Early lactate clearance as a reliable predictor of initial poor graft function after orthotopic liver transplantation
2011-07-03JianFengWuRongYaoWuJuanChenBinOuYangMinYingChenandXiangDongGuan
Jian-Feng Wu, Rong-Yao Wu, Juan Chen, Bin Ou-Yang, Min-Ying Chen and Xiang-Dong Guan
Guangzhou, China
Early lactate clearance as a reliable predictor of initial poor graft function after orthotopic liver transplantation
Jian-Feng Wu, Rong-Yao Wu, Juan Chen, Bin Ou-Yang, Min-Ying Chen and Xiang-Dong Guan
Guangzhou, China
BACKGROUND:Initial poor graft function (IPGF) following orthotopic liver transplantation is a major determinant of postoperative survival and morbidity. Lactate clearance is a good marker of liver function. In this study, we investigated the clinical utility of early lactate clearance as an early and accurate predictor for IPGF following liver transplantation.
METHODS:This was a prospective observational study of 222 patients referred to the surgical intensive care unit (SICU) after orthotopic liver transplantation. The IPGF group consisted of patients with alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) >1500 IU/L within 72 hours after orthotopic liver transplantation. Early lactate clearance was defined as lactate at SICU presentation (hour 0) minus lactate at hour 6, divided by lactate at SICU presentation. The model for end-stage liver disease (MELD) score, Child-Pugh score and laboratory data including AST, ALT, total bilirubin (TB) and prothrombin time (PT) were recorded at SICU presentation and compared between the non-IPGF and IPGF groups. Receiver operating characteristic (ROC) curves were plotted to measure the performance of early lactate clearance, MELD score, Child-Pugh score, TB and PT.
RESULTS:IPGF occurred in 45 of the 222 patients (20.3%). The early lactate clearance in the non-IPGF group was markedly higher than that in the IPGF group (43.2±13.8% vs 13.4±13.7%P<0.001). The optimum cut-off value for early lactate clearance predicting IPGF was 24.8% (sensitivity 95.5%, specificity 88.9%). The area under the curve of the ROC was 0.961, which was significantly superior to MELD score, Child-Pugh score, TB and PT. Patients with early lactate clearance≤24.8% had a higher IPGF rate (OR=169) and a higher risk of in-hospital mortality (OR=3.625).
CONCLUSIONS:Early lactate clearance can serve as a prompt and accurate bedside predictor of IPGF. Patients with early lactate clearance less than 24.8% are associated with a higher incidence of IPGF.
(Hepatobiliary Pancreat Dis Int 2011; 10: 587-592)
early lactate clearance; initial poor graft function; liver transplantation
Introduction
Nowadays, liver transplantation has evolved as an effective therapeutic modality for patients with end-stage liver disease. Early graft function after liver transplantation is an important prognostic marker for the individual outcome. However, liver procurement and implantation are inevitably associated with allograft damage due to long cold and warm ischemic times, the medical status of the recipient and the surgical complications.[1,2]Initial poor graft function (IPGF) may be related to the quality of the donor organ and is potentially associated with secondary complications, such as renal failure, severe bleeding or septic infections, and might have a negative effect on long-term health and employment.[2,3]The most serious end result of initial poor allograft function is primary graft nonfunction which has a high mortality.[4]For this reason, to identify a simple and reliable early indicator of IPGF could contribute to the improvement of outcomes after liver transplantation.
Researchers provided the first definition of IPGF that was exclusively based on a direct parameter of graft performance--the actual metabolic capacity.[5]Since circulating lactate is metabolized mainly in the liver, impaired liver function may lead to high blood lactate concentrations. The injured liver itself may act as a source of lactate as well.[6]Recently, lactate concentrationmonitoring in the perioperative liver transplant period was reported in a few studies.[7,8]However, since the lactate concentration in blood is a result of the dynamic balance between production and clearance, baseline blood lactate levels alone do not necessarily predict the initial graft function in liver transplant recipients, especially when compared with the clearance of lactate. Early lactate clearance, which dynamically reflects the production and elimination of lactate, is defined as the percent change in lactate level after six hours from a baseline measurement in the ICU and has a high sensitivity and specificity in predicting the outcomes of patients in critical care.[9,10]This has led to the question whether early lactate clearance is a good indicator for IPGF following liver transplantation. In this study, we tried to answer this question and go further to define an optimum early lactate clearance cutoff for predicting IPGF.
Methods
Patients
This study was carried out between January 1, 2007 and December 31, 2009 in the surgical intensive care unit (SICU) of the First Affiliated Hospital, Sun Yat-Sen University.
A total of 222 patients were enrolled after orthotopic liver transplantation. Indications for liver transplantation were hepatitis B with cirrhosis (110 patients), primary liver cancer (92), hepatitis C with cirrhosis (7), fulminant hepatic failure (3), polycystic liver and kidney disease (3), hepatoangioma (3), biliary cirrhosis (3), and cholangiocarcinoma (1).
Data collection
Data of all patients were collected by two trained physicians using a specially designed case report form. The data included a manual review of their laboratory data, progress notes, nursing notes and the SICU flow sheets, and the timing of specific events.
Age, gender, MELD score, Child-Pugh score, lactate at SICU presentation, lactate at hour 6 and laboratory data including aspartate aminotransferase (AST), alanine aminotransferase (ALT), platelet, albumin, total bilirubin (TB), prothrombin time (PT) and serum creatinine were recorded at SICU presentation, as well as ALT and AST levels within 72 hours after OLT.
Treatment and measurements
All hepatic allografts were perfused through the abdominal aorta and portal vein and preserved with cold University of Wisconsin solution after procurement. Among the 222 recipients, 146 received standard OLT and the other 76 received piggyback OLT.
Anesthetic techniques were standardized using vecuronium, midazolam and fentanyl for induction while fentanyl and midazolam were used for maintenance anesthesia.
The postoperative immunosuppressive protocols were tacrolimus, basiliximab and steroids. Piperacillin/ tazobactam was used as early prophylaxis for bacterial infections and caspofungin or itraconazole as prophylaxis for fungal infections, while lamivudine and anti-hepatitis B immunoglobulin were used for patients with positive HBsAg.
We used predetermined hemodynamic goals to adjust fluid administration in all post-transplantation patients admitted to SICU within 6 hours: central venous pressure approximately 8 cmH2O, mean arterial pressure >70 mmHg, urine output >1 mL/kg per hour.[11]Patients admitted to SICU received 5% albumin as fluid resuscitation within 6 hours besides blood products.
Definition
Early lactate clearance (percent) was defined using the following formula:[9]lactate at SICU presentation (hour 0) minus lactate at hour 6, divided by lactate at SICU presentation, then multiplied by 100%. A positive value denotes a decrease or clearance of lactate, whereas a negative value denotes an increase in lactate after 6 hours of SICU intervention.
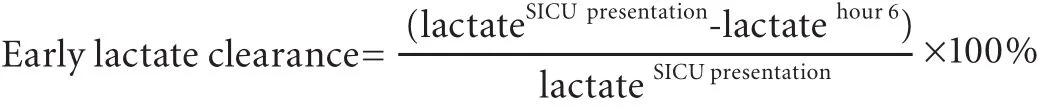
PGF was defined using Nanashima's classification:[12]ALT and/or AST levels >1500 IU/L within 72 hours after OLT. Those with values <1500 IU/L were assigned to the non-IPGF group.
Statistical analysis
Continuous variables were tested for normal distribution, expressed as mean±standard deviation, and compared by Student's t test. Nonparametric analysis with the Mann-Whitney U test was used for data with non-normal distributions. Categorical variables were compared with the chi-square test. A two-sided P value of <0.05 was considered to be statistically significant. Receiver operating characteristic (ROC) curves were plotted to measure the performance of early lactate clearance, the model for end-stage liver disease (MELD) score, Child-Pugh score, TB and PT for predicting the incidence of IPGF following liver transplantation. All of the statistical calculations were performed using SPSS (version 15.0; Chicago, USA).
Results
Patient demographics
A total of 222 consecutive patients were included in this observational study (Table 1), among whom 191 were males and 31 females. Their ages at the time of transplantation ranged from 23 to 75 (median 48.5) years. Our data showed that 45 recipients (20.3%) had IPGF.
Comparison of early lactate clearance and various parameters in patients with IPGF and those without IPGF
Baseline data in the non-IPGF and IPGF groups (age, gender, blood lactate level and laboratory examinations including AST, ALT, platelet, albumin and serum creatinine at SICU presentation) showed no statistically significant difference. The early lactate clearance in the non-IPGF group was markedly higher than that in the IPGF group. In addition, there were statistically significant differences between the non-IPGF and IPGF groups in TB, PT, MELD score and Child-Pugh score (Table 2).
Predictive value of early lactate clearance for IPGF following liver transplantation
We established an early lactate clearance rate of 24.8% as the best cut-off point with a sensitivity of 95.5% and a specificity of 88.9% for prediction of IPGF. The area under the ROC curve (AUC) of early lactate clearancefor IPGF following liver transplantation was 0.961±0.013, while the AUCs of MELD and Child-Pugh scores were 0.639 and 0.584 respectively. TB and PT also had a low sensitivity and specificity (Table 3, Figs. 1, 2). Early lactate clearance was superior to the other parameters in our study for predicting IPGF in terms of AUC, sensitivity and specificity. Most patients (83.3%, 40/48) with earlylactate clearance ≤24.8% experienced IPGF, while only 2.9% (5/174) of those with early lactate clearance >24.8% experienced IPGF (OR=169, 95% CI: 52.49-544.13).
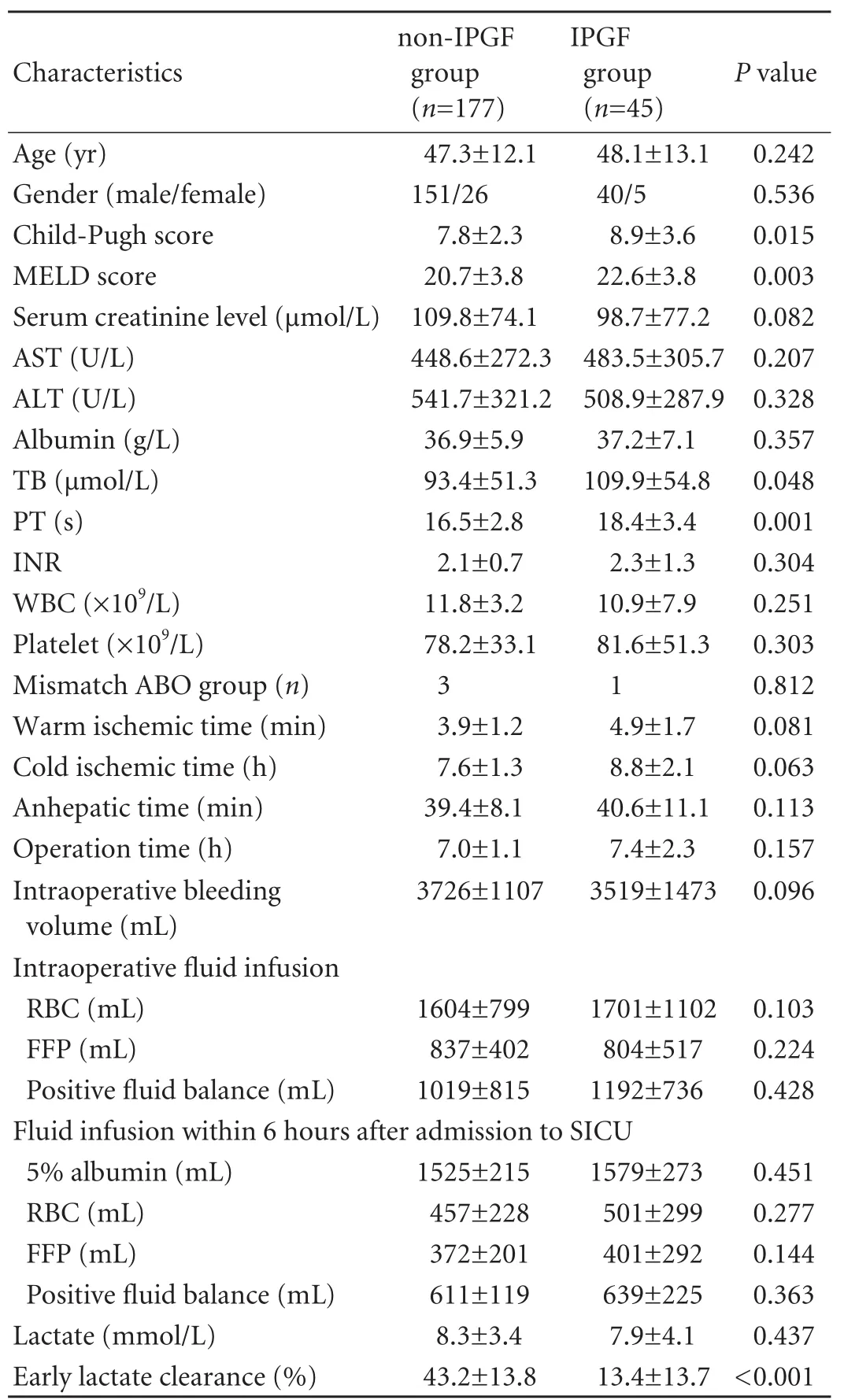
Table 2. Comparison of early lactate clearance rate and other parameters between non-IPGF group and IPGF groups
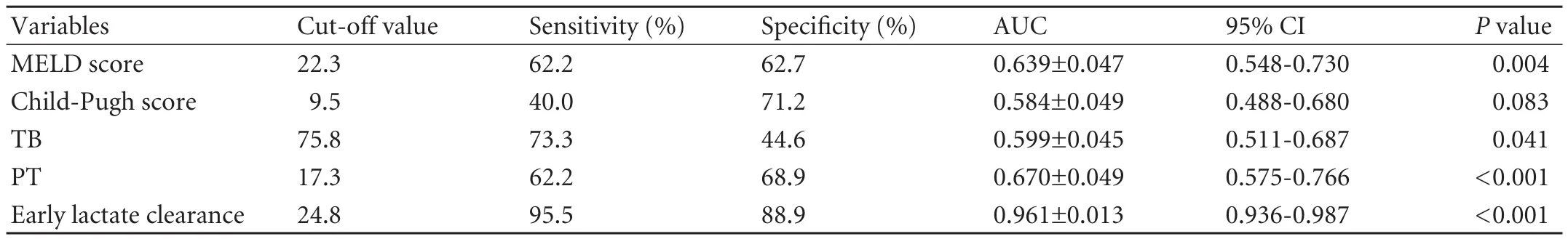
Table 3. Comparison of predictive value between early lactate clearance and other parameters
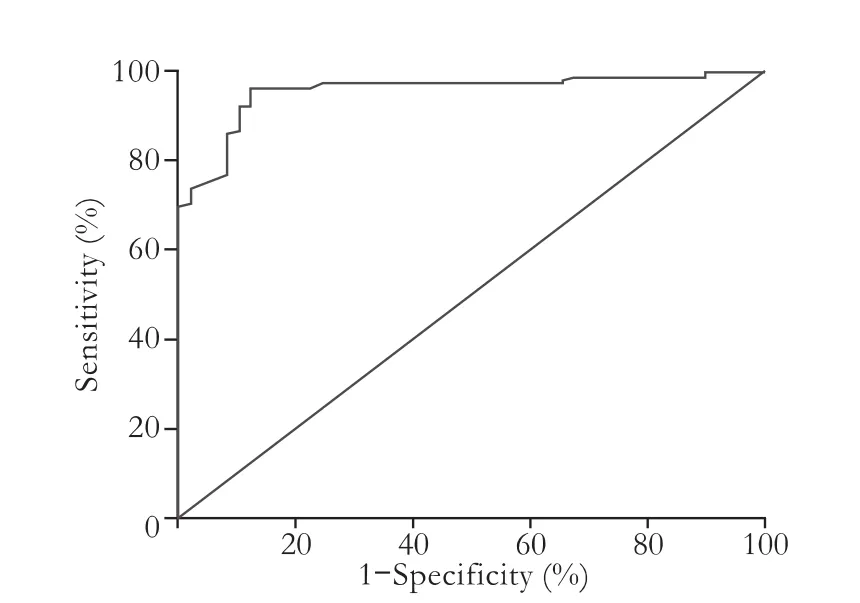
Fig. 1. ROC curve of early lactate clearance.

Fig. 2. ROC curves of MELD and Child-Pugh scores, TB and PT.
Clinical outcome
The overall in-hospital mortality after liver transplantation was 7.2% (16/222). Patients with early lactate clearance ≤24.8% had a in-hospital mortality (8/48) statistically significant higher than those with early lactate clearance >24.8% (8/174) (16.7% vs 4.6%, OR=3.625, 95% CI: 1.29-10.16). The overall one-year mortality after liver transplantation was 14.4% (32/222). There was no statistical difference in one-year mortality rate between patients with early lactate clearance ≤24.8% (11/48) and those with early lactate clearance >24.8% (21/174) (22.9% vs 12.1%, OR=1.89, 95% CI: 0.86-4.21).
Discussion
Liver transplantation has evolved into a successful treatment for patients with end-stage liver disease and acute liver failure. Improvements in immunosuppression, perioperative management, and surgical techniques have contributed to a 1-year patient survival of more than 90% and 70%-80% after 3 years.[13,14]The postoperative course and survival of patients depend in the first place on the initial function of the transplanted liver. However, IPGF is a frequent complication after liver transplantation with an incidence rate of 18.3% to 36.25%.[1,15,16]IPGF, which may be complicated by the patients' poor general condition and neglected by clinicians, can significantly compromise patient survival.[17,18]Therefore, new practical, rapid and specific tools for the early recognition of IPGF are needed for prompt intervention to improve the patients' prognosis.
The MELD and Child-Pugh scoring systems have been widely used in predicting the outcome of liver transplantation in recent years. The MELD score, which seems to be a more promising alternative to the Child-Pugh score, is significantly and independently correlated with short-term and mid-term survival. However, neither MELD nor Child-Pugh scores are able to predict organ function.[19-21]In addition, the principal limitation of MELD is the need for computation, limiting its usefulness at the bedside. Our study showed that MELD and Child-Pugh scores had a low sensitivity and specificity for prediction of IPGF after liver transplantation. Hence, we believe the preoperative Child and MELD scores cannot be considered as definitive parameters for postoperative liver function.
Currently, most surgeons rely on laboratory tests such as liver-specific transaminases (ALT and AST), TB and PT[22]for graft function monitoring. However, due to complicated circumstances encountered during surgery or at SICU admission, such as injury caused by surgery, bleeding and fresh-frozen plasma or prothrombin complex infusion, these parameters cannot predict initial graft function accurately.[23]
In critical illness, prolonged high blood lactate concentrations are associated with a poor prognosis.[24,25]The concentration of lactic acid in blood is a balance between production and clearance. Normally, this balance is optimally maintained. Healthy individuals produce approximately 1400 mmol/day of lactic acid. The lactate is then transported to other tissues, primarily the liver, where it is either converted to bicarbonate or used as a substrate for gluconeogenesis.[26]Since hepatic utilization of lactate depends upon uptake of substrate, gluconeogenic capacity and hepatic blood flow, impaired liver function may affect lactate metabolism, resulting in increased lactate levels. This explains why lactate concentrations are monitored in the perioperative liver transplant period. Orii et al[27]found that blood lactate levels in the immediate postoperative period may be useful for the early evaluation of the new liver's function. Bernal et al[28]reported that arterial blood lactate measurement rapidly and accurately identifies patients who will die from paracetamol-induced acute liverfailure and its use can improve the speed and accuracy of selection of appropriate candidates for transplantation.
Our previous experience of clinical practice confirmed that the monitoring of blood lactate is of great significance in predicting the prognosis and may serve as a guide for treatment in critically ill patients.[29]However, baseline blood lactate level and prognosis is not correlated closely in some patients, especially in liver transplant patients who had undergone surgery of long duration and a prolonged period with a non-functioning liver graft. We found that in this group of patients postoperative blood lactate did not predict severity. Moreover, a discrete sample of blood lactate measured at any particular moment can only show the balance between production and clearance at the time of sampling; it does not accurately reflect progression of the disease, especially when interventions may have dynamic impacts on the production and clearance of lactate.[30]Therefore, the interpretation of single lactate measurement has evident limitations. For example, a patient with IPGF may have a lactate concentration at SICU admission similar to patient without IPGF because they have a similar degree of stress. A single sample of the blood lactate level cannot by itself fully reflect the state of initial liver function after transplantation. One of our principal findings is that there is no significant difference in blood lactate levels between patients with IPGF and those without IPGF, therefore baseline blood lactate levels alone are not meaningful for the early prediction of IPGF in liver transplant patients.
In order to accurately assess initial graft function and patients' responses to interventions, we believe that dynamic monitoring of blood lactate clearance should be carried out. Lactate clearance, especially early clearance as an important index in severe sepsis and septic shock, is reported to perform well in the prediction of prognosis with a high sensitivity and specificity[9,10,31]and is increasingly applied in clinical practice. Similarly, in liver transplant patients, decreased lactate clearance may indicate that liver function is not improving and should be interpreted as a progression of disease, with an increased risk of IPGF. On the other hand, graft function rapidly improved by early and appropriate clinical interventions will result in decreased tissue concentrations of lactate and increased lactate clearance.
Our previous study showed that severe septic patients with early lactate clearance above 10% have better prognosis than those below 10%.[10]The significance of early lactate clearance in assessment of IPGF following liver transplantation is still poorly studied. The purpose of this study was to examine the clinical utility of lactate clearance as early as 6 hours after surgery as an indicator of IPGF. Our results confirmed that the occurrence of IPGF and early lactate clearance are closely related. In patients with IPGF, early lactate clearance was much lower than that in patients without IPGF, a difference that was statistically significant in our material. Due to the different conditions between liver transplantation and sepsis, an early lactate clearance cutoff of 10% cannot be blindly adopted in liver transplant patients. We used ROC analysis to determine the best cutoff of early lactate clearance rate and its sensitivity and specificity in liver transplant patients as a predictor of IPGF. The results showed that at a threshold level of 24.8%, the ROC area under the curve was 0.958, with a sensitivity of 96.5% and a specificity of 87.8% respectively, suggesting that early lactate clearance can effectively predict IPGF.
There are several limitations in our study. First, we could not obtain enough data about donor-related factors. Based on 7 donor variables, Feng et al[32]described a donor risk index (DRI) to quantitatively assess donor liver graft failure. DRI is a marker for evaluating the quality of a graft.[33,34]In our future research, we will compare the predictive performance of lactate clearance and DRI for IPGF. Second, since early lactate clearance reflects the status of patients within as early as six hours, it is better to be seen as a temporary functional index than a decisive determinant of final outcomes. This is why we have focused on the predictive performance of early lactate clearance for IPGF rather than clinical outcomes. We are planning to investigate whether the monitoring of lactate clearance can improve the outcome of liver transplant recipients by prompting ICU teams and surgeons to seek more aggressive interventions at early stages.
Early lactate clearance is a simple and feasible method to obtain an early prediction (within 6 hours) of IPGF. It hence prompts ICU teams and surgeons to diagnose IPGF at an early stage instead of waiting a few days until all the evidence pointing to IPGF emerges. In conclusion, early lactate clearance and the incidence of IPGF in liver transplantation are closely related; an early lactate clearance rate below 24.8% reliably predicts IPGF in liver transplant patients.
Funding:This study was supported by grants from the Natural Science Foundation of Guangdong Province (8151008901000079) and the Sun Yat-Sen University Clinical Research 5010 Program (2007015).
Ethical approval:Not needed.
Contributors:WJF and WRY contributed equally to this work. WJF, WRY and GXD wrote the first draft. All authors contributed to the design and interpretation of the study and to further drafts. GXD provided supportive work and is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Chen H, Peng CH, Shen BY, Deng XX, Shen C, Xie JJ, et al. Multi-factor analysis of initial poor graft function after orthotopic liver transplantation. Hepatobiliary Pancreat Dis Int 2007;6:141-146.
2 Tenza E, Bernardo CG, Escudero D, Otero J, Quindós B, Miyar A, et al. Liver transplantation complications in the intensive care unit and at 6 months. Transplant Proc 2009;41: 1050-1053.
3 Koffron A, Stein JA. Liver transplantation: indications, pretransplant evaluation, surgery, and posttransplant complications. Med Clin North Am 2008;92:861-888, ix.
4 Gaspari R, Cavaliere F, Sollazzi L, Perilli V, Melchionda I, Agnes S, et al. Molecular adsorbent recirculating system (Mars) in patients with primary nonfunction and other causes of graft dysfunction after liver transplantation in the era of extended criteria donor organs. Transplant Proc 2009; 41:253-258.
5 Stockmann M, Lock JF, Malinowski M, Seehofer D, Puhl G, Pratschke J, et al. How to define initial poor graft function after liver transplantation? - a new functional definition by the LiMAx test. Transpl Int 2010;23:1023-1032.
6 Jansen TC, van Bommel J, Bakker J. Blood lactate monitoring in critically ill patients: a systematic health technology assessment. Crit Care Med 2009;37:2827-2839.
7 Murphy ND, Kodakat SK, Wendon JA, Jooste CA, Muiesan P, Rela M, et al. Liver and intestinal lactate metabolism in patients with acute hepatic failure undergoing liver transplantation. Crit Care Med 2001;29:2111-2118.
8 De Gasperi A, Mazza E, Corti A, Zoppi F, Prosperi M, Fantini G, et al. Lactate blood levels in the perioperative period of orthotopic liver transplantation. Int J Clin Lab Res 1997;27: 123-128.
9 Nguyen HB, Rivers EP, Knoblich BP, Jacobsen G, Muzzin A, Ressler JA, et al. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med 2004;32:1637-1642.
10 Xu XD, Wu JF, Guan XD, Chen J, Ou YB, Yang CH. Clinical significance of early lactate clearance in patients with severe sepsis after surgery. Zhong Guo Shi Yong Wai Ke Za Zhi 2007;27:969-970.
11 Della Rocca G, Pompei L, Costa MG, Coccia C, Scudeller L, Di Marco P, et al. Fenoldopam mesylate and renal function in patients undergoing liver transplantation: a randomized, controlled pilot trial. Anesth Analg 2004;99:1604-1609.
12 Nanashima A, Pillay P, Verran DJ, Painter D, Nakasuji M, Crawford M, et al. Analysis of initial poor graft function after orthotopic liver transplantation: experience of an australian single liver transplantation center. Transplant Proc 2002;34:1231-1235.
13 Chen GH. Liver transplantation in China: retrospect and prospect. Chin Med J (Engl) 2009;122:2229-2230.
14 Dutkowski P, De Rougemont O, Müllhaupt B, Clavien PA. Current and future trends in liver transplantation in Europe. Gastroenterology 2010;138:802-809.e1-4.
15 Ploeg RJ, D'Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, et al. Risk factors for primary dysfunction after liver transplantation--a multivariate analysis. Transplantation 1993;55:807-813.
16 Chui AK, Shi LW, Rao AR, Anasuya A, Hagl C, Pillay P, et al. Primary graft dysfunction after liver transplantation. Transplant Proc 2000;32:2219-2220.
17 Chen CL, Concejero AM. Early post-operative complications in living donor liver transplantation: prevention, detection and management. Hepatobiliary Pancreat Dis Int 2007;6:345-347.
18 Clemente Ricote G, Díaz Sánchez A. Early complications after liver transplant. May we go as far as to predict them? Rev Esp Enferm Dig 2008;100:121-128.
19 Cholongitas E, Marelli L, Shusang V, Senzolo M, Rolles K, Patch D, et al. A systematic review of the performance of the model for end-stage liver disease (MELD) in the setting of liver transplantation. Liver Transpl 2006;12:1049-1061.
20 Graziadei I. Liver transplantation organ allocation between Child and MELD. Wien Med Wochenschr 2006;156:410-415.
21 Gallegos-Orozco JF, Vargas HE. Liver transplantation: from Child to MELD. Med Clin North Am 2009;93:931-950, ix.
22 Shaked A, Nunes FA, Olthoff KM, Lucey MR. Assessment of liver function: pre- and peritransplant evaluation. Clin Chem 1997;43:1539-1545.
23 Feng ZY, Xu X, Wu LJ, Wu J, Zhu SM, Zheng SS. Downregulation of endothelin-1 by somatostatin improves liver function of recipients undergoing adult-to-adult living donor liver transplantation. Chin Med J (Engl) 2010;123: 1961-1966.
24 Englehart MS, Schreiber MA. Measurement of acid-base resuscitation endpoints: lactate, base deficit, bicarbonate or what? Curr Opin Crit Care 2006;12:569-574.
25 Levy B. Lactate and shock state: the metabolic view. Curr Opin Crit Care 2006;12:315-321.
26 Marko P, Gabrielli A, Caruso LJ. Too much lactate or too little liver? J Clin Anesth 2004;16:389-395.
27 Orii R, Sugawara Y, Hayashida M, Yamada Y, Kubota K, Takayama T, et al. Peri-operative blood lactate levels in recipients of living-related liver transplantation. Transplantation 2000;69:2124-2127.
28 Bernal W, Donaldson N, Wyncoll D, Wendon J. Blood lactate as an early predictor of outcome in paracetamol-induced acute liver failure: a cohort study. Lancet 2002;359:558-563.
29 Guan XD, Chen J, Ou YB. Hemorrhagic hypovolemic shock: clinical evaluation of oxygen dynamics and blood lactate level. Zhong Guo Shi Yong Wai Ke Za Zhi 2000;20:401-403.
30 James JH, Luchette FA, McCarter FD, Fischer JE. Lactate is an unreliable indicator of tissue hypoxia in injury or sepsis. Lancet 1999;354:505-508.
31 Nguyen HB, Loomba M, Yang JJ, Jacobsen G, Shah K, Otero RM, et al. Early lactate clearance is associated with biomarkers of inflammation, coagulation, apoptosis, organ dysfunction and mortality in severe sepsis and septic shock. J Inflamm (Lond) 2010;7:6.
32 Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant 2006;6:783-790.
33 Vitale A, D'Amico F, Gringeri E, Valmasoni M, Pauletto A, Bonsignore P, et al. Prognostic evaluation of the donor risk index among a prospective cohort of Italian patients undergoing liver transplantation. Transplant Proc 2009;41: 1096-1098.
34 Palmiero HO, Kajikawa P, Boin IF, Coria S, Pereira LA. Liver recipient survival rate before and after model for end-stage liver disease implementation and use of donor risk index. Transplant Proc 2010;42:4113-4115.
Received January 3, 2011
Accepted after revision April 24, 2011
Author Affiliations: Department of Surgical Intensive Care Unit, First Affiliated Hospital, Sun Yat-Sen University, Guangzhou 510080, China (Wu JF, Wu RY, Chen J, Ou-Yang B, Chen MY and Guan XD)
Xiang-Dong Guan, MD, Department of Surgical Intensive Care Unit, First Affiliated Hospital, Sun Yat-Sen University, Guangzhou 510080, China (Tel: 86-20-87755766ext8454; Fax: 86-20-87755766ext8080; Email: carlg@163.net)
© 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(11)60100-8
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Harmine induces apoptosis in HepG2 cells via mitochondrial signaling pathway
- Clinicopathological analysis of 14 patients with combined hepatocellular carcinoma and cholangiocarcinoma
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Undifferentiated embryonal sarcoma of the liver presenting as a hemorrhagic cystic tumor in an adult
- Fulminant liver failure models with subsequent encephalopathy in the mouse
- Risk factors of intrahepatic cholangiocarcinoma in patients with hepatolithiasis: a case-control study
