YKL-40 expression in human hepatocellular carcinoma: a potential biomarker?
2011-07-03XinQiangXiaoTarekHassaneinQunFangLiWeiLiuYuHuangZhengandJunChen
Xin-Qiang Xiao, Tarek Hassanein, Qun-Fang Li, Wei Liu, Yu-Huang Zheng and Jun Chen
Changsha, China
YKL-40 expression in human hepatocellular carcinoma: a potential biomarker?
Xin-Qiang Xiao, Tarek Hassanein, Qun-Fang Li, Wei Liu, Yu-Huang Zheng and Jun Chen
Changsha, China
BACKGROUND:YKL-40 is a new biomarker with diagnostic value in many different cancers. Whether it may serve as a biomarker for hepatocellular carcinoma (HCC) is still unclear. This study aimed to examine the expression of YKL-40 in the serum and liver tissues of HCC patients and in HCC cell lines, in comparison with that in non-HCC liver disease patients and non-tumor hepatic cell lines, respectively.
METHODS:Immunohistochemical staining was used to detect YKL-40 protein expression in liver biopsy specimens from 8 HCC patients. ELISA was used to assess the serum YKL-40 level in 90 HCC patients, 90 inactive HBsAg carrier (IHC) patients with normal liver functions, and 90 liver cirrhosis patients. Real-time PCR was used to determine the YKL-40 mRNA expression in three HCC cell lines and two non-tumor hepatic cell lines.
RESULTS:Immunohistochemical staining of liver biopsy specimens from HCC patients showed that the YKL-40 protein expression in tumor tissue was higher than that in adjacent normal tissues. ELISA revealed that the YKL-40 serum level in the HCC group was significantly higher than that in the IHC group, but not significantly different from that in the cirrhosis group. Real-time PCR showed that YKL-40 mRNA levels in HCC cell lines were significantly higher than those in nontumor hepatic cells.
CONCLUSIONS:YKL-40 is highly expressed in HCC at the molecular, cellular and tissue levels. However, it may not serve as a serum biomarker for HCC because measurement of the serum YKL-40 level cannot distinguish HCC from cirrhosis.
(Hepatobiliary Pancreat Dis Int 2011; 10: 605-610)
YKL-40; hepatocellular carcinoma; cirrhosis
Introduction
Hepatocellular carcinoma (HCC) is a highly malignant tumor with an annual incidence of between 250 000 and 1.2 million cases in high-risk areas such as Southeast Asia, China, and sub-Saharan Africa. Worldwide, it is the seventh most common cancer with the highest incidence of adult malignancy in areas endemic for hepatitis B virus. Most symptomatic HCCs progress rapidly and have a very poor prognosis.[1,2]Treatment of HCC is frequently limited by advanced stages of the tumor at the time of diagnosis. In the early diagnosis of HCC, one of the main problems is the poor sensitivity and specificity of available tests, such as alpha-fetoprotein (AFP) and liver images. Prospective studies evaluating the AFP test for HCC screening reported a sensitivity of 39%-64%, a specificity of 76%-91%, and positive predictive values varying from 9% to 32%.[3-5]Thus, normal AFP does not exclude HCC, and abnormal AFP does not necessarily mean that a patient has HCC. Therefore, more sensitive and specific markers are needed for early diagnosis of HCC. Up to now, a large number of new biomarkers, including lens culinaris agglutinin reactive AFP (AFP-L3), des-c-carboxy prothrombin (DCP), glypican-3 (GPC3), chromogranin-A (CgA), transforming growth factorbeta 1 (TGF-β1), alpha-l-fucosidase (AFU) and Golgi protein 73 (GP73) have been developed to make an early diagnosis and improve patients' prognosis, but absolute positive and negative markers for HCC are still lacking, and even those characterized by very high sensitivity and specificity do not have universal diagnostic usefulness.[6]
YKL-40 is a secreted heparin-, chitin-, and collagenbinding glycoprotein belonging to a group of mammalian proteins with an amino acid sequence similar to the 18-glycosyl hydrolase group of bacterial chitinases.[7]YKL-40 is reportedly expressed in several types of carcinoma (breast, colon, lung, kidney, ovarian, prostate, uterine, osteosarcoma, oligodendroglioma, glioblastoma and germ cell tumors).[8,9]Elevated serum levels ofYKL-40 are associated with a poor prognosis in several localized and metastatic malignancies.[10]Then, if YKL-40 is expressed in HCC, it may serve as an effective biomarker for detecting HCC. To determine this, in the present study we examined the expression of YKL-40 in the serum and liver tissues of HCC patients and in HCC cell lines, in comparison with that in non-HCC liver disease patients and non-tumor hepatic cell lines, respectively.
Methods
Study subjects
After signing informed consent, 90 HCC patients who had undergone liver cancer surgery, 90 inactive HBsAg carrier (IHC) patients and 90 liver cirrhosis patients meeting relevant diagnostic criteria had their serum samples banked at the Second Xiangya Hospital of Central South University (China) from July 2007 to December 2009. There were 227 males and 43 females, aged between 15 and 73 years (mean 43.8±12.5 years). All patients were seropositive for HBsAg for more than 6 months. Those with evidence of concomitant hepatitis C or D virus infection, HIV infection, autoimmune, druginduced or other chronic liver diseases were excluded. HCC was diagnosed by liver biopsy. Inactive chronic HBV infection was defined as chronic hepatitis B with persistently normal ALT levels (≤40 U/L) for 6 months prior to enrollment. Cirrhosis was defined as the presence of fibrosis stage 4 or >4 in the HAI score. Those with HCC diagnosed within 3 months after initiation of assessment were excluded. The relevant clinical and laboratory data of the patients are summarized in Table 1. The liver biopsy tissues from eight HCC patients were banked at the Hillcrest Medical Center, University of California at San Diego from 2005 to 2006, and were kindly provided by Dr. Tarek Hassanein. This study was approved by the Ethics Committee of the Second Xiangya Hospital of Central South University.
Immunohistochemistry
Sections were deparaffinized, rehydrated in xylene followed by a series of graded alcohols, treated with a 0.5% solution of hydrogen peroxide in methanol for 15 minutes to quench endogenous peroxidase, and rinsed in Tris buffered saline (TBS, 5 mmol/L Tris-HCl, 146 mmol/L NaCl, pH 7.6). Nonspecific binding was inhibited by incubation for 30 minutes with blocking buffer (ChemMate antibody diluent S2022; DakoCytomation, Glostrup, Denmark) at room temperature. The sections were incubated overnight at 4 ℃ with a monoclonal mouse antibody against human YKL-40[6](MAb 201.F9,kindly provided by Dr. Paul A. Price, University of California at San Diego, La Jolla, CA) at 7.5 μg/mL in blocking buffer (ChemMate antibody diluent S2022). Then the sections were washed with TBS and incubated for 30 minutes with a peroxidase-labeled polymer conjugated to goat anti-mouse immunoglobulins (DAKO EnVision System/HRP K4007; Dako Cytomation). Positive staining was recognized as a brown color. The sections were counterstained with Mayer's hematoxylin, dehydrated in graded alcohols followed by xylene, and cover-slipped with DPX mounting medium. Positive controls were stained of tissues known to be immuno-reactive to YKL-40. Nonimmune mouse IgG1 (X0931; IgG1 concentration, 7.5 mg/mL, Dako Cytomation) was used as a negative control. Positive immunostaining of YKL-40 appeared as a cytoplasmic and granular brown color. YKL-40 expression levels were assessed by the percentage of positivelystained cells over the total cells. A mean percentage of positive tumor cells was determined in at least five areas at ×400 magnification. Numeric scores were calculated on the following scale by the percentage of YKL-40-expressing cells over the total cells: 0 (<5% positive cells), 1 (5%-25%), 2 (25%-50%), 3 (50%-75%), and 4 (>75%). The cumulative numeric score was calculated by adding individual numeric scores for tumor tissues or adjacent normal tissues in the 8 liver biopsy specimens. Positivelystained blood vessels were not included in the scoring.
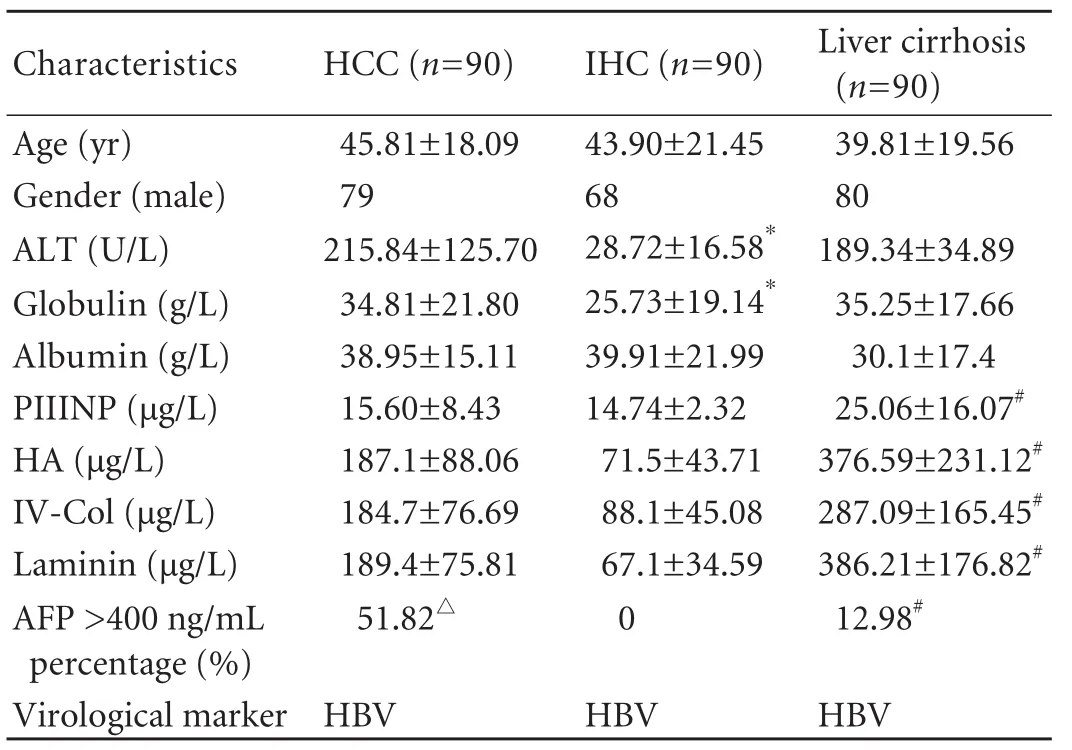
Table 1. Biochemical characteristics of patients according to pathoanatomical diagnosis
YKL-40 enzyme-linked immunosorbent assay (ELISA)
Serum YKL-40 levels were determined in duplicatefor all serum samples, per the manufacturer's protocol with the YKL-40 ELISA Kit (Metra Biosystems, Mountain View, CA). Protein concentrations were determined using the Bio-Rad Benchmark Microplate Reader (Bio-Rad Laboratories, Hercules, CA).
Cell culture and real-time quantitative RT-PCR analysis
QSG-7701, LX-2, HepG2, Huh-7, and LM-3 cells were seeded onto 60×15 mm cell culture dishes at a density of 5×105cells/dish and maintained in DMEM (Gibco/BRL, USA) supplemented with 10% FBS at 37 ℃in a 5% CO2incubator. After 72 hours, when reaching the confluency of 80%-90%, the cells were harvested. Total RNA was isolated from the cell homogenates using Trizol reagent; cDNA was synthesized using M-MLV reverse transcriptase (Promega, USA) containing equivalent amounts of total RNA (4 μg/sample). Realtime quantitative PCR reactions were performed with a 7500 real-time PCR system (Applied Biosystems Inc., USA) using the Thunderbird SYBR qPCR Mix (Toyobo, Japan). The results were normalized against that of the housekeeping gene β-actin in the same sample. The thermal profile was 95 ℃ for 1 minute, followed by 40 cycles of 95 ℃ for 15 seconds and 60 ℃ for 45 seconds, finally holding at 4 ℃. The sequences of the primers (Invitrogen, Shanghai) were as follows: β-actin forward: 5'-CAT CTC TTG CTC GAA GTC CA-3'; β-actin reverse: 5'-ATC ATG TTT GAG ACC TTC AAC A-3'; YKL40 forward: 5'-GGA TGG AAC TTT GGG TCT CA-3'; YKL40 reverse: 5'-GCT GTT TGT CTC TCC GTC CA-3'.
The fluorescence signal was plotted versus cycle number. The threshold cycle (Ct) was defined as the cycle number where the fluorescence signal was reliably detected above background. Each PCR run also included non-template controls containing all reagents except for cDNA. After cycling, a melting curve was produced by slow denaturation of the PCR end products to validate the specificity of amplification. The relative quantification (RQ) of expression of YKL-40 was determined by 2-ΔΔCt, as described by Pfaff l.[11]Each experiment was repeated twice in triplicate and results were expressed as mean±SE.
Statistical analysis
One-way ANOVA and Kruskal-Wallis tests were performed using SPSS for Windows 13.0. The significance level was set at 0.05. We evaluated the diagnostic value of YKL-40 by receiver operating characteristic (ROC) curve analysis (SPSS 13.0).
Results
Expression of YKL-40 in tumor tissues and adjacent tissues
Positive immunostaining of YKL-40 appeared as cytoplasmic and granular brown-colored staining (Fig. 1). Immunohistochemical staining of liver biopsy specimens from 8 HCC patients showed that YKL-40 protein expression levels in tumor tissues were significantly higher than those in adjacent normal tissues. In 7 out of the 8 (87.5%) liver biopsy specimens, YKL-40 positive cells in the tumor tissue accounted for over 50% of total cells, while YKL-40 positive cells in the adjacent normal tissue accounted for less than 50% of total cells in all of the 8 specimens (Table 2). The cumulative numeric score was 28 for the tumor tissue, and 7 for the adjacent normal tissue in the 8 specimens.
Serum YKL40 concentrations in patients with different liver diseases
The serum YKL-40 level in patients with liver cirrhosis or HCC was higher than that in IHC patients (P<0.01), with no significant difference between HCC and liver cirrhosis patients (Table 3). Patients with a serum YKL-40 level of ≥100 ng/mL were defined as patients with a high YKL-40 level. The percentages of patients with a high YKL-40 level among cirrhosis and HCC patientswere higher than those in IHC patients (P<0.01). The ROC curve analysis results showed that the areas under the ROC curves (AUROC) of HCC, liver cirrhosis and IHC were 0.716, 0.742 and 0.954, respectively (Fig. 2 and Table 4). All the differences were statistically significant (P<0.001). The AUROC, sensitivity and specificity of each group are shown in Table 4.
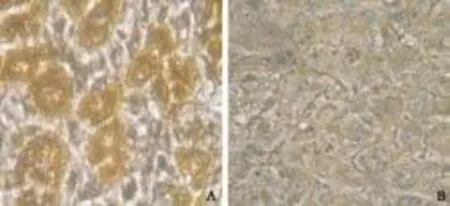
Fig. 1. Immunohistochemical staining for YKL-40 in HCC liver tissue (original magnification ×400). A: positive immunostaining of YKL-40 appeared as a cytoplasmic and granular brown color; B: negative control.
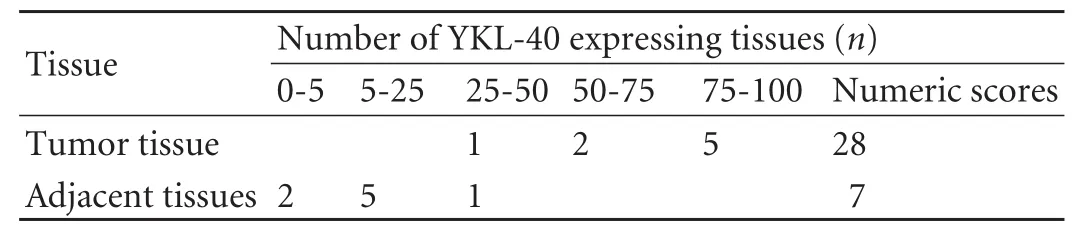
Table 2. Immunohistochemical analysis of YKL-40 expression in 8 HCC liver samples
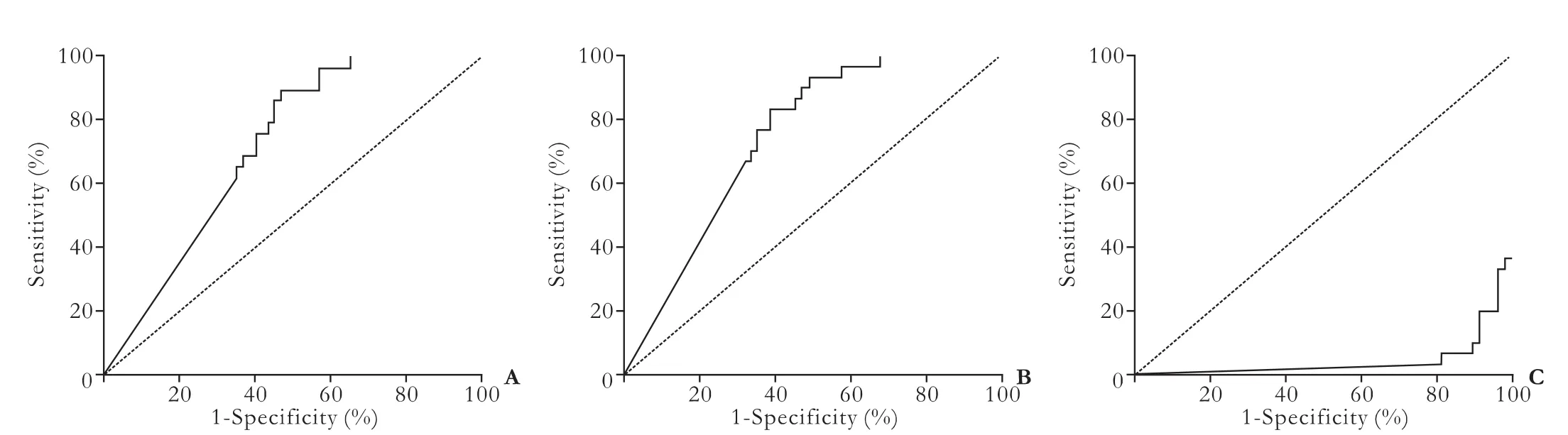
Fig. 2. Diagnostic value of YKL-40 with receiver operating characteristic curve analysis. YKL-40 had an AUROC of 0.716 for HCC (A), an AUROC of 0.742 for cirrhosis (B), and a lower AUROC of 0.046 for IHC (C). The AUROC of YKL-40 was significant for diagnosis of cirrhosis and HCC, but not for chronic hepatitis B.
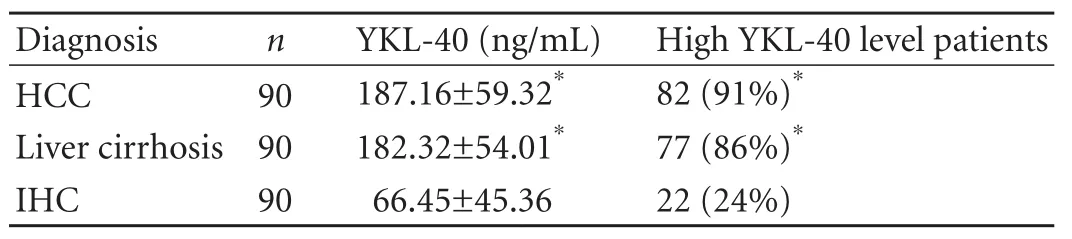
Table 3. Serum concentrations of YKL-40 in patients with three different liver diseases
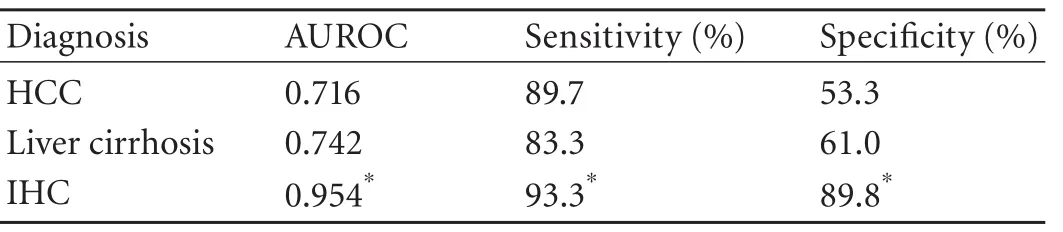
Table 4. AUROC, sensitivity and specificity in different patients
Expression of YKL-40 mRNA in HCC cell lines
YKL-40 mRNA levels in the normal human hepatic cell line QSG-7701, human hepatic stellate cell line LX-2, and HCC cell lines HepG2, Huh-7 and LM-3 were assessed with real-time quantitative RT-PCR. While YKL-40 mRNA expression was barely detectable in QSG-7701 and LX-2 cells, the relative quantification values were 0.191±0.03 and 0.233±0.04, respectively (Fig. 3). The HCC cell lines HepG2, Huh-7 and LM-3 showed substantial expression of YKL-40 mRNA. The relative quantification values were 1.037±0.2, 0.699±0.1 and 1.001±0.15, respectively.
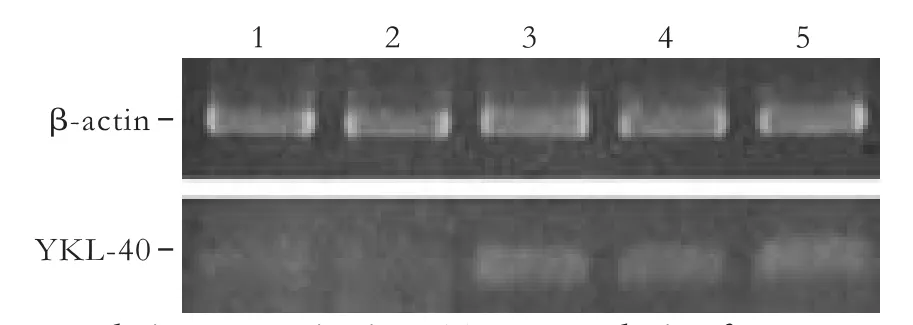
Fig. 3. Real-time quantitative RT-PCR analysis of YKL-40 mRNA expression in different hepatic cell lines. Lane 1: YKL-40 mRNA levels in normal human hepatic cell line QSG-7701; Lane 2: human hepatic stellate cell line LX-2; Lane 3: HCC cell lines HepG2; Lane 4: Huh-7; Lane 5: LM-3. β-actin was used as an internal control.
Discussion
In patients with chronic hepatitis B, C and alcoholic liver disease, elevated serum YKL-40 concentrations are often related to the presence of liver fibrosis and cirrhosis and the severity of fibrosis.[12-16]In chronic HBV-infected patients, serum YKL-40 levels were significantly different between patients with chronic hepatitis and those with cirrhosis. In HCV-infected patients, the YKL-40 serum level is associated with the severity of HCV-induced fibrosis[13]and may be a useful non-invasive serum marker to estimate the degree of fibrosis and to evaluate the efficacy of IFN therapies in patients with HCV-associated liver disease.[14,15]Patients with alcoholic cirrhosis, post-hepatitic cirrhosis and non-cirrhotic fibrosis have significantly higher serum YKL-40 than normal subjects and patients with fatty liver.[16]Nøjgaard et al[16]studied 370 patients withalcoholic liver disease in a trial with a median followup period of 470 days, and concluded that serum levels of YKL-40 and PIIINP are elevated in alcoholic patients, are related to the presence of fibrosis, and can provide prognostic information.
YKL-40 is believed to be involved in inflammation and remodeling of the extracellular matrix through growth factor activity. Several studies[6-10]have indicated a role for YKL-40 in cancer cell proliferation and invasiveness. A number of studies suggested that serum YKL-40 could be a useful biomarker for predicting the survival of cancer patients.[17,18]Whether YKL-40 may serve as a biomarker for HCC is still unclear. In the present study, we observed the expression of YKL-40 in HCC patients and found that YKL-40 protein expression in HCC tumor tissues was significantly higher than that in adjacent normal tissues. Compared with IHC patients, HCC patients showed significantly elevated serum YKL-40 levels; 93% of the HCC patients had a serum YKL-40 level ≥100 ng/mL (Table 3). The results indicated that YKL-40 was highly expressed in HCC at the molecular, cellular and tissue levels. Therefore, it has potential as a biomarker to detect HCC.
The cellular sources of YKL-40 in the liver are still unknown. The hepatic stellate cell is a possible source of secreted YKL-40 during active hepatic fibrogenesis; it plays a central role in fibrosis, and is found in the space of Disse in close contact with hepatocytes and endothelial cells.[19]However, YKL-40 mRNA was barely expressed in the human hepatic stellate cell line LX-2 (Fig. 3), probably because the LX-2 cell has just some of the morphologic features of early activated stellate cells.[20]Whether the stellate cell is a cellular source of YKL-40 needs to be confirmed. Lau et al[21]found that clusterin greatly upregulates YKL-40 gene expression in primary HCC H2P cells, suggesting that the CLUYKL-40 pathway may play an important role in HCC metastasis, and that HCC tumor cells may be one of the cellular sources of YKL-40. Indeed, in our study, the HCC cell lines HepG2, LM-3 and Huh-7 expressed high levels of YKL-40 mRNA.
The AUROC for YKL-40 was significantly superior for predicting the presence of cirrhosis and HCC, but chronic hepatitis B (Fig. 2). Although the sensitivity of serum YKL-40 at the selected cut-off points was satisfactory for both HCC and cirrhosis (89.7% and 83.3%), low specificities were found (53.3% and 61.0%) (Table 4). While the AUROC changed to 0.954, the sensitivity and specificity in IHC were 93.3% and 89.8%, respectively (Table 4), indicating that YKL-40 could be a test with high discriminant capabilities for the distinction between inactive carrier state and HCC or cirrhosis. Our data also showed no significant difference in the serum YKL-40 level between HCC patients and cirrhosis patients (Table 3), suggesting that serum YKL-40 is not a specific indicator for the diagnosis of liver cirrhosis or HCC. Since lack of specificity to distinguish it from cirrhosis, YKL-40 cannot serve as an ideal serum biomarker for HCC.
Acknowledgement
We thank Dr. Elliot Alpert for technical support and Dr. Paul A. Price for providing us with the mouse monoclonal anti-human YKL-40 antibody MAb 201.F9.
Funding:This study was supported by a grant from the National Natural Science Foundation of China (No. 81072035).
Ethical approval:Not needed.
Contributors:CJ proposed the study and wrote the first draft. LQF analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. CJ is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist 2010;15:5-13.
2 Long J, Lang ZW, Wang HG, Wang TL, Wang BE, Liu SQ. Glutamine synthetase as an early marker for hepatocellular carcinoma based on proteomic analysis of resected small hepatocellular carcinomas. Hepatobiliary Pancreat Dis Int 2010;9:296-305.
3 Tian L, Wang Y, Xu D, Gui J, Jia X, Tong H, et al. Serological AFP/ golgi protein 73 could be a new diagnostic parameter of hepatic diseases. Int J Cancer 2010 Dec 7.
4 El-Attar HA, Kandil MH, El-Kerm YM, El-Ghandour MK. Comparison of serum survivin and alpha fetoprotein in Egyptian patients with hepatocellular carcinoma associated with hepatitis C viral infection. Asian Pac J Cancer Prev 2010; 11:897-903.
5 Cabrera R, Nelson DR. Review article: the management of hepatocellular carcinoma. Aliment Pharmacol Ther 2010;31: 461-476.
6 Malaguarnera G, Giordano M, Paladina I, Berretta M, Cappellani A, Malaguarnera M. Serum markers of hepatocellular carcinoma. Dig Dis Sci 2010;55:2744-2755.
7 Johansen JS, Høyer PE, Larsen LA, Price PA, Møllgård K. YKL-40 protein expression in the early developing human musculoskeletal system. J Histochem Cytochem 2007;55: 1213-1228.
8 Horbinski C, Wang G, Wiley CA. YKL-40 is directly produced by tumor cells and is inversely linked to EGFR in glioblastomas. Int J Clin Exp Pathol 2010;3:226-237.
9 Thöm I, Andritzky B, Schuch G, Burkholder I, Edler L, Johansen JS, et al. Elevated pretreatment serum concentrationof YKL-40-An independent prognostic biomarker for poor survival in patients with metastatic nonsmall cell lung cancer. Cancer 2010;116:4114-4121.
10 Johansen JS, Schultz NA, Jensen BV. Plasma YKL-40: a potential new cancer biomarker? Future Oncol 2009;5:1065-1082.
11 PfafflMW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001; 29:e45.
12 Lee KG, Seo YS, An H, Um SH, Jung ES, Keum B, et al. Usefulness of non-invasive markers for predicting liver cirrhosis in patients with chronic hepatitis B. J Gastroenterol Hepatol 2010;25:94-100.
13 Berres ML, Papen S, Pauels K, Schmitz P, Zaldivar MM, Hellerbrand C, et al. A functional variation in CHI3L1 is associated with severity of liver fibrosis and YKL-40 serum levels in chronic hepatitis C infection. J Hepatol 2009;50:370-376.
14 Pungpapong S, Nunes DP, Krishna M, Nakhleh R, Chambers K, Ghabril M, et al. Serum fibrosis markers can predict rapid fibrosis progression after liver transplantation for hepatitis C. Liver Transpl 2008;14:1294-1302.
15 Saitou Y, Shiraki K, Yamanaka Y, Yamaguchi Y, Kawakita T, Yamamoto N, et al. Noninvasive estimation of liver fibrosis and response to interferon therapy by a serum fibrogenesis marker, YKL-40, in patients with HCV-associated liver disease. World J Gastroenterol 2005;11:476-481.
16 Nøjgaard C, Johansen JS, Christensen E, Skovgaard LT, Price PA, Becker U, et al. Serum levels of YKL-40 and PIIINP as prognostic markers in patients with alcoholic liver disease. J Hepatol 2003;39:179-186.
17 Shao R, Hamel K, Petersen L, Cao QJ, Arenas RB, Bigelow C, et al. YKL-40, a secreted glycoprotein, promotes tumor angiogenesis. Oncogene 2009;28:4456-4468.
18 Yang GF, Cai PY, Li XM, Deng HX, He WP, Xie D. Expression and clinical significance of YKL-40 protein in epithelial ovarian cancer tissues. Ai Zheng 2009;28:142-145.
19 Ku BM, Lee YK, Ryu J, Jeong JY, Choi J, Eun KM, et al. CHI3L1 (YKL-40) is expressed in human gliomas and regulates the invasion, growth and survival of glioma cells. Int J Cancer 2011;128:1316-1326.
20 Taimr P, Higuchi H, Kocova E, Rippe RA, Friedman S, Gores GJ. Activated stellate cells express the TRAIL receptor-2/ death receptor-5 and undergo TRAIL-mediated apoptosis. Hepatology 2003;37:87-95.
21 Lau SH, Sham JS, Xie D, Tzang CH, Tang D, Ma N, et al. Clusterin plays an important role in hepatocellular carcinoma metastasis. Oncogene 2006;25:1242-1250.
Received December 23, 2010
Accepted after revision March 30, 2011
Author Affiliations: Liver Diseases Center (Xiao XQ, Li QF, Zheng YH and Chen J), Department of Hepatobiliary Surgery (Liu W), Second Xiangya Hospital, Central South University, Changsha 410011, China; Liver Center, Hillcrest Medical Center, University of California at San Diego, CA 92103, USA (Hassanein T)
Jun Chen, MD, Liver Diseases Center, Second Xiangya Hospital, Central South University, Changsha 410011, China (Tel: 86-731-85599556; Fax: 86-731-85292173; Email: drchenjun@yahoo.com.cn) © 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(11)60103-3
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Harmine induces apoptosis in HepG2 cells via mitochondrial signaling pathway
- Clinicopathological analysis of 14 patients with combined hepatocellular carcinoma and cholangiocarcinoma
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Undifferentiated embryonal sarcoma of the liver presenting as a hemorrhagic cystic tumor in an adult
- Fulminant liver failure models with subsequent encephalopathy in the mouse
- Risk factors of intrahepatic cholangiocarcinoma in patients with hepatolithiasis: a case-control study
