Hepatitis C virus infection and biological falsepositive syphilis test: a single-center experience
2011-07-05WeiFangZhuShuiYingLeiandLanJuanLi
Wei-Fang Zhu, Shui-Ying Lei and Lan-Juan Li
Hangzhou, China
Hepatitis C virus infection and biological falsepositive syphilis test: a single-center experience
Wei-Fang Zhu, Shui-Ying Lei and Lan-Juan Li
Hangzhou, China
BACKGROUND:Rapid plasma regain positive and/or treponema pallidum hemagglutination negative [RPR(+)/TPHA(-)] results were designated as biologic false-positive (BFP). There are limited data about BFP reactions against syphilis in patients with hepatitis C virus (HCV) infection. This study aimed to determine the prevalence of BFP reactions for syphilis in patients with HCV infection in a large sample and assess the relationship between BFP reactions and HCV infection.
METHODS:A total of 2656 patients with positive anti-HCV and 5600 healthy control subjects were enrolled in this study. Hepatitis C serology was determined by a second generation ELISA test for HCV antibody. Syphilis serology was determined by the RPR test. Those subjects with reactive RPR positive underwent the TPHA test. Demographics and laboratory data were collected by trained clinicians.
RESULTS:Among 2656 patients, 111 (4.2%) had a reactive RPR test. Of the 111 patients who were subjected to reactive RPR test, 30 (27.0%) showed HCV(+)/RPR(+). Of 5600 healthy controls, 80 (1.4%) had a reactive RPR test. Fourteen (17.5%) controls with HCV(-)/RPR(+) had a non-reactive TPHA test. These represented 1.1% of all HCV-positive and 0.3% of all HCV-negative subjects (P<0.001). A significantly increased prevalence shown by false-positive tests for syphilis was observed in elderly HCV-seropositive patients. BFP-HCV positive group had a higher prevalence of eosinophilia. The eosinophil abnormality was compared between the patients and controls (66.7% vs 21.4%,P=0.0043). No significant results were observed in antinuclear antibodies, antiphospholipid and complement (C3, C4) (P>0.05).
CONCLUSIONS:The data of this study demonstrate that HCV infection is associated with a false-positive RPR test. In this study BFPs were significantly more common in HCV positive patients compared to HCV-negative ones. Eosinophil abnormality can be considered as a predictor for BFP. Excessive BFPs must be considered in assessing the frequency of syphilis in a HCV-positive population and the importance of the treponemal specific serologic test should be emphasized for a diagnosis of syphilis in such population.
(Hepatobiliary Pancreat Dis Int 2011; 10: 399-402)
hepatitis C virus; biological false-positive; syphilis
Introduction
The diagnosis of syphilis requires a two-step serological test. Not infrequently, sensitive screening is reactive, but it is not confirmed by a more specific test yielding a biological false-positive (BFP).[1]The rapid plasma reagin (RPR) test for syphilis screening may, under certain circumstances, yield positive results in patients not infected by Treponemapallidum. Occasional BFP may occur in any of the serological tests for syphilis. In general, BFP is more likely to be seen in autoimmune disease, HIV infection, pregnancy, and intravenous drug abuse.[2-5]The nonrecognition of BFP reaction for syphilis may have negative social and economic implications.
An array of infectious and noninfectious conditions has been reported to cause BFP reaction.[2-5]Hepatitis C virus (HCV) infection has been associated with mixed cryoglobulinemia and immune complex glomerulonephriris,[6,7]but has rarely been investigated as a cause of BFP reactions in a large sample. This study aimed to describe the prevalence of BFP in a large HCV-infected or non-infected population.
Methods
A total of 2656 patients with HCV infection (patientsgroup) and 5600 healthy outpatients (control group) were enrolled in this study. The patients had been treated at the First Affiliated Hospital, Zhejiang University School of Medicine from December 1, 2008 to April 1, 2011.
Healthy controls of comparable age and gender were recruited from our hospital. They had no history of cardiovascular, liver or kidney diseases. Clinical examination and laboratory test (liver and kidney function, hepatitis B surface antigen, HCV antibody and HIV antibody) were normal. The main exclusion criteria of both groups should included incomplete clinical data, current pregnancy, pre-existing hepatitis B, and HIV infection or connective tissue diseases.
HCV antibody of all enrollees was determined by a second generation ELISA (Ortho Diagnostics) test. Syphilis detection was done using the RPR test; those with reactive RPR positive underwent the treponema pallidum hemagglutination (TPHA) test. Autoantibodies [antinuclear antibodies (ANA), antiphospholipid (aPL)] were detected in sera by indirect immunofluorescence. Eosinophil count was determined by an automated blood cells counter (Sysmex XS800i). The levels of complement (C3, C4) were measured by the photometry immuno-turbidimetric test (BECKMAN). The levels of γ-globulin were measured by cellulose acetate membrane electrophoresis (INTERLAB).
Clinical and laboratory data were collected from records through an Excel spreadsheet (version 2003). Stratified analysis was made using the chi-square test or Fisher's exact test. A P value less than 0.05 was considered statistically significant.
Results
Of the 2656 HCV seropositive patients, 1100 were female and 1556 male, with an average age of 37 (range 20-69) years. Among the 5600 healthy subjects, 2101 were female and 3499 male, with an average age of 35 (range 19-71) years (Table 1). No significance was observed in gender and age between the two groups.
One hundred and eleven (4.2%) patients with HCV seropositive and 80 (1.4%) controls with HCV seronegative were RPR reactive (P<0.0001). Thirty (27.0%) patients were HCV(+)/RPR(+) and 14 (17.5%) controls were HCV(-)/RPR(+). All of them had a nonreactive TPHA test and represented 1.1% of HCV-positive and 0.3% of HCV-negative subjects (P<0.001) (Fig. 1).
Laboratory data were compared between the 30 BFP-HCV positive patients and 14 BFP-HCV negative controls (Table 2). False-positive tests for syphilis showed a significantly increased prevalence in elderly HCV seropositive patients (≥60 years old). The BFP-HCV positive group had a higher prevalence of eosinophilia. The eosinophil abnormality was compared between theBFP-HCV positive group and BFP-HCV negative group (66.7% vs 21.4%, P=0.0043). To investigate whether there is an association between BFP and immunological abnormality, we analyzed the results of ANA, aPL and complement (C3, C4). Seven (23.3%) HCV-positive BFP patients were ANA positive. Two (1.4%) patients with HCV-negative BFP were ANA positive. Four (13.3%) HCV-positive BFP patients were aPL positive. One (7.1%) of the HCV-negative BFP patients were aPL positive. Four (1.4%) of the HCV-negative BFP patients were aPL positive. But no significant results were observed in ANA, aPL and complement (C3, C4) (P>0.05, Fig. 2).

Table 1. Demographic and laboratory characteristics of the patients and control groups

Table 2. Demographic characteristics of the BFP group by HCV serostatus
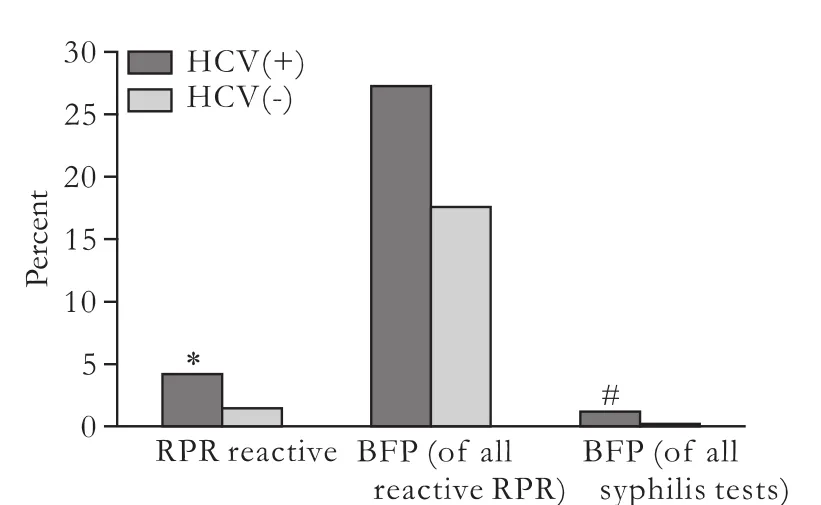
Fig. 1. BFP results as a proportion of reactive syphilis serological tests by HCV serostatus. *: A significantly increased prevalence in RPR reactive HCV between seropositive and controls with HCV seronegative. #: Significance was noted between HCV seropositive patients and HCV seronegative controls.
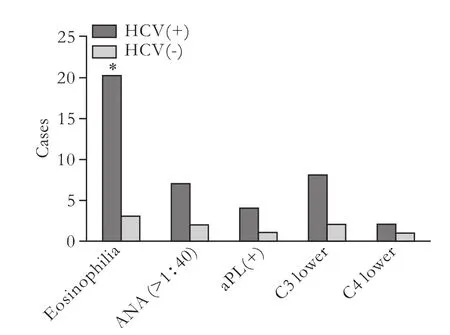
Fig. 2. Laboratory findings of the BFP group by HCV serostatus. *: P<0.05, BFP-HCV positive group vs BFP-HCV negative group.
We measured γ-globulin in 23 HCV-positive BFP patients and 10 HCV-negative BFP. Six (26.1%) of the HCV-positive BFP patients had a high level of serum γ-globulin. Two (20.0%) of the HCV-negative BFP patients had an abnormal level of serum γ-globulin.
Discussion
BFP serological test for syphilis has long been recognized. Reviewing the pertinent literature revealed that BFP may be associated with vaccinations, infections (infectious mononucleosis, measles, typhoid, varicella, influenza, lymphogranuloma venereum, malaria, etc), pregnancy, connective tissue diseases, multiple blood transfusions.[3,8-11]But the association of BFP reactions for syphilis with HCV has rarely been discussed. HCV infection associated with false-positive RPR test has been found mainly in patients with sexually transmitted disease.[12,13]In African-American women, Augenbraun et al[14]found that patients with HCV infection were 4-4.5 times more likely to have a false-positive reaction for syphilis than anti-HCV-negative patients. However, in this study we investigated HCV infection and BFP in Chinese including males and females in a local center. The HCV-positive group was 5 times more likely to have BFP for syphilis than the HCV-negative control group; this finding is similar to the results reported elsewhere.[12-14]
BFP is known to be associated with autoimmune disease, HIV infection, pregnancy, and hepatitis B.[12,15-17]In our study, we excluded such populations in design. Thus we can explain why there was no difference in the prevalence of autobodies (ANA, aPL) and the level of complement (C3, C4) between the two groups. We observed that the BFP-HCV positive group had a higher prevalence of eosinophil abnormality (P=0.0043). The association with HCV and eosinophilia abnormality in the BFP group was not reported. This association has not been elucidated. We infer that eosinophilia may be associated with active hepatitis, but this needs further investigation. Studies[5,6,12]have confirmed the association of immunoglobulin production disorder with HCV infection. In this study, we also found that a higher prevalence of serum γ-globulin abnormality in the BFP-HCV positive patients compared with the BFPHCV negative patients.
The proposed mechanism by which infectious pathogens cause BFP reactions includes coincidental cross-reactivity of an idiotype of the infecting pathogen with nontreponemal antigens and nonspectifically increased production of antibodies associated with infectious diseases.[16]HCV may be conclusively considered a cause of BFP. It may make an already nonspecific treponemal test less specific. HCV might alter the operational parameters of other treponemal-specific serological tests or serological tests used for other nonsyphilis clinical conditions. In our study, BFP may result from the cross-reactivity of HCV-induced antibodies with nontreponemal antigens in the RPR test.
According to these data, RPR positive in hepatitis C may be BFP with regard to the reaginic tests. Therefore, therapeutic measures should not be initiated without confirmation with a TPHA test.
This study has drawbacks. We have small positive BFP samples because of the lower prevalence rate of BFP in China. Traditionally BFP reactions were classified as acute and chronic ones. We couldn’t get the result because we failed to follow up further.
In conclusion, the data of this study demonstrate that HCV infection is associated with false-positive RPR test results. BFP was more common in HCV-positive patients than in HCV-negative patients. Eosinophil abnormality can be considered as a predictor for BFP. Excessive BFP must be considered in assessing the frequency of syphilis in HCV-positive population as well as the importance of the specific serologic TPHA test in confirming a diagnosis of syphilis in such population.
Acknowledgement
We thank to Dr. Da-Gan Yang and Shu-Juan Song for their technical assistance.
Funding:None.
Ethical approval:Not needed.
Contributors:ZWF and LSY contributed equally to this study. ZWF proposed the study. ZWF and LSY wrote the first draft. All authors contributed to the design and interpretation of the study and to further drafts. LLJ is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Nandwani R, Evans DT. Are you sure it's syphilis? A review of false positive serology. Int J STD AIDS 1995;6:241-248.
2 Geusau A, Kittler H, Hein U, Dangl-Erlach E, Stingl G, Tschachler E. Biological false-positive tests comprise a high proportion of Venereal Disease Research Laboratory reactions in an analysis of 300,000 sera. Int J STD AIDS 2005; 16:722-726.
3 Frederick T, Burian P, Terrault N, Cohen M, Augenbraun M, Young M, et al. Factors associated with prevalent hepatitis C infection among HIV-infected women with no reported history of injection drug use: the Women's Interagency HIV Study (WIHS). AIDS Patient Care STDS 2009;23:915-923.
4 Rompalo AM, Cannon RO, Quinn TC, Hook EW 3rd. Association of biologic false-positive reactions for syphilis with human immunodeficiency virus infection. J Infect Dis 1992;165:1124-1126.
5 Koike T, Sueishi M, Funaki H, Tomioka H, Yoshida S. Antiphospholipid antibodies and biological false positive serological test for syphilis in patients with systemic lupus erythematosus. Clin Exp Immunol 1984;56:193-199.
6 Operskalski EA, Mack WJ, Strickler HD, French AL, Augenbraun M, Tien PC, et al. Factors associated with hepatitis C viremia in a large cohort of HIV-infected anduninfected women. J Clin Virol 2008;41:255-263.
7 Li YD, Lin JJ, Zheng SS. Inflammatory bowel diseases and hepatitis C virus infection. Hepatobiliary Pancreat Dis Int 2010;9:398-401.
8 Augenbraun MH, DeHovitz JA, Feldman J, Clarke L, Landesman S, Minkoff HM. Biological false-positive syphilis test results for women infected with human immunodeficiency virus. Clin Infect Dis 1994;19:1040-1044.
9 Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, et al. The Women's Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology 1998;9:117-125.
10 Hernández-Aguado I, Bolumar F, Moreno R, Pardo FJ, Torres N, Belda J, et al. False-positive tests for syphilis associated with human immunodeficiency virus and hepatitis B virus infection among intravenous drug abusers. Valencian Study Group on HIV Epidemiology. Eur J Clin Microbiol Infect Dis 1998;17:784-787.
11 Cabrini JM, Bottasso OA, Margasin S, Schujman L, Mangiaterra L, Morini JC. Biological false positive test for syphilis in lepromatous leprosy patients with concomitant hepatitis B virus infection. J Investig Allergol Clin Immunol 1991;1:45-48.
12 Thomas DL, Rompalo AM, Zenilman J, Hoover D, Hook EW 3rd, Quinn TC. Association of hepatitis C virus infection with false-positive tests for syphilis. J Infect Dis 1994;170:1579-1581.
13 Sonmez E, Ozerol IH, Senol M, Kizilkaya N, Sahin K, Ozbilge H. False-positive reaction between syphilis and hepatitis C infection. Isr J Med Sci 1997;33:724-727.
14 Augenbraun M, French A, Glesby M, Sanchez-Keeland L, Young M, Greenblatt R, et al. Hepatitis C virus infection and biological false-positive syphilis tests. Sex Transm Infect 2010;86:97-98.
15 Huh HJ, Lee KK, Kim ES, Chae SL. Analysis of positive results in mediace rapid plasma reagin and Treponema pallidum latex agglutination as the automated syphilis test. Korean J Lab Med 2007;27:324-329.
16 Erbelding EJ, Vlahov D, Nelson KE, Rompalo AM, Cohn S, Sanchez P, et al. Syphilis serology in human immunodeficiency virus infection: evidence for false-negative fluorescent treponemal testing. J Infect Dis 1997;176:1397-1400.
17 Conley CL, Savarese DM. Biologic false-positive serologic tests for syphilis and other serologic abnormalities in autoimmune hemolytic anemia and thrombocytopenic purpura. Medicine (Baltimore) 1989;68:67-84.
Received May 21, 2011
Accepted after revision July 13, 2011
Author Affiliations: State Key Laboratory for Diagnosis and Treatment of Infectious Diseases; Key Laboratory of Infectious Diseases, Zhejiang Province; Department of Infectious Diseases, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Zhu WF, Lei SY and Li LJ)
Lan-Juan Li, MD, State Key Laboratory for Diagnosis and Treatment of Infectious Diseases; Key Laboratory of Infectious Diseases, Zhejiang Province; Department of Infectious Diseases, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Tel: 86-571-87236340; Email: ljli@zju.edu.cn)
© 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Surgical treatment of Budd-Chiari syndrome: analysis of 221 cases
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Success rate and complications of endoscopic extraction of common bile duct stones over 2 cm in diameter
- Donor liver natural killer cells alleviate liver allograft acute rejection in rats
- Enteral supplementation with glycyl-glutamine improves intestinal barrier function after liver transplantation in rats
- Noninvasive indocyanine green plasma disappearance rate predicts early complications, graft failure or death after liver transplantation
