almost all hepatic FDC tumors are EBV-positive.[11,12]They also have marked inflammatory infiltrates, while others do not. Other difference between FDC tumors in the liver and other locations is the predominance in females. Eleven out of 13 reported cases have been in females.[5]It has been suggested that hepatic FDC tumors might not be as aggressive as the reported intra-abdominal cases because local recurrence only occurred in 1 of the 5 cases over 1 to 5 years of follow-up. No metastases were found in a period of 3 years.[13]In our review of the literature we found that all except one patient underwent hepatic resection, only 2 recurrences were reported during a short follow-up, and all patients were alive at the time of publication (mean survival more than 2 years). Surgical resection is the treatment of choice. Local recurrence (36%) and metastases (28%) are relatively common.[6]The role of adjuvant chemotherapy and radiation needs to be defined. The general consensus is that neoadjuvant chemotherapy and/or radiation is reserved for tumors with aggressive pathological features, evidence of recurrence, or incompletely resected lesions.[14]
Funding:None.
Ethical approval:Not needed.
Contributors:MPN wrote the first draft of this report. All authors contributed to the intellectual context and approved the final version. MPN is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Monda L, Warnke R, Rosai J. A primary lymph node malignancy with features suggestive of dendritic reticulum cell differentiation. A report of 4 cases. Am J Pathol 1986;122: 562-572.
2 Bai LY, Kwang WK, Chiang IP, Chen PM. Follicular dendritic cell tumor of the liver associated with Epstein-Barr virus. Jpn J Clin Oncol 2006;36:249-253.
3 Grogg KL, Lae ME, Kurtin PJ, Macon WR. Clusterin expression distinguishes follicular dendritic cell tumors from other dendritic cell neoplasms: report of a novel follicular dendritic cell marker and clinicopathologic data on 12 additional follicular dendritic cell tumors and 6 additional interdigitating dendritic cell tumors. Am J Surg Pathol 2004; 28:988-998.
4 Fonseca R, Yamakawa M, Nakamura S, van Heerde P, Miettinen M, Shek TW, et al. Follicular dendritic cell sarcoma and interdigitating reticulum cell sarcoma: a review. Am J Hematol 1998;59:161-167.
5 Chen TC, Kuo TT, Ng KF. Follicular dendritic cell tumor of the liver: a clinicopathologic and Epstein-Barr virus study of two cases. Mod Pathol 2001;14:354-360.
6 Perez-Ordoñez B, Rosai J. Follicular dendritic cell tumor: review of the entity. Semin Diagn Pathol 1998;15:144-154.
7 Selves J, Meggetto F, Brousset P, Voigt JJ, Pradère B, Grasset D, et al. Inflammatory pseudotumor of the liver. Evidence for follicular dendritic reticulum cell proliferation associated with clonal Epstein-Barr virus. Am J Surg Pathol 1996;20: 747-753.
8 Toyoda K, Taniguchi J, Kikawa K, Uike N, Haraoka S, Ooshima K, et al. Follicular dendritic cell sarcoma: ultrastructural and immunohistochemical studies. Intern Med 2000;39:950-955.
9 Shek TW, Liu CL, Peh WC, Fan ST, Ng IO. Intra-abdominal follicular dendritic cell tumour: a rare tumour in need of recognition. Histopathology 1998;33:465-470.
10 Shek TW, Ho FC, Ng IO, Chan AC, Ma L, Srivastava G. Follicular dendritic cell tumor of the liver. Evidence for an Epstein-Barr virus-related clonal proliferation of follicular dendritic cells. Am J Surg Pathol 1996;20:313-324.
11 Torres U, Hawkins WG, Antonescu CR, DeMatteo RP. Hepatic follicular dendritic cell sarcoma without Epstein-Barr virus expression. Arch Pathol Lab Med 2005;129:1480-1483.
12 Cheuk W, Chan JK, Shek TW, Chang JH, Tsou MH, Yuen NW, et al. Inflammatory pseudotumor-like follicular dendritic cell tumor: a distinctive low-grade malignant intraabdominal neoplasm with consistent Epstein-Barr virus association. Am J Surg Pathol 2001;25:721-731.
13 Chan JK, Fletcher CD, Nayler SJ, Cooper K. Follicular dendritic cell sarcoma. Clinicopathologic analysis of 17 cases suggesting a malignant potential higher than currently recognized. Cancer 1997;79:294-313.
14 Azim HA, Elsedewy E, Azim HA Jr. Imatinib in the treatment of follicular dendritic sarcoma: a case report and review of literature. Onkologie 2007;30:381-384.
Received March 16, 2010
Accepted after December 10, 2010
Author Affiliations: Department of Surgery, Division of Hepatobiliary Surgery and Transplantation, New York Medical College (Martins PN, Martins AB and Facciuto M); Department of Surgery, New York Medical College, St. Vincent's Catholic Medical Center (Reddy S), New York, USA
Paulo N Martins, MD, PhD, New York Medical College, Westchester Medical Center, 95 Grassland Road, Valhalla, New York 10595, USA (Tel: +1-914-493-5930; Fax: +1-914-493-1097; Email: martinsp@wcmc.com, drpauloney@yahoo.com)
© 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Prognostic role of diabetes mellitus in hepatocellular carcinoma patients after curative treatments: a meta-analysis
- Risk factors of severe ischemic biliary complications after liver transplantation
- Surgical treatment of Budd-Chiari syndrome: analysis of 221 cases
- Efficacy of liver transplantation for acute hepatic failure: a single-center experience
- Combined invagination and duct-to-mucosa techniques with modifications: a new method of pancreaticojejunal anastomosis
- Large regenerative nodules in a patient with Budd-Chiari syndrome after TIPS positioning while on the liver transplantation list diagnosed by Gd-EOB-DTPA MRI
