Noninvasive indocyanine green plasma disappearance rate predicts early complications, graft failure or death after liver transplantation
2011-07-05LutzSchneiderMartinSpiegelSebastianLatanowiczMarkusWeigandJanSchmidtJensWernerWolfgangStremmelandChristophEisenbach
Lutz Schneider, Martin Spiegel, Sebastian Latanowicz, Markus A Weigand, Jan Schmidt, Jens Werner, Wolfgang Stremmel and Christoph Eisenbach
Heidelberg, Germany
Original Article / Transplantation
Noninvasive indocyanine green plasma disappearance rate predicts early complications, graft failure or death after liver transplantation
Lutz Schneider, Martin Spiegel, Sebastian Latanowicz, Markus A Weigand, Jan Schmidt, Jens Werner, Wolfgang Stremmel and Christoph Eisenbach
Heidelberg, Germany
BACKGROUND:Early detection of graft malfunction or postoperative complications is essential to save patients and organs after orthotopic liver transplantation (OLT). Predictive tests for graft dysfunction are needed to enable earlier implementation of organ-saving interventions following transplantation. This study was undertaken to assess the value of indocyanine green plasma disappearance rates (ICG-PDRs) for predicting postoperative complications, graft dysfunction, and patient survival following OLT.
METHODS:Eighty-six patients undergoing OLT were included in this single-centre trial. ICG-PDR was assessed daily for the first 7 days following OLT. Endpoints were graft loss or death within 30 days and postoperative complications, graft loss, or death within 30 days.
RESULTS:Postoperative complications of 31 patients included deaths (12 patients) or graft losses. ICG-PDR was significantly different in patients whose endpoints were graft loss or death beginning from day 3 and in those whose endpoints were graftloss, death, or postoperative complications beginning from day 4 after OLT. For day 7 measurements, receiver operating characteristic curve analysis revealed an ICG-PDR cut-off for predicting death or graft loss of 9.6% per min (a sensitivity of 75.0%, a specificity of 72.6%, positive predictive value 0.35, negative predictive value 0.94). For prediction of graft loss, death, or postoperative complications, the ICG-PDR cut-off was 12.3%per min (a sensitivity of 68.9%, a specificity of 66.7%, positive predictive value 0.57, negative predictive value 0.77).
CONCLUSIONS:ICG-PDR measurements on postoperative day 7 are predictive of early patient outcomes following OLT. The added value over that of routinely determined laboratory parameters is low.
(Hepatobiliary Pancreat Dis Int 2011; 10: 362-368)
indocyanine green; liver function; liver transplantation
Introduction
Improvements in surgical techniques, immunosuppression treatments, and management of postoperative complications have considerably enhanced survival rates following orthotopic liver transplantation (OLT). To date 1-year survival rates of patient and graft are as high as 90%.[1,2]However, early postoperative complications have a high impact on patient and graft survival. Postoperative hemorrhage, vascular dysfunction, anastomotic leakage, early graft rejection, and graft dysfunction are the main postoperative problems.[3-6]Despite intensive care for postoperative liver transplant patients, there is still a need for a diagnostic tool which can distinguish between patients who are likely to develop complications from those who are not. After the appearance of postoperative complications, early intervention including immunosuppressant therapy, reoperation, and endoscopic or radiological intervention is of great importance for organ rescue.
Liver function and hepatic blood flow can be evaluated by the clearance of the nontoxic compound indocyanine green (ICG).[7]ICG is eliminated exclusively by hepatocytes in the bile, without an enterohepatic cycle. The plasma disappearance rate of ICG (ICG-PDR) can bemeasured non-invasively by pulse-densitometry (LiMON, Pulsion Medical System, Munich, Germany).[8-11]ICGPDR is assessed and visualized using special monitors and correlates with graft function following liver transplantation.[12-16]
The present study aimed to determine whether daily noninvasive ICG-PDR measurements for the first seven days after OLT can predict graft survival, 30-day patient mortality, and postoperative complications which require intervention. Furthermore, we determined ideal time points for ICG-PDR measurements to reduce the number of measurements and costs.
Methods
This study was carried out according to the guidelines of the Declaration of Helsinki, relevant to the principles of good clinical practice. The study was an observational, prospective, single-centre study. The study protocol was approved by the Ethics Committee of the University of Heidelberg. Patients involving in the study signed informed consent forms before the study was initiated. The trial was registered at ClinicalTrails.gov under the identifying number NCT00408889.
Patients undergoing OLT at Heidelberg University from December 2006 to January 2009 were screened regardless of their reasons for transplantation. Patients were eligible if they were between 18 and 70 years of age, mentally healthy and able to give informed consent, had no known allergies to iodine or ICG-Pulsion® nor evidence of thyroid diseases. Patients who had measurements available through day 7 post-transplantation were included in the analysis. Transplantation was performed using sideto-side cavo-caval anastomosis and biliary anastomosis was made by either choledocho-choledochostomy or choledocho-jejunostomy, depending on the recipient diagnosis. Immunotherapy consisted of triple protocols using either cyclosporine or tacrolimus.
Endpoints
Primary endpoints were defined as listing for re-transplantation (graft loss) or death within 30 days following transplantation. Secondary endpoints were defined as the need for potentially organ-saving interventions (acute rejection treatment, surgical intervention, arteriography for anastomotic stenosis, or endoscopy for biliary anastomosis complications) within 30 days. For data analysis, two patient groups were defined: one consisting of patients meeting the primary endpoint and the other consisting of patients meeting the primary or secondary endpoints.
Experimental protocol
In the patients undergoing OLT, noninvasive ICG-PDR rates, APACHE II, Child-Pugh, SOFA, and MELD scores, and liver function parameters (aspartate aminotransferase, alanine aminotransferase, prothrombin time, gamma glutamyl transpeptidase, and bilirubin) were assessed immediately after operation and 7 days after operation. In this period, hepatic perfusion duplex ultrasound was performed daily.
ICG-PDR
ICG binds to plasma proteins, is taken up by hepatocytes from the plasma, and excretes to the bile.[17]Therefore, ICG clearance is a marker for hepatic perfusion and liver function.[7]Because the assay includes sodium iodide, its application must be avoided in patients with allergic reactions to iodine or thyroidal autonomy.
Pulse dye densitometry was performed using a noninvasive densitometer (LiMON Leberfunktionsmonitor, Pulsion Medical Systems AG, Munich, Germany). This instrument detects arterial ICG concentrations based on differences in absorbance between oxyhaemoglobin and ICG (wavelengths 905 nm and 805 nm, respectively). ICG-PDR was determined by monoexponential transformation of the original ICG concentration curve and backward extrapolation to the 'zero' time point (100%), followed by analyzing the decay as percentage change over time.
A 25 mg bolus of ICG (ICG-Pulsion®, Pulsion Medical AG, Munich, Germany) was injected through an intravenous catheter, followed immediately by flushing with physiological saline. A fixed dose was chosen to reduce complexity and thus there was a source of error in the determination of ICG-PDR. It was reported that ICG doses between 0.25 and 0.5 mg/kg BW showed accurate results in critically ill patients.[18]In our study, 5 patients weighed between 100 and 105 kg, and only 1 patient exceeded the target weight (118 kg).
Blood ICG concentrations were monitored via an optical probe attached to the patient's finger in this study. ICG-PDR is defined as the change in ICG concentration over time (recorded as percent change per minute) and reflects the percentage of initial dye eliminated.
Statistical analysis
A Mann-Whitney U test was used to compare baseline parameters and measurements of different groups of patients. Cut-off values were determined using the highest Youden index (sensitivity+specificity—1) retrieved from receiver operating characteristic (ROC) curves. All statistical computations were performed using SPSS forWindows, release 15.0 (SPSS, Chicago, IL). A P value of less than 0.05 was considered statistically significant.
Results
The study group comprised 86 patients with liver failure who had undergone OLT between December 2006 and January 2009. Among them 59 patients were male and 27 female; their median age was 52 years (range 19-66 years). The transplantation was due to liver failure caused by viral hepatitis in 31 patients, alcohol abuse in 25, cryptogenic cirrhosis in 7, biliary disease in 6, metabolic liver disease in 4, acute liver failure in 3, and others in 10 (Wilson's disease, metastasis, autoimmune hepatitis, amyloidosis and liver abscess). Nineteen patients were operated upon for hepatocellular carcinoma. Endpoints, defined as death, graft loss or postoperative complications, were reached by 31 patients overall (36%): primary endpoints of death or graft loss occurred in 12 patients (14%) and secondary endpoints of postoperative complications with need of intervention in 19 (22%) (Table 1).
Patients who had death or graft loss within 30 days (primary endpoint group) did so after a median of 11 days (range 8-30) (n=12). Because of the low number of patients in this group, sub-group analysis was not performed (Table 2). In the postoperative complications group (secondary endpoint group), an additional operation was performed in 8 patients, of whom 7 were due to hemorrhage and 1 was due to suspected biliary leakage. Acute rejection episodes were detected and treated by steroid bolus in 2 patients (BANFF-scores of 5 and 6). Three angiographic interventions were required because of the narrowing of arterial anastomosis. Endoscopic retrograde cholangiography was performed in 7 patients postoperatively for the treatment of suspected biliary anastomotic leakage (2 patients) and the narrowing of bile duct anastomosis (5). One of these patients required both angiographic and endoscopic intervention. These interventions were most commonly performed in patients with alcoholic cirrhosis.
Duplex ultrasound was performed daily for all patients in the observation period. Hepatic enzymes in serum were analyzed 3 times per day. ICG-PDR was assessed once a day for 7 days. During the study period, no adverse events attributable to ICG application were noted. After postoperative day 3, a significant difference in ICG-PDR was observed in the primary endpoint group (who had graft loss or died) compared to other groups (Fig. 1). In addition, ICG-PDR was significantly different from postoperative day 4 onward in patients with complications and graft loss, or in deaths (both primary and secondary endpoint groups) compared to those with an eventless postoperative recovery (Fig. 2). This difference in ICG-PDR between the groups was increased on postoperative day 7 (Figs. 1 and 2).
Using ROC curve analysis on the ICG-PDR measurements recorded on postoperative day 7 following OLT, the best ICG-PDR cut-off for the prediction of graft loss or death within 30 days was determined to be 9.6%per min with a sensitivity of 75.0% and a specificity of 72.6% (Fig. 3A). For prediction of graft loss, death or postoperative complications requiring intervention within 30 days, the cut-off was determined to be 12.3% per min with a sensitivity of 68.9% and a specificity of 66.7% (Fig. 3B). Although the positive predictive values were somewhat low (0.35 for prediction of graft loss or death and 0.57 for prediction of graft loss, death or postoperative complications requiring intervention), the negative predictive values were high (0.94 and 0.77, respectively).
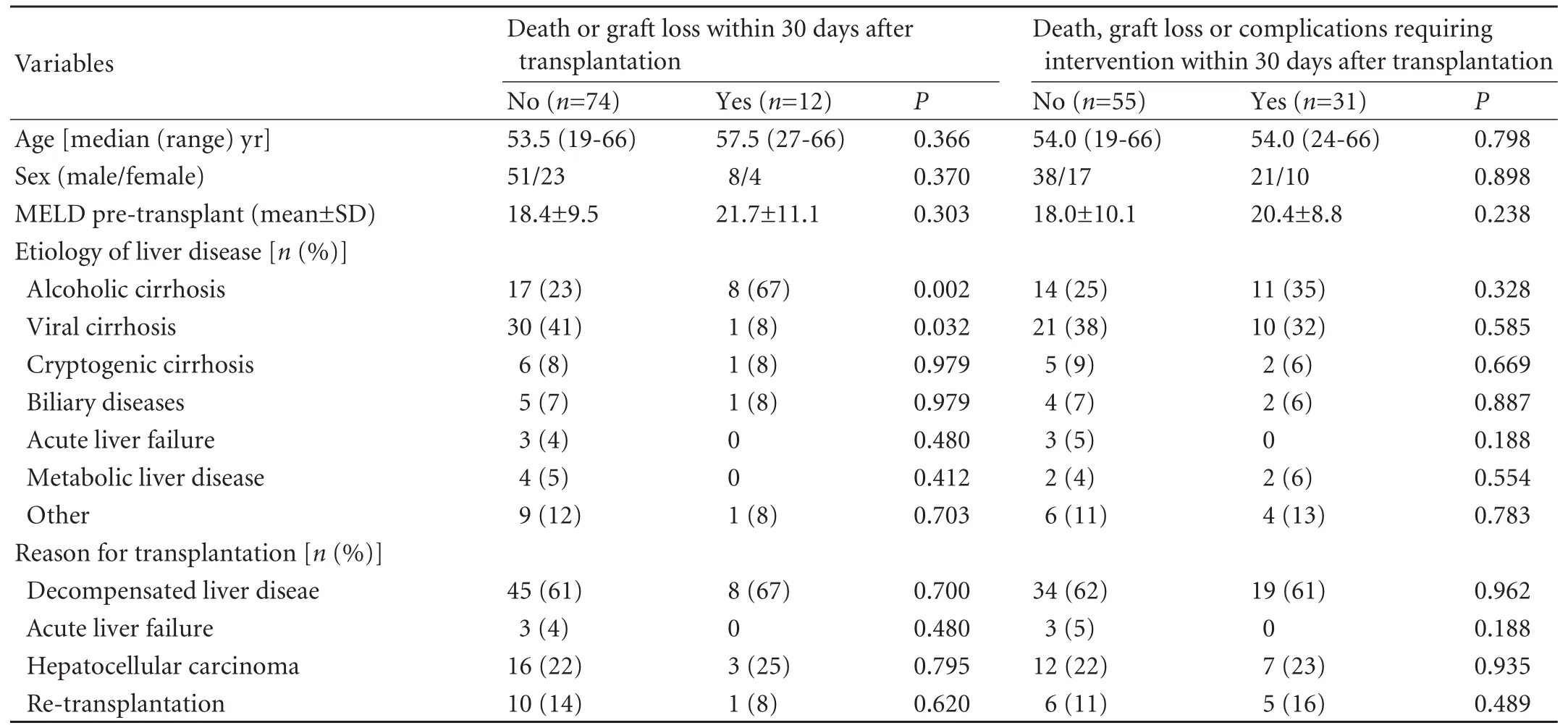
Table 1. Baseline characteristics
Over time, the predictive value of ICG-PDR increased through postoperative day 7. For comparison, the predictive value of the routinely determined laboratory parameters, bilirubin, aspartate aminotransferase and international normalized ratio, was calculated and found to be only minimally inferior to the determination of ICG-PDR (Table 3).

Fig. 1. Sequential changes in indocyanine green plasma disappearance rates (ICG-PDR). : group of patient and graft survival for more than 30 days (n=74), : group of death or graft loss within 30 days (n=12). Data are expressed as mean± SEM. *: P≤0.05; #: P≤0.01.

Fig. 2.Sequential changes in indocyanine green plasma disappearance rates (ICG-PDR). : group of patient and graft survival without complications requiring intervention for more than 30 days (n=55), : group of death, graft loss or complications requiring intervention within 30 days (n=31). Data are expressed as mean±SEM. *:P≤0.05; #:P≤0.01.
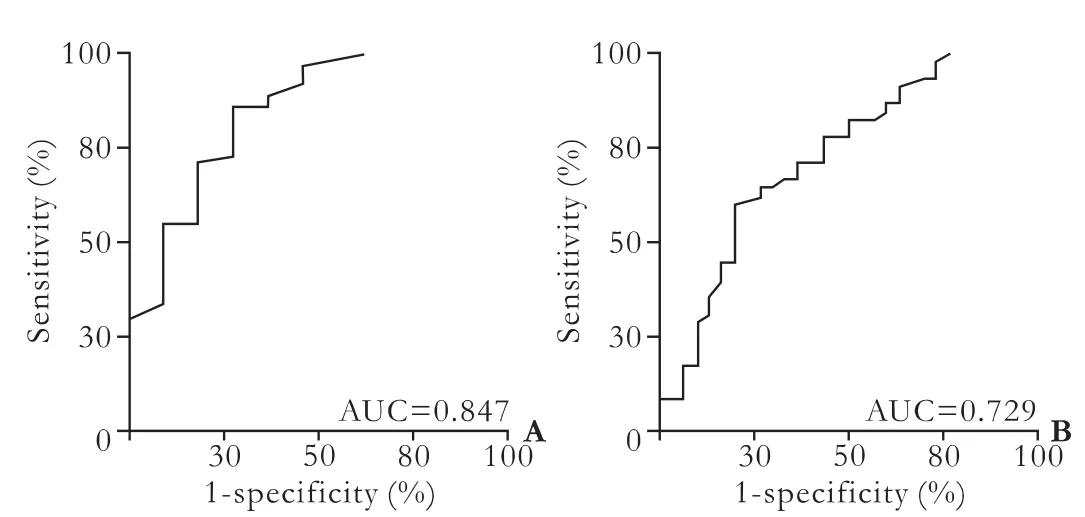
Fig. 3. Receiver operating characteristic (ROC) curves for the prediction of outcomes following liver transplantation. ICG-PDR measurements on postoperative day 7 after OLT were analysed. A: Analysis for the prediction of death or graft loss within 30 days. The area under the curve is 0.847 (95% CI, 0.712-0.982); B: Analysis for the prediction of death or graft loss or complications requiring intervention within 30 days. The area under the curve is 0.729 (95% CI, 0.608-0.850).
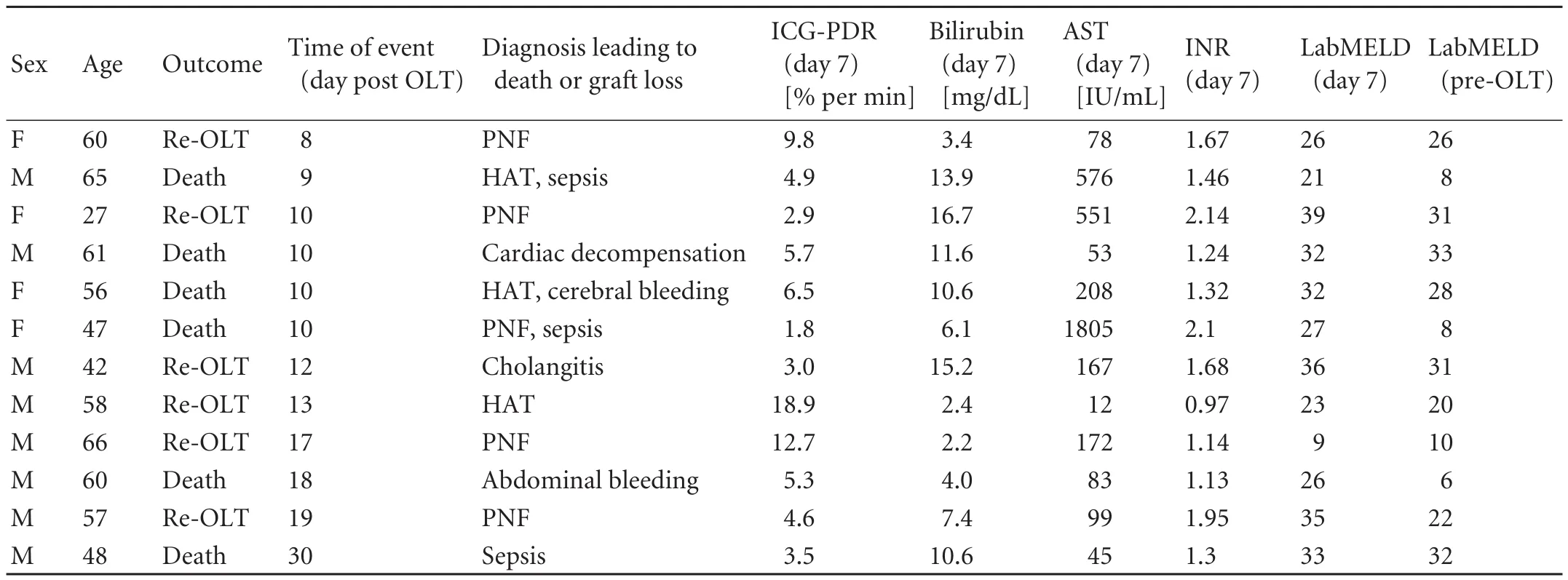
Table 2. Details of patients meeting the primary endpoint (death or graft loss within 30 days after transplantation)
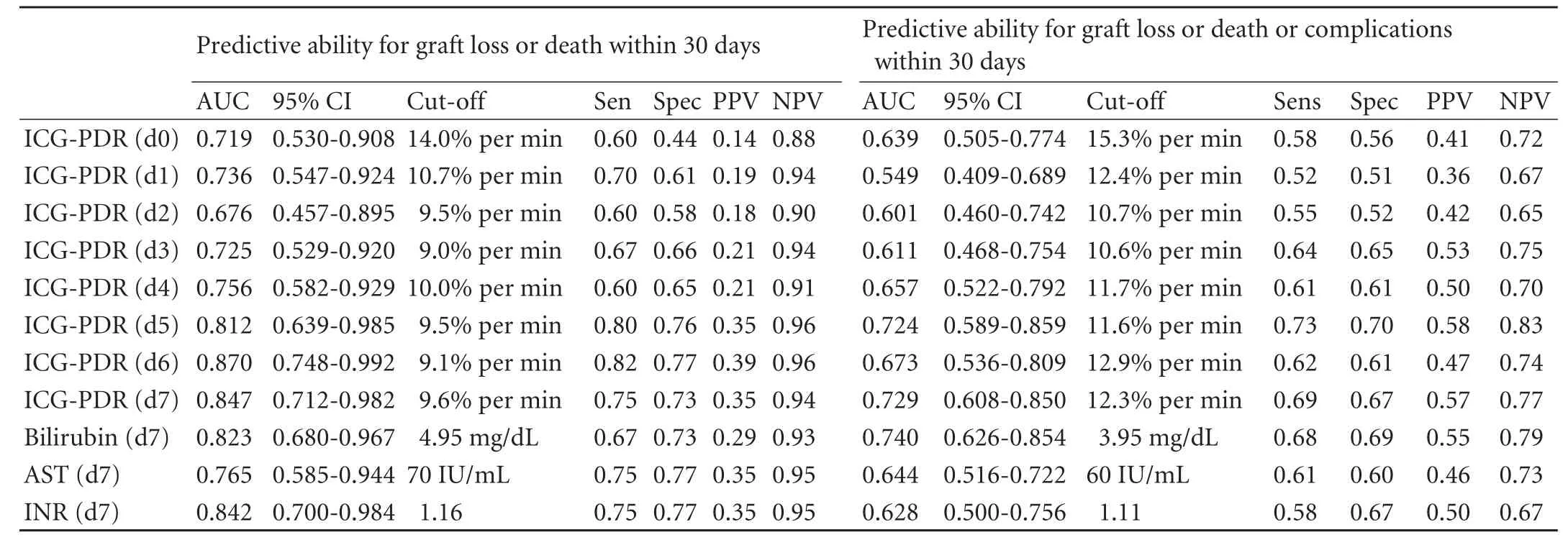
Table 3. Ability of different parameters and ICG-PDR over time to predict graft loss or death or graft loss, death or complications
Discussion
The present study was designed to prospectively evaluate the predictive value of ICG-PDR measurements in the early postoperative phase following OLT, to predict 30-day patient or graft survival and/or postoperative complications. In addition, we attempted to determine the ideal time-point for ICG-PDR measurements within the first postoperative week.
No selection was applied to our cohort of patients with respect to the cause of hepatic failure requiring OLT. All patients were screened and patient characteristics in the final cohort are listed in Table 1. Approximately one third required OLT because of alcoholic or viral cirrhosis. This is in accordance with published data from Mehrabi et al,[19]who reviewed 500 liver transplantations performed at our center, and found the same overall distribution of diagnoses leading to hepatic insufficiency. Thus there was no selection bias in this study.
Reduced ICG-PDR is known to be a marker of hepatic dysfunction. Nevertheless, it is not specific for the underlying cause. ICG-PDR measurements depend only on hepatosplanchnic blood flow and bile secretion, and can be altered by any causative factor changing these variables. For example, Kimura et al[20]associated reduced ICG clearance rates with fatal patient outcomes in sepsis. This correlation was due to reduced hepatosplanchnic blood flow, independent of cardiac output and adequate fluid management.[21,22]
In this study, we have demonstrated the usefulness of ICG-PDR measurements from postoperative day 4 onward for the identification of patients likely to suffer from postoperative complications, death, or graft loss, with significant differences in ICG-PDR values in the event-free group compared to patients with the above postoperative complications. Nevertheless, the sensitivity and specificity of the test was only 68.9% and 66.7%, respectively. ROC curve analysis defined a cut-off level of 12.3% per min and correlates well with data reported by Levesque et al.[23]However, although we were unable to reproduce the constant difference between the group reaching an endpoint and patients with no postoperative complications from day 0 onward, our data do show significant differences between the two groups from postoperative day 4 onward following liver transplantation.[23]Another study found a cut-off of around 10% per min to be predictive of severe graft dysfunction.[24]In that study, measurements as early as one hour post-reperfusion and within the first 24 hours were highly predictive. This very early predictability could not be confirmed by our data.
In our study cohort, we were unable to differentiate separate cut-off levels for different postoperative complications. However, this was not evaluated because the cohort reaching these different endpoints was too small for analysis. Nevertheless, we tried to differentiate between patients reaching any endpoint (i.e. patients suffering from postoperative complications, graft loss or death) from those reaching the primary endpoint (i.e. patients suffering from graft loss and/or death). We were able to demonstrate that graft loss or death is associated with an earlier decrease in ICG-PDR. We also determined a lower cut-off value of 9.6% per min for patients suffering from graft loss or death, comparedto 12.3% per min when postoperative complications were included. The sensitivity and specificity of these groups was comparable but low. This finding suggests that, regardless of the cut-off level, a lower ICG-PDR value is associated with more severe hepatic injury. We cannot rule out that the death process itself may have an impact on ICG-PDR measurements. However, as values were low in all patients meeting endpoints and not only in patients who died, we believe that ICG-PDR values mainly depended on graft function.
Early detection of postoperative complications following liver transplantation is essential to give healthcare workers an opportunity to save the organ, and relies on accurate and fast diagnosis and intervention. The sensitivity and specificity of ICGPDR measurements is not high enough to be used as a stand-alone parameter for the identification of early graft failure or postoperative complications. However, the high negative predictive values (0.94 and 0.77, respectively) reliably allow for the identification of patients unlikely to develop an adverse outcome. In conjunction with other clinical parameters, ICG-PDR may help to determine the prognosis and the ICGPDR test may be used as an additional criterion for transferring patients from the ICU to the peripheral ward. This approach would allow for very close surveillance and mandatory clinical workup in patients with ICG-PDR values predictive of an adverse outcome and may prevent late detection of complications and organ loss in the advanced hospitalization period.
Our study is limited by the size of the study population and thus the low number of patients having adverse events and meeting the primary endpoint. If a more homogenous endpoint (e.g. severe graft dysfunction or vascular complications) could be defined and tested in a larger cohort, diagnostic accuracy may have been higher. Also, a test yielding predictive results as early as possible following transplantation would be desirable. However, based on the data obtained in the present study we conclude that very early utilization of ICG-PDR for prediction of outcome is not feasible.
In conclusion, we have shown that ICG-PDR values measured on day 7 after transplantation are predictive of graft loss, death or serious complications within 30 days. The added value over that of routinely determined laboratory parameters is low.
Funding:This study was supported in part by a grant from the Else Kröner-Fresenius-Stiftung to Christoph Eisenbach (EKFS A 72/06). Pulsion Medical Systems AG, Munich, Germany, provided the LiMON-Monitor, but did not participate in the design or performance of this study, nor the analysis or interpretation of the data.
Ethical approval:This study was approved by the institutional Review Board of the University of Heidelberg, Germany.
Contributors:SL, WMA and EC proposed the study. SM and LS acquired the data. LS and EC wrote the first draft. SJ, WJ and SW revised it critically for important intellectual content. All authors contributed to the design and interpretation of the study and to further drafts. EC is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation 1988;45:673-676.
2 Starzl TE, Demetris AJ, Van Thiel D. Liver transplantation (1). N Engl J Med 1989;321:1014-1022.
3 Bilbao I, Armadans L, Lazaro JL, Hidalgo E, Castells L, Margarit C. Predictive factors for early mortality following liver transplantation. Clin Transplant 2003;17:401-411.
4 González FX, Rimola A, Grande L, Antolin M, Garcia-Valdecasas JC, Fuster J, et al. Predictive factors of early postoperative graft function in human liver transplantation. Hepatology 1994;20:565-573.
5 Ploeg RJ, D'Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, et al. Risk factors for primary dysfunction after liver transplantation--a multivariate analysis. Transplantation 1993;55:807-813.
6 Chen H, Peng CH, Shen BY, Deng XX, Shen C, Xie JJ, et al. Multi-factor analysis of initial poor graft function after orthotopic liver transplantation. Hepatobiliary Pancreat Dis Int 2007;6:141-146.
7 Caesar J, Shaldon S, Chiandussi L, Guevara L, Sherlock S. The use of indocyanine green in the measurement of hepatic blood flow and as a test of hepatic function. Clin Sci 1961;21: 43-57.
8 Faybik P, Krenn CG, Baker A, Lahner D, Berlakovich G, Steltzer H, et al. Comparison of invasive and noninvasive measurement of plasma disappearance rate of indocyanine green in patients undergoing liver transplantation: a prospective investigator-blinded study. Liver Transpl 2004; 10:1060-1064.
9 Hsieh CB, Chen CJ, Chen TW, Yu JC, Shen KL, Chang TM, et al. Accuracy of indocyanine green pulse spectrophotometry clearance test for liver function prediction in transplanted patients. World J Gastroenterol 2004;10:2394-2396.
10 Sakka SG, Reinhart K, Meier-Hellmann A. Comparison of invasive and noninvasive measurements of indocyanine green plasma disappearance rate in critically ill patients with mechanical ventilation and stable hemodynamics. Intensive Care Med 2000;26:1553-1556.
11 von Spiegel T, Scholz M, Wietasch G, Hering R, Allen SJ, Wood P, et al. Perioperative monitoring of indocyanine green clearance and plasma disappearance rate in patients undergoing liver transplantation. Anaesthesist 2002;51:359-366.
12 Hori T, Iida T, Yagi S, Taniguchi K, Yamamoto C, Mizuno S, et al. K(ICG) value, a reliable real-time estimator of graft function, accurately predicts outcomes in adult living-donor liver transplantation. Liver Transpl 2006;12:605-613.
13 Jalan R, Plevris JN, Jalan AR, Finlayson ND, Hayes PC. A pilot study of indocyanine green clearance as an early predictor of graft function. Transplantation 1994;58:196-200.
14 Tsubono T, Todo S, Jabbour N, Mizoe A, Warty V, Demetris AJ, et al. Indocyanine green elimination test in orthotopic liver recipients. Hepatology 1996;24:1165-1171.
15 Yamanaka N, Okamoto E, Kato T, Fujihara S, Sasase S, Oriyama T, et al. Usefulness of monitoring the ICG retention rate as an early indicator of allograft function in liver transplantation. Transplant Proc 1992;24:1614-1617.
16 Krenn CG, Schafer B, Berlakovich GA, Steininger R, Steltzer H, Spiss CK. Detection of graft nonfunction after liver transplantation by assessment of indocyanine green kinetics. Anesth Analg 1998;87:34-36.
17 Wheeler HO, Cranston WI, Meltzer JI. Hepatic uptake and biliary excretion of indocyanine green in the dog. Proc Soc Exp Biol Med 1958;99:11-14.
18 Sakka SG, Koeck H, Meier-Hellmann A. Measurement of indocyanine green plasma disappearance rate by two different dosages. Intensive Care Med 2004;30:506-509.
19 Mehrabi A, Mood ZA, Fonouni H, KashfiA, Hillebrand N, Müller SA, et al. A single-center experience of 500 liver transplants using the modified piggyback technique by Belghiti. Liver Transpl 2009;15:466-474.
20 Kimura S, Yoshioka T, Shibuya M, Sakano T, Tanaka R, Matsuyama S. Indocyanine green elimination rate detects hepatocellular dysfunction early in septic shock and correlates with survival. Crit Care Med 2001;29:1159-1163.
21 Fink MP, Cohn SM, Lee PC, Rothschild HR, Deniz YF, Wang H, et al. Effect of lipopolysaccharide on intestinal intramucosal hydrogen ion concentration in pigs: evidence of gut ischemia in a normodynamic model of septic shock. Crit Care Med 1989;17:641-646.
22 Navaratnam RL, Morris SE, Traber DL, Flynn J, Woodson L, Linares H, et al. Endotoxin (LPS) increases mesenteric vascular resistance (MVR) and bacterial translocation (BT). J Trauma 1990;30:1104-1115.
23 Levesque E, Saliba F, Benhamida S, Ichaï P, Azoulay D, Adam R, et al. Plasma disappearance rate of indocyanine green: a tool to evaluate early graft outcome after liver transplantation. Liver Transpl 2009;15:1358-1364.
24 Olmedilla L, Pérez-Pena JM, Ripoll C, Garutti I, de Diego R, Salcedo M, et al. Early noninvasive measurement of the indocyanine green plasma disappearance rate accurately predicts early graft dysfunction and mortality after deceased donor liver transplantation. Liver Transpl 2009;15:1247-1253.
Received February 11, 2011
Accepted after revision May 25, 2011
Author Affiliations: Department of General Surgery (Schneider L, Schmidt J and Werner J); Department of Anaesthesiology (Weigand MA), University of Heidelberg, Im Neuenheimer Feld 110, Heidelberg 69110, Germany; Department of Gastroenterology, University of Heidelberg, Im Neuenheimer Feld 410, Heidelberg 69120, Germany (Spiegel M, Latanowicz S, Stremmel W and Eisenbach C)
Present address: Department of Anaesthesiology, University of Giessen, Rudolf-Buchheim-Str. 7, 35392 Giessen, Germany (Weigand MA)
Christoph Eisenbach, MD, Department of Gastroenterology, University of Heidelberg, Im Neuenheimer Feld 410, Heidelberg 69120, Germany (Tel: 49-6221-5638849; Fax: 49-6221-566858; Email: Christoph_Eisenbach@med.uni-heidelberg.de)
© 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Prognostic role of diabetes mellitus in hepatocellular carcinoma patients after curative treatments: a meta-analysis
- Risk factors of severe ischemic biliary complications after liver transplantation
- Surgical treatment of Budd-Chiari syndrome: analysis of 221 cases
- Efficacy of liver transplantation for acute hepatic failure: a single-center experience
- Combined invagination and duct-to-mucosa techniques with modifications: a new method of pancreaticojejunal anastomosis
- Large regenerative nodules in a patient with Budd-Chiari syndrome after TIPS positioning while on the liver transplantation list diagnosed by Gd-EOB-DTPA MRI
