Enteral supplementation with glycyl-glutamine improves intestinal barrier function after liver transplantation in rats
2011-07-05JianWenJiangZhiGangRenLuYanChenLiJiangHaiYangXieLinZhouandShuSenZheng
Jian-Wen Jiang, Zhi-Gang Ren, Lu-Yan Chen, Li Jiang, Hai-Yang Xie, Lin Zhou and Shu-Sen Zheng
Hangzhou, China
Enteral supplementation with glycyl-glutamine improves intestinal barrier function after liver transplantation in rats
Jian-Wen Jiang, Zhi-Gang Ren, Lu-Yan Chen, Li Jiang, Hai-Yang Xie, Lin Zhou and Shu-Sen Zheng
Hangzhou, China
BACKGROUND:Most patients after liver transplantation (LT) suffer from intestinal barrier dysfunction. Glycyl-glutamine (Gly-Gln) by parenteral supplementation is hydrolyzed to release glutamine, which improves intestinal barrier function in intestinal injury. This study aimed to investigate the effect of Gly-Gln by enteral supplementation on intestinal barrier function in rats after allogenetic LT under immunosuppressive therapy.
METHODS:Twelve inbred Lewis rats were selected randomly as donors, and 24 inbred Brown Norway (BN) rats as recipients of allogenetic LT. The recipients were divided into a control group (Ala,n=12) and an experimental group (Gly-Gln,n=12). In each group, 6 normal BN rats were sampled for normal parameters on preoperative day 3. The 6 recipients in the control group received alanine (Ala) daily by gastric perfusion for 3 preoperative days and 7 postoperative days, and the 6 recipients in the experimental group were given Gly-Gln in the same manner. The 12 BN recipients underwent orthotopic LT under sterile conditions after a 3-day fast and were given immunosuppressive therapy for 7 days. They were harvested for sampling on postoperative day 8. The following parameters were assessed: intestinal mucosal protein content, mucosal ultrastructure, ileocecal sIgA content, portal plasma levels of endotoxin and TNF-α, and bacterial translocation.
RESULTS:All recipients were alive after LT. On preoperative day 3, all parameters were similar in the two groups. On postoperative day 8, all parameters in the two groups were remarkably changed from those on preoperative day 3. However, compared to the Ala group, supplementation withGly-Gln increased the levels of intestinal mucosal protein and ileocecal sIgA, improved mucosal microvilli, and decreased portal plasma levels of endotoxin and TNF-αas well as bacterial translocation.
CONCLUSION:Enteral supplementation with Gly-Gln improved intestinal barrier function after allogenetic LT in rats.
(Hepatobiliary Pancreat Dis Int 2011; 10: 380-385)
liver transplantation; glycyl-glutamine; bacterial translocation; intestinal barrier function
Introduction
Most patients waiting for liver transplantation (LT) have end-stage liver disease along with various degrees of nutritional deficiency.[1]At present, most surgeons adopt orthotopic LT without veno-venous by-pass.[2]During LT surgery, the recipients inevitably suffer from an anhepatic phase and gastrointestinal congestion lasting 45-65 minutes or longer.[2]However, the epithelial cells of the gastrointestinal mucosa are so susceptible to the hypoxia-ischemia caused by intestinal ischemia-reperfusion injury that they are prone to apoptosis and necrosis after the surgery.[3]Meanwhile, the liver graft itself must also be subjected to ischemiareperfusion injury during the operation. In addition, inflammatory cascade reactions are activated by intestinal and hepatic injury during the operation, and thus aggravate intestinal and hepatic injury.[4]These factors are closely associated with many postoperative complications and most likely lead to endogenous infections and intestinal barrier dysfunction, increasing the postoperative mortality rate. Thus, it is essential to improve the nutritional status and intestinal barrier function of recipients during the peri-operative period.
Glutamine is a specific nutrient of enterocytes and rapidly proliferating cells, and is also actively involved in energy metabolism.[5]However, glutamine monomer has low aqueous solubility and chemical heat-instability, and easily forms poisonous pyroglutamic acid, which limits its application in clinical practice. Currently, the synthetic dipeptides glycyl-glutamine (Gly-Gln) and alanyl-glutamine (Ala-Gln) are frequently used and decompose to release glutamine in vivo. With high solubility and stability, they can be sterilized at a high temperature.[6]Moreover, substantial dipeptide carriers exist on the cell surface of the mucosal epithelium, and can effectively transport dipeptides even if the intestinal barrier is impaired.[7]
So far, little is known about the effect of enteral supplementation with Gly-Gln on intestinal barrier function in recipients after LT. We explored the effects of Gly-Gln by enteral supplementation on intestinal protein metabolism, mucosal ultrastructure and intestinal barrier function after LT.
Methods
Animals
Specific pathogen-free (SPF) male inbred Lewis rats (weighing 220-240 g) and Brown Norway (BN) rats (weighing 250-280 g) (purchased from Beijing Vital River Laboratories), were housed in an SPF room in the Laboratory Animal Center of Zhejiang Province, China. The rats were caged at 25 ℃ in a 12-hour light/dark cycle, and fed with sterilized water and standard rat chow. All animals received humane care and the study was conducted according to the Guide for the Care and Use of Laboratory Animals (NIH publication No. 85-23, revised 1985).
Experimental protocol and operation methods
Twelve male inbred Lewis rats were selected as donors, and 24 male inbred BN rats as recipients of allogenetic LT. The recipients were randomly divided into two groups: control (Ala,n=12) and experimental (Gly-Gln, n=12). In each group, 6 normal BN rats were sampled for normal parameters on preoperative day 3. The 6 recipients in the control group received 600 mg/kg alanine (Ala) daily by gastric perfusion for 3 preoperative days and 7 postoperative days, and the 6 recipients in the experimental group were given Gly-Gln in the same manner. Orthotopic LT was performed in the 12 recipients under aseptic conditions after a 3-day fast (free access to water with 0.23% sodium chloride) and they were subcutaneously injected with 2 mg/kg Cyclosporin A daily after the operation.
Surgical procedures
The orthotopic LT was performed according to the modified method of Kamada and Calne[8]without anastomosis to the hepatic artery. Briefly, both donor and recipient were anesthetized by intraperitoneal injection of pentobarbital sodium (40 mg/kg, Shanghai No. 1 Biochemical & Pharmaceutical, China), and then ether was inhaled to maintain anesthesia. After the donor liver was segregated, the graft was perfused through the portal vein with chilled saline containing 25 U/mL heparin. The graft was placed into cold saline for about 40 minutes until being placed in the recipient abdomen. After anastomosis of the supra-hepatic vein cava and portal vein was completed, the liver was reperfused. The common bile duct was reconstructed by tying the duct over a stent, and 2 mL normal saline was injected through the penile vein of the recipient after the operation; the anhepatic phase lasted for 22-25 minutes. Upon awakening from anesthesia, rats had free access to sterilized water and standard rodent chow.
Sample collections
On postoperative day 8, all recipients were sampled under strictly sterile conditions. The portal vein was punctured and a blood sample was collected for the measurement of plasma endotoxin and TNF-α. Tissue samples from the left lobe of the liver were harvested for the study of bacterial translocation. Ileal mucosal samples were biopsied from about 2 cm from the ileocecal valve, and fixed in 2.5% glutaraldehyde for later histological study by electron microscopy. About 20 cm of terminal ileum was obtained for testing intestinal mucosal protein content, and ileocecal content was weighed for the study of sIgA level.
Intestinal mucosal protein content
About 20 cm of terminal ileum was washed with normal saline, the intestinal mucous layer was removed using a sterile cotton swab, and about 0.2-0.3 g of the mucosa was scraped off with a scalpel. The obtained mucosa was ground and diluted with a nine-fold volume of normal saline, and then centrifuged at 800×g for 10 minutes. The supernatant was obtained and mucosal protein content was determined by the method of Lowry. The results are presented as milligrams protein per gram wet weight of tissue (mg/g wet weight).
Investigation of intestinal mucosal ultrastructure
Ileal mucosal specimens were collected from the different groups and prepared for electron microscopy by the standard technical procedures.[9]Briefly, theywere fixed in 2.5% glutaraldehyde (4 ℃, pH 7.4), postfixed in 1% osmium tetroxide, and embedded in an epon-araldite mixture. Ultrathin sections were prepared, placed on mesh copper grids, and stained with uranyl acetate and lead citrate. The ultrastructure of the mucosa was analyzed on a Tecnai 10 electron microscope (Philips, Eindhoven, Netherlands). Particular attention was paid to the ultrastructure of microvilli.
Ileocecal sIgA content
Ileocecum contents (0.5 g) were homogenized in 1 mL PBS (pH 7.4) and centrifuged at 12 000×g for 20 minutes. The supernatant was taken for the measurement of sIgA by ELISA kit (RnD Ltd., USA) following the manufacturer's instructions. The sIgA content was presented as micrograms per gram of intestinal content (sIgA μg/g).
Portal plasma endotoxin and TNF-α
The portal blood sample (100 μL) was placed in a pyrogen-free heparin-containing tube and centrifuged at 3000×g for 15 minutes at 4 ℃. The level of plasma endotoxin was measured with a quantitative, chromogenic Limulus amebocyte lysate assay (Eihua Medical, Shanghai, China) according to the manufacturer's instructions.[9]The results were expressed as nanograms per liter (ng/L). Plasma levels of TNF-α were assessed with enzyme-linked immunosorbent assay (ELISA) (Groundwork Biotechnology Diagnosticate Ltd., USA) in accordance with the manufacturer's protocol. The value was expressed as ng/L.
Bacterial culture and identification
Liver samples were weighed and placed in a sterile glass homogenizer containing a nine-fold volume of anaerobic buffer (phosphate-buffered saline with 0.5 g cysteine HCl, 0.5 mL Tween 80, and 0.5 g agar/L), and 50 μL of 10% homogenate was placed on Colombian culture within 30 minutes of sample collection and incubated for 48 hours at 37 ℃. Bacterial colonies were counted and the results were expressed as bacterial colony forming units (CFU) per gram tissue (log10CFU/g). In addition, bacterial colonies from liver samples were identified by an automatic analyzer of bacteria (Model Viger 60, France) to identify the species.
Statistical analysis
All data were presented as mean±SD and were evaluated using analysis of variance. All analyses were performed with the statistical software SPSS16.0. APvalue of less than 0.05 was considered statistically significant.
Results
During the LT surgery, no complications of blood vessels or bile duct occurred, and all recipients in each group were still alive on postoperative day 8.
Intestinal mucosal protein content
Compared to preoperative day 3, mucosal protein content was reduced in both groups on postoperative day 8 (both P<0.05). Furthermore, the value was higher in the Gly-Gln group (87.2±6.99 mg/g) than in the Ala group (76.2±6.40 mg/g) on postoperative day 8 (P<0.05) (Fig. 1).
Ultrastructure of intestinal mucosa
Intestinal epithelial cells in the two groups on preoperative day 3 showed normal ultrastructure with many homogenously and compactly distributed microvilli (Fig. 2 A, B). However, epithelial cells in the Ala group on postoperative day 8 were damaged, mainly seen as microvilli disruption and loss (Fig. 2C). In contrast, the mucosal microvilli damage was markedly ameliorated in rats in the Gly-Gln group on postoperative day 8 as compared with that in the Ala group (Fig. 2D).
Ileocecal sIgA content
There was no significant difference between the two groups on preoperative day 3. Compared to preoperative day 3, the sIgA content in the ileocecum was significantly reduced in rats after LT in both groups on postoperative day 8. Moreover, the sIgA content was higher in the Gly-Gln group (34.8±2.41 μg/g) than in the Ala group (31.1±2.99 μg/g) (P<0.05) (Fig. 3).
Portal plasma endotoxin and TNF-α
Compared to preoperative day 3, plasma endotoxin in all recipients in both groups on postoperative day 8 increased (bothP<0.05). There was a clear reduction inendotoxin level in the Gly-Gln group (63.0±31.2 ng/L) versus that in the Ala group (135.0±70.48 ng/L) on postoperative day 8 (P<0.05) (Fig. 4A).
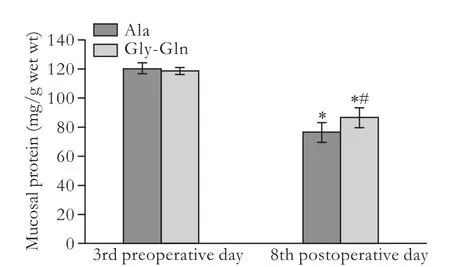
Fig. 1. Mucosal protein content in the LT+Ala and LT+Gly-Gln groups. *: P<0.05, vs the counter-group on preoperative day 3; #: P<0.05, vs the Ala group; P not significant for the remaining parameters.
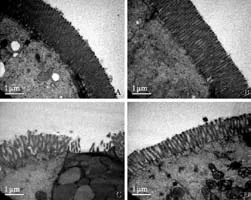
Fig. 2. Transmission electron micrographs of ileal mucosal structure in the two groups (original magnification ×12 500, bar=1 μm). Ala group (A) and Gly-Gln (B) group on preoperative day 3, many microvilli homogenously and compactly distributed on epithelial cells. C: Ala group on postoperative day 8, many microvilli sparsely distributed on epithelial cells with partial disarticulation; D: Gly-Gln group on postoperative day 8, many microvilli sparsely but integrally distributed on epithelial cells.
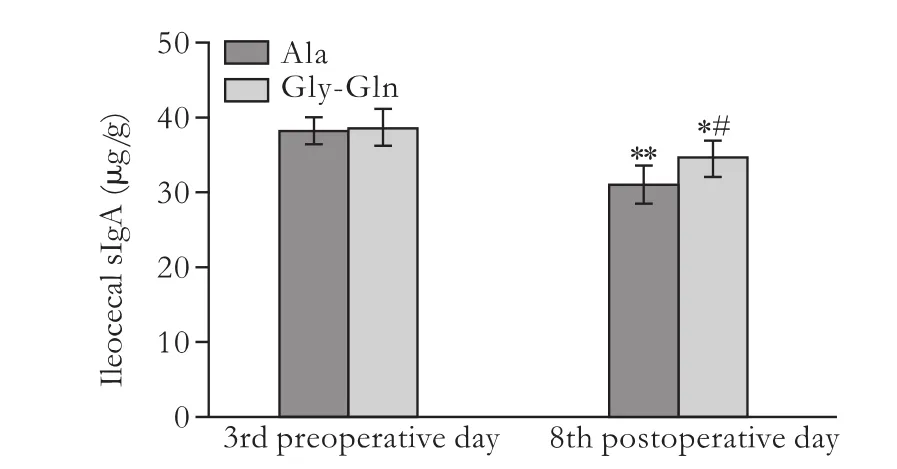
Fig. 3. Ileocecal sIgA content in the LT+Ala and LT+Gly-Gln groups. *: P<0.05, **: P<0.01, vs the counter-group on preoperative day 3; #: P<0.05, vs the Ala group.
The plasma levels of TNF-α in the two groups on postoperative day 8 were much higher than those on preoperative day 3 (Fig. 4B). However, the plasma level of TNF-α in the Gly-Gln group was lower than that in the Ala group on postoperative day 8 (P<0.05).
Bacterial culture and identification
Bacteria in samples from the liver were cultured, and the results rarely showed bacteria on preoperative day 3, while bacterial counts on postoperative day 8 were significantly increased. Compared to the Ala group (3.48±0.24 log10CFU/g), however, bacterial counts of translocation in the liver were remarkably decreased in the Gly-Gln group (2.62±0.45 log10CFU/g) on postoperative day 8 (P<0.05).
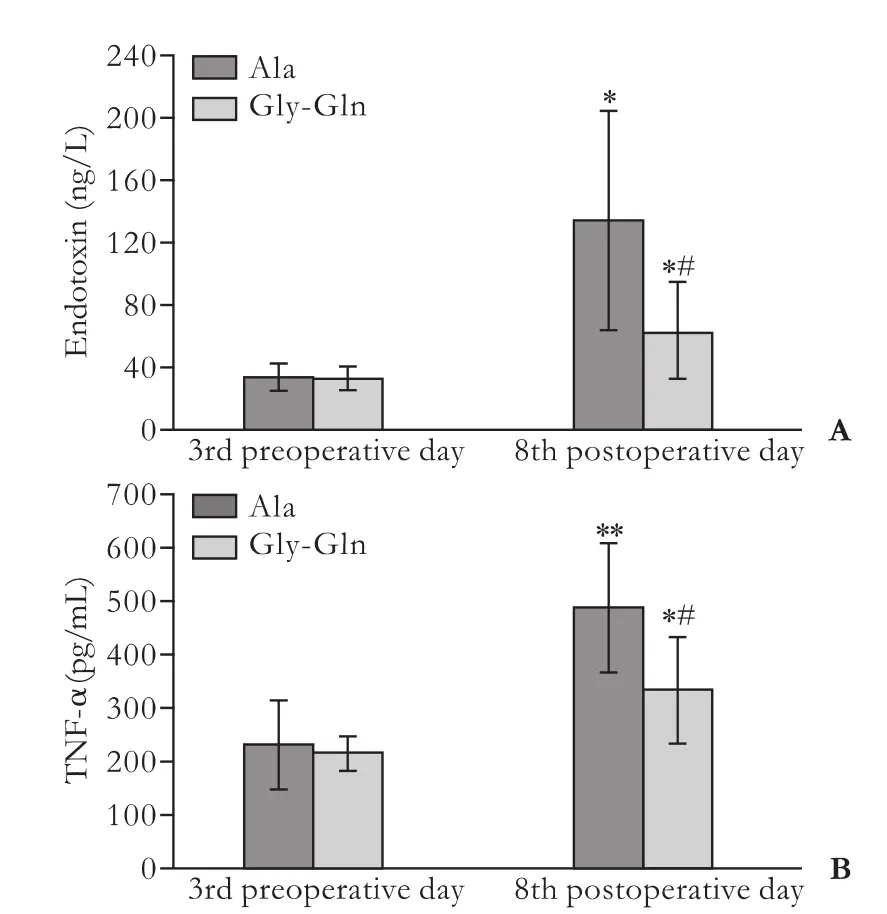
Fig. 4. Portal plasma levels of endotoxin (A) and TNF-α (B) in the LT+Ala and LT+Gly-Gln groups. *: P<0.05, **:P<0.01, vs the counter-group on preoperative day 3; #: P<0.05, vs the Ala group.

Table. Bacterial identification from hepatic samples and rates of bacterial translocation in the different groups on postoperative day 8
Meanwhile, bacterial identification of hepatic samples on postoperative day 8 suggested that application of enteral Gly-Gln slightly decreased the translocation rates of the aerobic bacteriaEscherichia coli, Enterococcus,Proteus vulgaris, Streptococcus agalactiae, Proteus mirabilis and Viridans streptococci compared with those in the Ala group (Table).
Discussion
Malnutrition is almost universally present in patients with end-stage liver disease undergoing LT and is associated with increased morbidity and mortality. It is essential to identify and correct nutritional deficiencies in these patients and provide adequate nutritional support during the peri-operative period.
In the present experiment, we used rats with slight malnutrition as the research model, which approached clinical practice more closely than using normal rats. After 3 days of fasting, the weight of all recipients decreased by 12%-15% and the recipients showed slight malnutrition.
Under physiological conditions, the gastrointestinal system not only plays a vital role in the digestion and absorption of food, but also functions as a significantly defensive barrier function.[10]During LT surgery, recipients have to undergo ischemia-reperfusion injury of the intestine and liver,[2]and the intestinal barrier is damaged to different degrees. The reduction of mucous secretion, damage to intestinal structure and weakening of intestinal motility impair the physical barrier;[11]the imbalance of intestinal microflora and the reduction of secreted digestive juice abate the biological and chemical barrier.[12]Atrophy of gut-associated lymphoid tissue and reduction of intestinal sIgA secretion remarkably impair the immune barrier.[10]Thus destruction of intestinal barrier function might lead to translocation of bacteria and endotoxin as well as activation of hepatic and peritoneal macrophages, which produce large amounts of inflammatory mediators and cytokines, inducing intestinal infection and systemic inflammatory response syndrome.[10,12]
The amino acid glutamine is the most abundant free alpha-amino acid in the bloodstream and skeletal muscle.[13]This nutrient plays important roles in nucleotide synthesis, the regulation of gene expression, protein turnover, anti-catabolic and anti-oxidative functions, nutrient metabolism, immunoregulatory functions, and acid-base balance.[13]Meanwhile, as a synthetic dipeptide with a high solubility and stability, Gly-Gln is decomposed to release glutaminein vivo. Thus in this study, we assessed whether Gly-Gln by enteral supplementation has a beneficial effect on intestinal barrier function following LT in rats.
Our study showed that enteral application of Gly-Gln significantly increased mucosal protein content. As a vital fuel for enterocytes, glutamine is a carrier of nitrogen between tissues and is involved in the synthesis of amino acids for maintaining tight junctions and mucosal cell proliferation of the intestine.[14]Meanwhile, glutamine serves as a nitrogen carrier of purine and pyrimidine synthesis is essential for the synthesis of DNA and RNA, and provides the prerequisites for protein synthesis in the intestinal mucosa.[5,13]Kim et al[15]found that glutamine stimulates the translation of mRNAs to increase protein synthesis through phosphorylation and activation of components of the mammalian target of the rapamycin signaling pathway.
In terms of ultrastructure, rats after LT showed intestinal mucosal destruction, mainly seen as abnormal microvilli with partial loss and broadening of tight junctions between epithelial cells. However, enteral supplementation with Gly-Gln remarkably ameliorated this condition and promoted the restoration of mucosal microvilli. Similarly, our previous research revealed that enteral supplementation with Gly-Gln improves intestinal mucosal structure, enhances Na+-K+-ATP enzyme activity, and promotes the absorptive function of the small intestine.[16]Li et al[17]also indicated that Gly-Gln-supplemented long-term total parenteral nutrition selectively improves structure and function in heterotopic small-bowel autotransplantation in pigs and oral glutamine ameliorates chemotherapy-induced changes of intestinal permeability in patients with breast cancer.[18]The observation of mucosal ultrastructure indicated that supplementation with Gly-Gln protects the intestinal mechanical barrier, which is likely correlated with the improvement of intestinal protein metabolism.
The intestinal immune barrier plays a pivotal role in defense. We measured sIgA levels in the ileocecum in the different groups to reflect alteration of the immune barrier. We found that ileocecal sIgA was significantly reduced after LT in the two groups, which might be closely associated with the atrophy of gut-associated lymphoid tissue and the reduction of intestinal sIgA secretion following intestinal ischemia-reperfusion injury.[19]However, enteral supplementation with Gly-Gln increased the intestinal sIgA level after LT, which is consistent with the report that glutamine-enriched enteral nutrition maintains small intestine gut-associated lymphoid tissue and protects against intestinal mucosal barrier injury after LT.[20]
To further clarify the protective role of enteral supplementation with Gly-Gln, we measured the plasma levels of endotoxin and TNF-α in the portal vein as well as bacterial cultures for translocation and identification.
Inflammation plays a vital role in dysfunction of the intestinal barrier.[4]TNF-α is an important proinflammatory factor and can directly, or by inducing inflammatory cascades and promoting microvascular dysfunction of the intestine, aggravate intestinal injury.[4]Meanwhile, the measurement of plasma endotoxin also provided similar evidence. Thus, we concluded that enteral supplementation with Gly-Gln was helpful in reducing portal plasma endotoxin and TNF-α after LT in rats.
We performed the LT under sterile conditions and carried out bacterial culture and identification from liver tissue. We found that bacterial counts in postoperative rats were significantly increased and supplementation with Gly-Gln decreased the counts. We also noted that the reduction was mainly in the aerobic bacteria Escherichia coli, Enterococcus, Proteus vulgaris, Streptococcus agalactiae,Proteus mirabilis and Viridans streptococci. Thus, we concluded that enteral supplementation with Gly-Gln is helpful in reducing bacterial translocation after LT in rats, which is consistent with the result that enteral supplementation with Gly-Gln helps to improve intestinal mucosal protein metabolism, the mechanical barrier, and the immune barrier.
In conclusion, our study has revealed that enteral supplementation with Gly-Gln improves intestinal barrier function after LT in rats, and might have a vital positive impact on the recovery of patients after LT.
Acknowledgments
We are grateful to Hai-Shen Kong and Chao-Rong Ge for their help with the bacterial culture and identification. We are grateful to Xiao-Ying Sa, Xian-Fu Ke, Qiao-Juan Shi and Qi Lou in Zhejiang Academy of Medical Sciences for their advice in this experiment.
Funding:This study was supported by grants from the National Basic Research Program (973) of China (2007CB513005 and 2009CB522406) and by the Health Bureau Fund of Zhejiang Province (2008A050).
Ethical approval:The study was conducted according to the Guide for the Care and Use of Laboratory Animals (NIH publication No. 85-23, revised 1985).
Contributors:JJW, XHY, ZL and ZSS designed the research. JJW, RZG, CLY and JL performed the research. JJW and RZG analyzed the data and wrote the first draft. All authors contributed to the design and interpretation of the study and to further drafts. ZSS is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Ferreira LG, Anastácio LR, Lima AS, Correia MI. Assessment of nutritional status of patients waiting for liver transplantation. Clin Transplant 2011;25:248-254.
2 Sakai T, Matsusaki T, Marsh JW, Hilmi IA, Planinsic RM. Comparison of surgical methods in liver transplantation: retrohepatic caval resection with venovenous bypass (VVB) versus piggyback (PB) with VVB versus PB without VVB. Transpl Int 2010;23:1247-1258.
3 Puglisi F, Lacitigniola L, Loverre A, Capuano P, Martines G, Staffieri F, et al. Activation of PI3-kinase/Akt induced small bowel cell apoptosis during laparoscopic ischaemia-reperfusion of swine jejunum. Acta Chir Belg 2009;109:216-223.
4 Chiba Y, Shida K, Nagata S, Wada M, Bian L, Wang C, et al. Well-controlled proinflammatory cytokine responses of Peyer's patch cells to probiotic Lactobacillus casei. Immunology 2010;130:352-362.
5 van Zwol A, Neu J, van Elburg RM. Long-term effects of neonatal glutamine-enriched nutrition in very-low-birthweight infants. Nutr Rev 2011;69:2-8.
6 Fürst P. A thirty-year odyssey in nitrogen metabolism: from ammonium to dipeptides. JPEN J Parenter Enteral Nutr 2000;24:197-209.
7 Minami H, Morse EL, Adibi SA. Characteristics and mechanism of glutamine-dipeptide absorption in human intestine. Gastroenterology 1992;103:3-11.
8 Kamada N, Calne RY. A surgical experience with five hundred thirty liver transplants in the rat. Surgery 1983;93:64-69.
9 Xing HC, Li LJ, Xu KJ, Shen T, Chen YB, Sheng JF, et al. Protective role of supplement with foreign Bifidobacterium and Lactobacillus in experimental hepatic ischemiareperfusion injury. J Gastroenterol Hepatol 2006;21:647-656.
10 Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol 2009;124:3-22.
11 Fasano A, Shea-Donohue T. Mechanisms of disease: the role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases. Nat Clin Pract Gastroenterol Hepatol 2005;2:416-422.
12 Baumgart DC, Dignass AU. Intestinal barrier function. Curr Opin Clin Nutr Metab Care 2002;5:685-694.
13 Mondello S, Galuppo M, Mazzon E, Italiano D, Mondello P, Aloisi C, et al. Glutamine treatment attenuates the development of organ injury induced by zymosan administration in mice. Eur J Pharmacol 2011;658:28-40.
14 Panigrahi P, Gewolb IH, Bamford P, Horvath K. Role of glutamine in bacterial transcytosis and epithelial cell injury. JPEN J Parenter Enteral Nutr 1997;21:75-80.
15 Kim J, Burghardt RC, Wu G, Johnson GA, Spencer TE, Bazer FW. Select Nutrients in the Ovine Uterine Lumen. IX. Differential Effects of Arginine, Leucine, Glutamine, and Glucose on Interferon Tau, Ornithine Decarboxylase, and Nitric Oxide Synthase in the Ovine Conceptus. Biol Reprod 2011;84:1139-1147.
16 Jiang JW, Zheng SS, Xue F, Gao LH, Jiang GP, Xie HY. Enteral feeding of glycyl-glutamine dipeptide improves the structure and absorptive function of the small intestine after allogenetic liver transplantation in rats. Hepatobiliary Pancreat Dis Int 2006;5:199-204.
17 Li Y, Li J, Jiang J, Li N, Wang X, Wang Z, et al. Glycylglutamine-supplemented long-term total parenteral nutrition selectively improves structure and function in heterotopic small-bowel autotransplantation in the pig. Transpl Int 2003; 16:866-871.
18 Li Y, Yu Z, Liu F, Tan L, Wu B, Li J. Oral glutamine ameliorates chemotherapy-induced changes of intestinal permeability and does not interfere with the antitumor effect of chemotherapy in patients with breast cancer: a prospective randomized trial. Tumori 2006;92:396-401.
19 Fukatsu K, Sakamoto S, Hara E, Ueno C, Maeshima Y, Matsumoto I, et al. Gut ischemia-reperfusion affects gut mucosal immunity: a possible mechanism for infectious complications after severe surgical insults. Crit Care Med 2006;34:182-187.
20 Li Y, Chen Y, Zhang J, Zhu JF, Liu ZJ, Liang SY, et al. Protective effect of glutamine-enriched early enteral nutrition on intestinal mucosal barrier injury after liver transplantation in rats. Am J Surg 2010;199:35-42.
Received March 3, 2011
Accepted after revision June 10, 2011
Author Affiliations: Key Laboratory of Combined Multi-organ Transplantation, Ministry of Public Health; Department of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Jiang JW, Ren ZG, Chen LY, Jiang L, Xie HY, Zhouland Zheng SS)
Shu-Sen Zheng, MD, PhD, FACS, Key Laboratory of Combined Multi-organ Transplantation, Ministry of Public Health; Department of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Tel: 86-571-87236567; Fax: 86-571-87236884; Email: shusenzheng@ zju.edu.cn)
© 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Surgical treatment of Budd-Chiari syndrome: analysis of 221 cases
- Hepatitis C virus infection and biological falsepositive syphilis test: a single-center experience
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Success rate and complications of endoscopic extraction of common bile duct stones over 2 cm in diameter
- Donor liver natural killer cells alleviate liver allograft acute rejection in rats
- Noninvasive indocyanine green plasma disappearance rate predicts early complications, graft failure or death after liver transplantation
