Protective effects of Ligustrazine, Kakonein and Panax Notoginsenoside on the small intestine and immune organs of rats with severe acute pancreatitis
2011-07-03XiPingZhangJunJiangQiHuiChengQianYeWeiJuanLiHuaZhuandJunYaShen
Xi-Ping Zhang, Jun Jiang, Qi-Hui Cheng, Qian Ye, Wei-Juan Li, Hua Zhu and Jun-Ya Shen
Hangzhou, China
Original Article / Pancreas
Protective effects of Ligustrazine, Kakonein and Panax Notoginsenoside on the small intestine and immune organs of rats with severe acute pancreatitis
Xi-Ping Zhang, Jun Jiang, Qi-Hui Cheng, Qian Ye, Wei-Juan Li, Hua Zhu and Jun-Ya Shen
Hangzhou, China
BACKGROUND:Severe acute pancreatitis (SAP) is characterized by fatal pathogenic conditions and a high mortality. It is important to study SAP complicated with multiple organ injury. In this study we compared the protective effects of three traditional Chinese medicines (Ligustrazine,KakoneinandPanax Notoginsenoside) on the small intestine and immune organs (thymus, spleen and lymph nodes) of rats with SAP and explored their mechanism of action.
METHODS:One hundred forty-four rats with SAP were randomly divided into model control,Ligustrazine-treated,Kakonein-treated, andPanax Notoginsenoside-treated groups (n=36 per group). Another 36 normal rats comprised the shamoperated group. According to the different time points after operation, the experimental rats in each group were subdivided into 3-, 6- and 12-hour subgroups (n=12). At various time points after operation, the mortality rate of rats and pathological changes in the small intestine and immune organs were recorded and the serum amylase levels were measured.
RESULTS:Compared to the model control groups, the mortality rates in all treated groups declined and the pathological changes in the small intestine and immune tissues were relieved to different degrees. The serum amylase levels in the three treated groups were significantly lower than those in the model control group at 12 hours. The pathological severity scores for the small intestinal mucosa, thymus and spleen (at 3 and 12 hours) in theLigustrazine-treated group, for the thymus (at 3 and 12 hours) and spleen (at 3 and 6 hours) in theKakonein-treated group, and for the thymus (at 3 hours)and spleen (at 3 hours) in thePanax Notoginsenoside-treated group were significantly lower than those in the model control group. The pathological severity scores of the small intestinal mucosa (at 6 and 12 hours) and thymus (at 6 hours) in theLigustrazine-treated group were significantly lower than those in theKakonein- andPanax Notoginsenoside-treated groups.
CONCLUSIONS:All the three traditional Chinese drugs significantly alleviated the pathological changes in the small intestine and immune organs of SAP rats.Ligustrazinewas the most effective one among them.
(Hepatobiliary Pancreat Dis Int 2011; 10: 632-637)
severe acute pancreatitis; traditional Chinese medicine; small intestine; multiple organs; apoptosis
Introduction
Severe acute pancreatitis (SAP) is a systemic disease mainly characterized by pancreatic self-necrosis, and can induce multiple organ dysfunction in severe cases.[1,2]SAP is also characterized by dangerous onset, many complications and a high mortality rate.[3-5]Since the gastrointestinal tract and the pancreas belong to the digestive system and are adjacent, the intestine is more vulnerable to injury when multiple organ dysfunction develops.[6]In turn, SAP-induced intestinal mucosal injury is one of the major contributing factors to aggravation of the illness.[7]With advances in research, the impairment of immune function in SAP patients has gradually attracted the attention of researchers.[8,9]In SAP patients, the pancreas and others develop severe microcirculatory disturbance,[10]which is a trigger factor for the progression of SAP.[11]Indispensable measures for a comprehensive therapy for SAP are to modulate immune function and improvemicrocirculation. In the auxiliary treatment of SAP, traditional Chinese medicine has the advantages of low cost, extensive pharmacological effects, few sideeffects and definite efficacy. Ligustrazine, Kakonein and Panax Notoginseng saponins resist lipid oxidation, enhance oxygen free radical-scavenging ability, inhibit platelet aggregation and improve microcirculation.[12-17]Clinical and animal experiments have shown that both Ligustrazine and Panax Notoginseng saponins can achieve good efficacy in the treatment of SAP by improving microcirculation.[18,19]It has also been reported that Kakonein protects the functions of vascular endothelium in rats with acute pancreatitis.[18]Moreover, treatment of SAP patients with Kakonein increase the clinical cure rate by more than 10%.[20]However, no studies in China or overseas on the protective effects and mechanisms of action of these three traditional Chinese medicines on the small intestinal mucosa and immune organs of SAP rats have been reported. For this reason, this study was designed to provide an experimental basis for the clinical application of these medicines.
Methods
Healthy male Sprague-Dawley rats of clean grade, weighing 250-300 g, were provided by the Laboratory Animal Research Center of Zhejiang Chinese Medical University (China). Sodium taurocholate and sodium pentobarbital were from Sigma (Sigma-Aldrich, St. Louis, MO, USA); the serum amylase testing kit was from Jingmei Bioengineering Corp., China (units, IU/mL); Ligustrazine hydrochloride injection (each 2 mL ampoule contained active components equivalent to 40 mg) was from Wuxi No. 7 Pharmaceutical Co. Ltd., China; Puerarin powder (0.2 g per-ramus) was from Zhenyuan Pharmaceutical Co. Ltd., China; Panax Notoginsenosides injection (main component, Panax Notoginsenosides; each 5 mL ampoule contained active components equivalent to 250 mg) was from Xingzhong Pharmaceutical Co. Ltd., Kunming, China.
Animal grouping
One hundred eighty rats were randomly divided into sham-operated, model control, Ligustrazine-treated, Kakonein-treated and Panax Notoginsenoside-treated groups (n=36), which were further randomly subdivided into 3-, 6-, and 12-hour groups (n=12).
Preparation of SAP models
The rats were anesthetized by an intraperitoneal injection of 2.5% sodium pentobarbital (0.2 mL/100 g). First, a femoral vein was cannulated, and a microinfusion pump was used to continuously transfuse physiological saline (1 mL/h per 100 g). Then 3.5% sodium taurocholate was used to prepare the SAP model by retrograde injection into the pancreatic duct through an epidural catheter in the duodenal papilla. In the sham-operated groups the pancreas and duodenum were just moved after opening the abdominal cavity.
Therapeutic regimen
Ten minutes after successful operation, a single dose of the corresponding drug was given via the femoral vein, and then continued through the microinfusion pump. In the Ligustrazine-treated group, a single dose of 1 mL/kg body weight Ligustrazine was injected into the vein (20 mg/mL active components), and then continuously infused via the microinjection pump at 10 mL/kg per hour (5 mg/mL active components).[13,21]In the Kakonein-treated group, a single dose of 1 mL/kg body weight Kakonein was injected into the vein (3 mg/mL active components), and then continuously infused via microinjection pump at 10 mL/kg per hour (1 mg/mL active components).[22,23]In the Panax Notoginsenoside-treated group, a single dose of 1 mL/100 g body weight physiological saline solution was injected into the vein, and then Panax Notoginsenoside was continuously infused via the microinjection pump at 10 mL/kg per hour (1 mg/mL active components).[24,25]
Collection of specimens
At the corresponding time points after operation, experimental rats were anesthetized with 2.5% sodium pentobarbital and sacrificed. Blood samples, small intestine, spleen, thymus and lymph nodes were then collected.
Statistical analysis
The statistical analysis was conducted with SPSS 11.5. The Kruskal-Wallis and Mann-Whitney U tests were performed for comparisons among the five groups. Yates' correction for continuity and the Chi-square test were used for intergroup comparisons of mortality rates and the associations between all indices at different time points were analyzed. P<0.05 was considered statistically significant.
Results
Mortality rates
The sham-operated group survived at all time points. The mortality rates of the model control group were 8% (1/12), 42% (5/12), and 50% (6/12) at 3, 6 and 12hours, respectively. They were 0, 0, and 25% (3/12) in the Ligustrazine-treated group, 0, 0, and 25% (3/12) in theKakonein-treated group, and 0, 0, and 33% (4/12) in the Panax Notoginsenoside-treated group at 3, 6 and 12 hours, respectively. At 12 hours, the mortality rate was higher in the model control group than in the shamoperated and all treated groups (P<0.005 or P<0.001). No difference was noted among the treated groups.
Serum amylase levels
The serum amylase levels in the model control and treated groups were higher than those in the shamoperated group at all time points (P<0.01). The levels in the three treated groups were lower than those in the model control group at 12 hours (P<0.05). There was no difference among the treated groups at all time points (Table 1).
Pathological severity scores in small intestine and immune organs
The criteria for the pathological severity scores for multiple organs are described in our previous reports,[26-28]except for the spleen. The criteria for the spleen were: 0, normal structure; 1, necrosis in the follicular center; 2, blood sinus expansion and arteriolosclerosis; 3, necrosis in the follicular center, blood sinus expansion and arteriolosclerosis. In the sham-operated groups at all time points, the scores for small intestine, thymus and spleen were lower than those in the model control groups (P<0.01), and the scores for thymus and lymph nodes were lower than those in the treated groups (P<0.01). The scores for the small intestine (at all time points), thymus (at all time points) and spleen (at 3 and 12 hours) in the Ligustrazine-treated groups were lower than those in the model control groups (P<0.05). The scores for the thymus (at 3 and 12 hours) and spleen (at 3 and 6 hours) in the Kakonein-treated group were lower than those in the model control groups (P<0.05). The scores for the thymus (at 3 hours) and spleen (at 3 hours) in the Panax Notoginsenoside-treated groups were lower than those in the model control groups (P<0.05). At 6 and 12 hours, the scores for the small intestine and thymus in the Ligustrazine-treated groups were lower than those in the Kakonein-treated groups (P<0.05). The scores for the small intestine (at all time points), thymus (at 6 hours) and spleen (at 12 hours) were lower than those in thePanaxNotoginsenoside-treated groups (P<0.05). The scores forthe small intestine (at 3 hours) in the Kakonein-treated group was lower than those in the Panax Notoginsenosidetreated group (P<0.05) (Table 2 and Figs. 1 and 2).
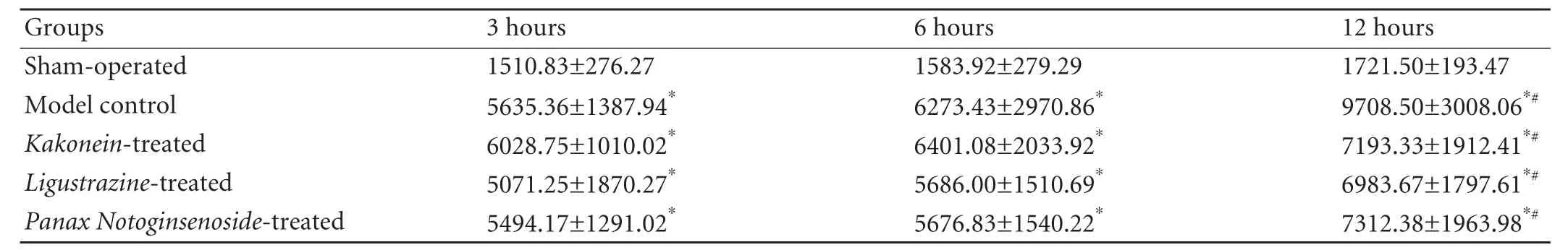
Table 1. Serum amylase levels at different time points (IU/mL, mean±SD)
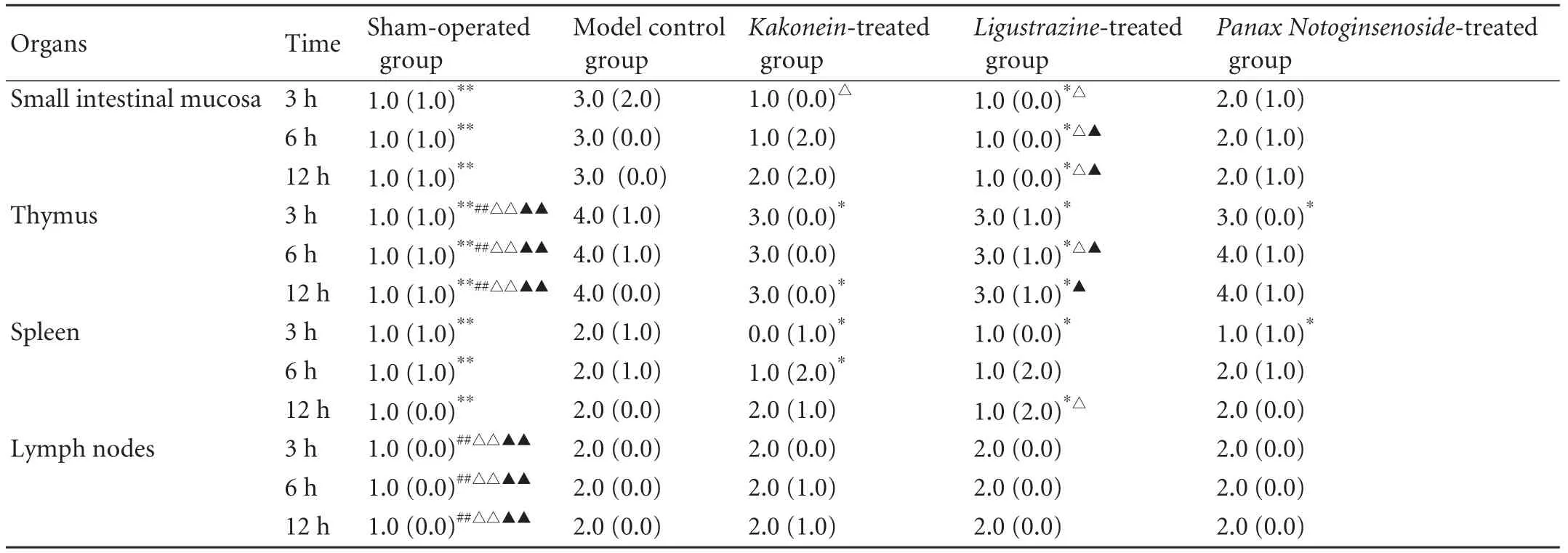
Table 2. Pathological severity scores (M(QR))
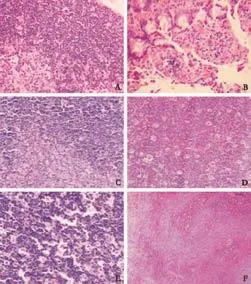
Fig. 1. Pathological changes in sham-operated and model control groups (HE, A-E, original magnification ×200; F, original magnification ×100). A: Sham-operated group at 12 hours, normal thymus tissue. B-F: In the model control group, shedding of some small intestinal epithelial cells, increased inflammatory cells in the proper layer and vascular congestion at 12 hours (B). Starry sky-like changes in thymic cortical epithelial cells and vacuolelike changes in the nuclei of medullary epithelial cells at 6 hours (C). Patchy necrosis of lymphocytes in the thymic cortex at 12 hours (D). Enlargement and spotty necrosis of germinal centers in lymph nodes, expansion of lymph node sinuses, severe hyperplasia of sinus cells and infiltration of a few neutrophils in lymph node sinuses at 12 hours (E). Spotty necrosis in the germinal centers of lymphoid follicles of the spleen as well as expansion, congestion and local hemorrhage of red pulp sinus at 12 hours (F).
Discussion
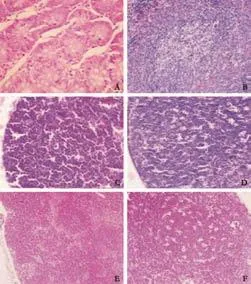
Fig. 2. Pathological changes in three traditional Chinese drug groups (HE, original magnification ×200). In the Ligustrazinetreated group (at 12 hours), normal small intestinal mucosa (A), focal necrosis in lymph nodule of spleen (B). In the Kakoneintreated group (at 12 hours), necrosis of lymphocytes in the thymic cortex (C), enlargement and spotty necrosis of germinal centers in lymph nodes, expansion of lymph node sinuses and hyperplasia of sinus cells (D). In the Panax Notoginsenoside-treated group, enlargement of germinal centers in lymph nodes, expansion of lymph node sinuses and hyperplasia of sinus cells at 6 hours (E), enlargement and spotty necrosis of germinal centers in lymph nodes at 12 hours (F).
When SAP develops, premature activation of pancreatic enzymes in the acini and an excessive inflammatory response induce the production of large numbers of cytokines and vasoactive substances, which directly or indirectly result in decreased local blood flow, leukocyte adhesion and increased capillary permeability. Leukocyteendothelium, platelet-endothelium and platelet-leukocyte interactions and vascular thrombosis lead to microcirculatory disturbance,[29-32]thereby inducing pathological changes in multiple organs.[33-35]Microcirculation disturbance-induced ischemia, hypoxia and ischemiareperfusion injury play important roles in the development of extrapancreatic organ injury in SAP. Through improving the microcirculation of the kidney, liver and lung, the pathological changes in these organs are significantly mitigated and the mortality rates in experimental animals are reduced.[36-39]The results of our study indicated that the small intestine and immune organs of rats in the model control group showed varying degrees of pathological damage and the pathological changes in the small intestinal mucosa, spleen and thymus were aggravated and increased with time. Interlobular and interacinar edema, acinar necrosis and inflammatory cell infiltration were also found in the model control group, while the pancreas of a few rats showed hemorrhage and fatty necrosis. However, in the sham-operated group, the pancreas was normal in themajority. Focal edema and infiltration of small numbers of inflammatory cells were seen in the pancreas of a few rats (data submitted for publication). The pathological changes in multiple organs and the changes in serum amylase levels in the different groups demonstrated that the method of SAP model preparation was successful.
We speculate that the results of this study are due to impairment of the intestinal mucosal barrier in SAP, which enables endotoxin to enter other tissues via blood and lymphatic vessels and thereby gives rise to varying degrees of pathological damage to the small intestinal mucosa, spleen and thymus. The impairment of intestinal immunity and barrier function can further result in small intestinal mucosal injury and increased permeability, thus forming a vicious cycle.[40]In this study, no significant differences in the pathological severity scores in lymph nodes at each time point were found, which may be due to the strong defense capacity of lymph nodes.
Ligustrazine and Panax notoginseng saponins, having some therapeutic effects on SAP, are effective extracts from two Chinese herbs that promote blood circulation and eliminate blood clot, namely, Szechuan lovagerhizomeand root ofPanax pseudo-ginseng, respectively. Animal studies have shown that both Ligustrazine and Panax notoginseng saponins protect the structure and function of multiple organs, such as the pancreas, lung, kidney and gastrointestinal tract, reduce the incidence of complications, and improve the survival rates of experimental animals.[24,25,41,42]Furthermore, Ligustrazine inhibits the production of inflammatory mediators in macrophages and lymphocytes of rats and prevents the formation of immune complexes,[43]whilePanax Notoginseng saponinsenhance immune function in rats.[44,45]Therefore, these two drugs mitigate the pathological injury in the immune organs and small intestine of SAP rats to elevate survival rate.
Although Kakonein is extracted from kudzu vine root, drugs for relieving exterior syndrome, our study showed that the scores for the thymus (at 3 and 12 hours) and spleen (at 3 and 6 hours) in the Kakonein-treated groups were significantly lower than those in the model control groups. The pathological severity scores in thePanaxNotoginseng saponin-treated groups were significantly higher than those in the Kakonein-treated group in small intestinal mucosa, suggesting that the therapeutic effect of Kakonein on SAP was stronger than that of Panaxnotoginseng saponins.
In summary, all three traditional Chinese medicines can provide varying degrees of protection for the immune organs. Ligustrazine has the most protective effects on the small intestine, thymus and spleen, followed byKakonein, which may be due to their antiinflammatory effect, regulation of immune function and improvement of microcirculation. Therefore, in the auxiliary treatment of SAP, the three medicines should be selectively used as appropriate so as to achieve the best efficacy.
Funding:The study was supported by grants from the Technological Foundation Project of Traditional Chinese Medicine Science of Zhejiang Province (2003C130) and Zhejiang First Level 151 Talent Foundation (2010382).
Ethical approval:This study was approved by the Ethics Committee of the hospital.
Contributors:ZXP proposed the study. ZXP, JJ and CQH wrote the first draft. All authors contributed to the intellectual context and approved the final version. ZXP is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Morel DR, Frossard JL, Cikirikcioglu B, Tapponnier M, Pastor CM. Time course of lung injury in rat acute pancreatitis. Intensive Care Med 2006;32:1872-1880.
2 Rau BM, Bothe A, Kron M, Beger HG. Role of early multisystem organ failure as major risk factor for pancreatic infections and death in severe acute pancreatitis. Clin Gastroenterol Hepatol 2006;4:1053-1061.
3 Isaji S, Takada T, Kawarada Y, Hirata K, Mayumi T, Yoshida M, et al. JPN Guidelines for the management of acute pancreatitis: surgical management. J Hepatobiliary Pancreat Surg 2006;13:48-55.
4 Dambrauskas Z, Giese N, Gulbinas A, Giese T, Berberat PO, Pundzius J, et al. Different profiles of cytokine expression during mild and severe acute pancreatitis. World J Gastroenterol 2010;16:1845-1853.
5 De Campos T, Braga CF, Kuryura L, Hebara D, Assef JC, Rasslan S. Changes in the management of patients with severe acute pancreatitis. Arq Gastroenterol 2008;45:181-185.
6 Liu H, Li W, Wang X, Li J, Yu W. Early gut mucosal dysfunction in patients with acute pancreatitis. Pancreas 2008;36:192-196.
7 Zhang XP, Zhang J, Song QL, Chen HQ. Mechanism of acute pancreatitis complicated with injury of intestinal mucosa barrier. J Zhejiang Univ Sci B 2007;8:888-895.
8 Allan SE, Broady R, Gregori S, Himmel ME, Locke N, Roncarolo MG, et al. CD4+ T-regulatory cells: toward therapy for human diseases. Immunol Rev 2008;223:391-421.
9 Kylänpää ML, Repo H, Puolakkainen PA. Inflammation and immunosuppression in severe acute pancreatitis. World J Gastroenterol 2010;16:2867-2872.
10 Zhang XP, Zhang L, Chen LJ, Cheng QH, Wang JM, Cai W, et al. Influence of dexamethasone on inflammatory mediators and NF-kappaB expression in multiple organs of rats with severe acute pancreatitis. World J Gastroenterol 2007;13:548-556.
11 Cuthbertson CM, Christophi C. Disturbances of the microcirculation in acute pancreatitis. Br J Surg 2006;93:518-530.
12 Yang Z, Zhang Q, Ge J, Tan Z. Protective effects of tetramethylpyrazine on rat retinal cell cultures. NeurochemInt 2008;52:1176-1187.
13 Zhang JX, Dang SC. Ligustrazine alleviates acute lung injury in a rat model of acute necrotizing pancreatitis. Hepatobiliary Pancreat Dis Int 2006;5:605-609.
14 Park EK, Shin J, Bae EA, Lee YC, Kim DH. Intestinal bacteria activate estrogenic effect of main constituents puerarin and daidzin of Pueraria thunbergiana. Biol Pharm Bull 2006;29: 2432-2435.
15 Wang J, Huang ZG, Cao H, Wang YT, Hui P, Hoo C, et al. Screening of anti-platelet aggregation agents from Panax notoginseng using human platelet extraction and HPLCDAD-ESI-MS/MS. J Sep Sci 2008;31:1173-1180.
16 Wu L, Qiao H, Li Y, Li L. Protective roles of puerarin and Danshensu on acute ischemic myocardial injury in rats. Phytomedicine 2007;14:652-658.
17 Sun K, Wang CS, Guo J, Liu YY, Wang F, Liu LY, et al. Effect of Panax notoginseng saponins on lipopolysaccharideinduced adhesion of leukocytes in rat mesenteric venules. Clin Hemorheol Microcirc 2006;34:103-108.
18 Zhang JX, Dang SC, Qu JG, Wang XQ. Preventive effect of tetramethylpyrazine on intestinal mucosal injury in rats with acute necrotizing pancreatitis. World J Gastroenterol 2006;12:6386-6390.
19 Pezzilli R, Ceciliato R, Barakat B, Corinaldesi R. Immunemanipulation of the inflammatory response in acute pancreatitis. What can be expected? JOP 2004;5:115-121.
20 Pan F. Observation in curative effects of kakkonein treating 48 cases of patients with acute pancreatitis. Henan J Surg 2002;8:72.
21 Wu B, Wang CY. Role of apoptosis in pathogenesis of acute pancreatitis and effect of Ligustrazine. Guo Zhong Xi Yi Jie He Wai Ke Za Zhi 2005;11:23-26.
22 Hao LN, Ling YQ, Luo XM, Mao YX, Mao QY, He SZ, et al. Puerarin decreases lens epithelium cell apoptosis induced partly by peroxynitrite in diabetic rats. Sheng Li Xue Bao 2006;58:584-592.
23 Zhang S, Chen S, Shen Y, Yang D, Liu X, Sun-Chi AC, et al. Puerarin induces angiogenesis in myocardium of rat with myocardial infarction. Biol Pharm Bull 2006;29:945-950.
24 Wang HZ, Liu J, Song H, Li L, Hou XH. The therapeutic effect of Ulinastatin combined with Panax Notoginsenosidum on nitric oxide in rats with acute pancreatitis. World Chin J Digestol 2007;15:1956-1959.
25 Zhang XP, Wang C, Wu DJ, Ma ML, Ou JM. Protective effects of ligustrazine, kakonein and Panax notoginsenosides on multiple organs in rats with severe acute pancreatitis. Methods Find Exp Clin Pharmacol 2010;32:631-644.
26 Zhang XP, Xu HM, Jiang YY, Yu S, Cai Y, Lu B, et al. Influence of dexamethasone on mesenteric lymph node of rats with severe acute pancreatitis. World J Gastroenterol 2008;14:3511-3517.
27 Xiping Z, Li C, Miao L, Hua T. Protecting effects of dexamethasone on thymus of rats with severe acute pancreatitis. Mediators Inflamm 2007;2007:72361.
28 Zhang X, Chen L, Luo L, Tian H, Feng G, Cai Y, et al. Study of the protective effects of dexamethasone on ileum mucosa injury in rats with severe acute pancreatitis. Pancreas 2008;37:e74-82.
29 von Dobschuetz E, Pahernik S, Hoffmann T, Kiefmann R, Heckel K, Messmer K, et al. Dynamic intravital fluorescence microscopy--a novel method for the assessment of microvascular permeability in acute pancreatitis. Microvasc Res 2004;67:55-63.
30 Strate T, Mann O, Kleinhans H, Schneider C, Knoefel WT, Yekebas E, et al. Systemic intravenous infusion of bovine hemoglobin significantly reduces microcirculatory dysfunction in experimentally induced pancreatitis in the rat. Ann Surg 2003;238:765-771.
31 Hackert T, Pfeil D, Hartwig W, Fritz S, Schneider L, Gebhard MM, et al. Platelet function in acute experimental pancreatitis. J Gastrointest Surg 2007;11:439-444.
32 Keck T, Friebe V, Warshaw AL, Antoniu BA, Waneck G, Benz S, et al. Pancreatic proteases in serum induce leukocyteendothelial adhesion and pancreatic microcirculatory failure. Pancreatology 2005;5:241-250.
33 Dobosz M, Hac S, Mionskowska L, Dymecki D, Dobrowolski S, Wajda Z. Organ microcirculatory disturbances in experimental acute pancreatitis. A role of nitric oxide. Physiol Res 2005;54: 363-368.
34 Yan WW, Zhou ZG, Chen YD, Gao HK. Role of COX-2 in microcirculatory disturbance in experimental pancreatitis. World J Gastroenterol 2004;10:2095-2098.
35 Johansson M, Carlsson PO, Jansson L. Caerulein-induced pancreatitis and islet blood flow in anesthetized rats. J Surg Res 2003;113:13-20.
36 Liu XM, Liu QG, Xu J, Pan CE. Microcirculation disturbance affects rats with acute severe pancreatitis following lung injury. World J Gastroenterol 2005;11:6208-6211.
37 Freise H, Lauer S, Konietzny E, Hinkelmann J, Minin E, Van Aken HK, et al. Hepatic effects of thoracic epidural analgesia in experimental severe acute pancreatitis. Anesthesiology 2009;111:1249-1256.
38 Zhang XP, Shi Y, Zhang L. Progress in the study of therapeutic effects of traditional Chinese medicine and extracts in treating severe acute pancreatitis. JOP 2007;8:704-714.
39 Panek J, Zasada J, Poźniczek M. Microcirculatory disturbance in the course of acute pancreatitis. Przegl Lek 2007;64:435-437.
40 Vasilescu C, Herlea V, Buttenschoen K, Beger HG. Endotoxin translocation in two models of experimental acute pancreatitis. J Cell Mol Med 2003;7:417-424.
41 Zhang JX, Dang SC, Qu JG, Wang XQ. Ligustrazine alleviates acute renal injury in a rat model of acute necrotizing pancreatitis. World J Gastroenterol 2006;12:7705-7709.
42 Dang SC, Zhang JX, Qu JG, Wang XQ, Fan X. Ligustrazine alleviates gastric mucosal injury in a rat model of acute necrotizing pancreatitis. Hepatobiliary Pancreat Dis Int 2007; 6:213-218.
43 Abu-Zidan FM, Bonham MJ, Windsor JA. Severity of acute pancreatitis: a multivariate analysis of oxidative stress markers and modified Glasgow criteria. Br J Surg 2000;87: 1019-1023.
44 Sun H, Yang Z, Ye Y. Structure and biological activity of protopanaxatriol-type saponins from the roots of Panax notoginseng. Int Immunopharmacol 2006;6:14-25.
45 Wang Q, Liu J, Wang G, Wu B, Hu L, Li J, et al. Effects of Panax notoginseng saponins on immune-neuroendocrine network of SD rats in experimental navigation and intensive exercise. Zhongguo Zhong Yao Za Zhi 2010;35:1612-1618.
Received October 14, 2010
Accepted after revision April 6, 2011
Author Affiliations: Department of General Surgery (Zhang XP), Department of Gynecology and Obstetrics (Cheng QH) and Central Laboratory Department (Li WJ, Zhu H and Shen JY), Hangzhou First People's Hospital, Hangzhou 310006, China; Zhejiang Chinese Medical University, Hangzhou 310053, China (Jiang J and Ye Q)
Xi-Ping Zhang, MD, Department of General Surgery, Hangzhou First People's Hospital, Hangzhou 310006, China (Tel: 86-571-87065701; Fax: 86-571-87914773; Email: zxp99688@vip.163.com) © 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(11)60107-0
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Harmine induces apoptosis in HepG2 cells via mitochondrial signaling pathway
- Clinicopathological analysis of 14 patients with combined hepatocellular carcinoma and cholangiocarcinoma
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Undifferentiated embryonal sarcoma of the liver presenting as a hemorrhagic cystic tumor in an adult
- Fulminant liver failure models with subsequent encephalopathy in the mouse
- Risk factors of intrahepatic cholangiocarcinoma in patients with hepatolithiasis: a case-control study
