Pathological changes at early stage of multiple organ injury in a rat model of severe acute pancreatitis
2010-12-14XiPingZhangJieZhangMeiLiMaYangCaiRuJunXuQiXieXinGeJiangandQianYe
Xi-Ping Zhang, Jie Zhang, Mei-Li Ma, Yang Cai, Ru-Jun Xu, Qi Xie,Xin-Ge Jiang and Qian Ye
Hangzhou, China
Pathological changes at early stage of multiple organ injury in a rat model of severe acute pancreatitis
Xi-Ping Zhang, Jie Zhang, Mei-Li Ma, Yang Cai, Ru-Jun Xu, Qi Xie,Xin-Ge Jiang and Qian Ye
Hangzhou, China
(Hepatobiliary Pancreat Dis Int 2010; 9: 83-87)
severe acute pancreatitis;pathological changes;multiple organs;injury
Introduction
The features of severe acute pancreatitis (SAP) are fatal pathogenic conditions, rapid progression and high mortality. It is usually complicated by systemic in fl ammatory response syndrome and multiple organ dysfunction syndrome.[1,2]Current studies have shown that some in fl ammatory mediators play an important role in SAP complicated with multiple organ injury.[3-5]The pathological changes in multiple organs and in fl ammatory mediators in plasma at early stage of SAP were studied in rats, and the causes of multiple organ injury were analyzed. We explored the underlying mechanism of SAP, provided a theoretical basis for its early pathological changes, and identi fi ed relevant in fl ammatory mediators.
MethodsExperimental animals and reagents
Thirty clean grade healthy male Sprague-Dawley rats weighing 250-300 g were purchased from the Experimental Animal Center of Zhejiang University School of Medicine (Hangzhou, China).
Sodium taurocholate and sodium pentobarbital were from Sigma-Aldrich China Inc. (Shanghai, China).A fully automatic biochemical analyzer was used to determine the concentration of plasma amylases (U/L). The kits used were plasma endotoxin tachypleus amebocyte lysate kit, Shanghai Yihua Medical Science and Technology Corp. (Institute of Medical Analysis,Shanghai, China); IL-6 ELISA, pg/ml (ng/L), Shanghai Shenxiong Biotech Co. (China); TNF-α ELISA, pg/ml(ng/L), Jingmei Bioengineering Corp. (China); serum secretory phospholipase A2 enzyme assay ELA (PLA2),U/ml, R&D Systems Inc. (USA); serum nitrogen oxide(NO), μmol/L, Nanjing Jiancheng Bioengineering Research Institute, China; serum endothelin-1 ELA(ET-1), ng/L (pg/ml), Cayman Chemical Co. (USA;Catalog Number: 583151); and serum malonaldehyde(MDA), nmol/ml, Nanjing Jiancheng Bioengineering Research Institute. All tests were made according to the instructions with the kits.
Empirical methods and grouping
The improved Aho method[6]was used to induce SAP in 15 rats.[7,8]The method of animal model preparation was as follows: Rats were anesthetized by intraperitoneal injection of 2% sodium pentobarbital (0.25 ml/100 g).In the model control group, we identi fi ed the duodenal papilla inside the duodenum duct wall, and then used a No. 5 needle to drill a hole in the avascular area of the mesentery. A segmental epidural catheter was inserted into the duodenum cavity through the hole, and inserted retrogradely into the biliary-pancreatic duct through the papilla. This was followed by retrograde transfusion of 3.5% sodium taurocholate (0.1 ml/100 g) by a microinjection pump at 0.2 ml/min. The hole in the lateral duodenal wall was then sutured.
Another 15 normal rats were randomly assigned to the sham-operated group. We opened the abdominal cavity in the sham-operated group, turned over the pancreas and duodenum, and closed the abdomen. The rats in all groups were sacri fi ced by euthanasia 3 hours after operation.
After blood was collected from the heart and tissue sample from multiple organs, we observed pathological changes in the pancreas, liver, kidney, terminal ileum,lung, spleen, lymph nodes, thymus, heart and brain; and assessed the levels of IL-6, TNF-α, PLA2, NO, ET-1 and MDA in serum, and these of amylase and endotoxin in plasma.
Statistical analysis
Statistical analysis was made with SPSS 11.5 statistical software (SPSS, Chicago, IL). The Kruskal-Wallis test or analysis of variance data were recorded as mean±SD (PLA2 only), the others blood indices were recorded as M(QR)). All of them were applied for the comparison of the two groups. Bonferroni's correction was also applied for comparison. A P value ≤0.05 was considered statistically signi fi cant.
ResultsBlood level indices
In the model control group, the levels of IL-6, TNF-α and NO in serum and the levels of amylases and endotoxin in plasma were higher than those in the sham-operated group (P<0.001). In contrast, the levels of ET-1 and MDA in the model control group were higher than in the sham-operated group (P<0.01). The level of serum PLA2 in the model group was higher than that in the sham-operated group (P<0.001) (Table).
Pathological changes in multiple organs of the sham-operated groupGross changes
The overall structure of the pancreas remained intact and neither hemorrhage nor evident abnormalities were seen. The color and morphology of the lung were normal. There were no bleeding points on the lung surface or effusions in the thoracic cavity. No swelling was evident in the liver, and its color was normal.The appearance of the kidney was normal without swelling. Neither bleeding points on the surface of the renal cortex nor visible intestinal dilation, intestinal wall hyperemia or edema were found. The intestinal mucosal surface was smooth, without bleeding and ulcers. The morphology of thymus tissues, spleen, heart(myocardium), brain and lymph nodes was normal.
Changes under a light microscope
Pancreas samples showed normal intact gland structure and mild interstitial edema in a few cases.Neutrophil in fi ltration was occasional. No acinar cell, fat necrosis, and hemorrhage were observed. The structure of lung tissues was normal. Some tissuesshowed slight edema and in fl ammatory cell in fi ltration of the interstitium and alveolar wall. Complete structure of hepatic lobules and occasional in fi ltration of in fl ammatory cells in the portal area were also found.Hepatocytes were normal morphologically, but some liver tissues showed local swelling of hepatocytes, cholestasis and stenosis of the sinus hepaticus. Renal glomeruli were normal pathologically in addition to tubules and interstitium in most rats. Swelling and blurry boundaries of renal tubular epithelial cells and stenosis of lumens were found in a few rats. Integrated epidermis and microvillus structure of intestinal mucosa were seen without exfoliation, necrosis or edema in the propria layer,submucosa and placenta percreta in most rats. Thymus structure was normal, with a clear boundary between the cortex and medulla, evident lobules, and an intact envelope. A few epithelial cells with nuclei stained slightly in the medulla, and a few cells with "vacuoles" were seen. Mild dilation of the splenic pulp blood sinus, blood stagnation, and thickened splenic arteriole walls were seen in a few rats. No pathological changes were found in the myocardium, brain tissue and lymph nodes in all rats.

Table. Index levels in blood (M(QR) or mean±SD)
Pathological changes of multiple organs in the model control groupGross changes
Pathological changes were more severe in the pancreas tail than in the head, and congestion, edema,hemorrhage and necrosis were evident. Mild liver swelling was found, and local, gray plaques with obscure borders occurred in the liver of individual rats. There were congestion and edema in the pulmonary lobes on both sides, red bleeding points on the surface of local pulmonary lobes, and mild effusions in the thoracic cavity. The color and texture of the spleen were normal in most rats, but congestion was found in a few. Ileum,heart, lymph nodes, thymus and brain had no visible pathological changes.
Changes under a light microscope
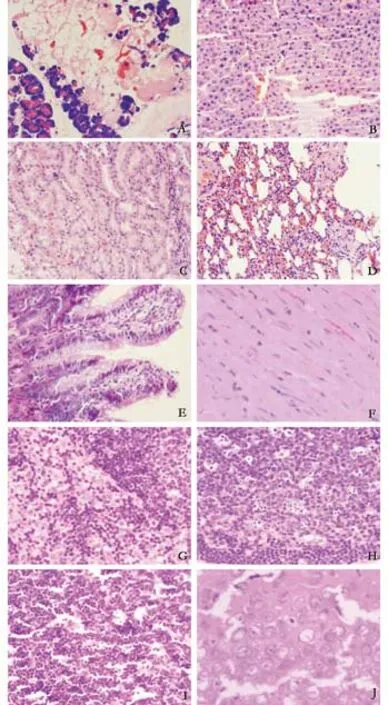
Fig. The multiple organ injury of the model control group at 3 hours (HE, all original magnification ×400). A: pancreas(massive necrosis of acini); B: liver (lamellar necrosis of hepatic cells); C: kidney (scattered necrosis in the epithelium of renal tubules); D: lung (interstitial edema); E: intestinal mucosa(necrosis and exfoliation of endothelial cells of intestinal villi);F: myocardium (unclear transverse striations); G: Lymph node(dilation of lymphatic sinus and sinus cell hyperplasia); H: lymph node (dilation and spotty necrosis of germinal center); I: thymus(starry sky change of thymic cortex); J: brain (swelling of cerebral pyramidal cells and vacuolated nuclei).
In pancreas samples, interstitial congestion and edema, mild in fl ammatory cell in fi ltration, focal necrosis and mild interstitial hemorrhage were observed.Local hemorrhage and necrosis became con fl uent fl akes. There were also capillary congestion, thrombosis and in fi ltration of red cells into the interstitium (Fig.A). In liver tissue, there were hepatocyte swelling,acidophilic denaturation, concentration of apoptotic bodies, in fi ltration of in fl ammatory cells and leukocyte adhesion to vessel walls in the portal area, white clots in venules, dilation and congestion of the sinus hepaticus,and scattered spotty necrosis in hepatic lobules (Fig. B).In kidney samples, capillary congestion in renal glomeruli,swelling, scattered necrosis and blurry boundaries in renal tubule epithelial cells, stenosis or atresia of lumens,visible protein casts, interstitial edema, in fl ammatory cell in fi ltration and thrombosis were found (Fig. C). There were edema in the lung interstitium and alveolar space,broadened alveolar wall interstitium, in fl ammatory cell in fi ltration, telangiectasis and congestion in the alveolar wall, and broadened alveolar septa (Fig. D). In intestinal mucosa, focal necrosis of the ileal mucosa and in fl ammatory cell in fi ltration in various mucosal layers were found in most rats. Con fl uence, exfoliation and defection of villi, broadened intervillous lacunae, parce arrangement of mucosal glands, decrease of beaker cells and atrophic mucosa were observed in a few rats (Fig.E). Focal necrosis of white pulp and lymphoid follicles and thickened walls of splenic arterioles were found in the spleen of most rats. There were visible dilation and congestion of blood sinuses in the red pulp. The spleen tissue was normal in a few rats. Granulation or lysis of the cytoplasm of cardiac muscle fi bers was found in individual rats. Mild in fl ammatory cell in fi ltration of the myocardial interstitium was found occasionally. There was mild in fl ammatory cell in fi ltration of the epicardium in a few rats (Fig. F). Swelling of lymph nodes, dilation of germinal centers in the nodes and lymphatic sinuses,and hyperplasia of sinus cells were seen (Fig. G). Spotty necrosis in the mantle zone and germinal centers of lymphatic follicles was found in most rats but mild in fl ammatory cell in fi ltration in a few (Fig. H). Mild histological changes were found in the thymus, with"starry sky" epithelial cells, fragmentation of nuclei and decreased lymphocytes. The nuclei of epithelial cells in the medulla were stained slightly, and vacuolated epithelial cells occurred in some rats (Fig. I). The brain was normal pathologically in most rats. Mild swelling of neurons and brain edema were only found in a few rats (Fig. J).
Discussion
Current studies have con fi rmed that pancreatic injury during SAP is complicated by injury of multiple organs including the liver, lung, kidney, ileum, brain and heart.[8-10]The pathological changes in these organs aggravate with time. There have been no reports about injury of the thymus, lymph nodes and spleen or the corresponding pathological changes. In this study,pathological changes were found 3 hours after operation in multiple organs, mainly edema, necrosis, hemorrhage and in fl ammatory cell in fi ltration. Thrombosis was also found in the pancreas, liver and kidney, and congestion was found in the lung and spleen. The multiple organ injuries occurred in the early stage of SAP in rats. To understand the severity of multiple organ injury in the early stage is very important for SAP treatment.
The following are believed to be the underlying mechanisms of multiple organ injury in SAP: (1)Various causative agents may cause injury of pancreatic acinar cells. With the release of pancreatin and activation of mononuclear macrophages, the excess of neutrophilic leukocytes may produce or release a great deal of in fl ammatory mediators which form a network to cause in fl ammatory "cascade effects". This results in multiple organ injury.[11,12](2) These in fl ammatory mediators include endotoxin, IL-6, TNF-α, PLA2, NO, ET-1 and MDA. Endotoxin permeates into blood through damaged intestinal mucosa at early stages. The mononuclear phagocyte system is activated by endotoxin in blood and initiates an in fl ammatory reaction, which aggravates the injury of the intestinal mucosal epithelium, inhibits the proliferation of intestinal endothelial cells and further delays recovery from SAP. Therefore, this causes endotoxin and cytokines to form a vicious cycle.[13,14]We suggest that endotoxin plays an important role in promoting SAP during the progression of multiple organ injury. As one of the important cytokines participating in the pathogenesis of SAP,[15]TNF-α, a primary proin fl ammatory factor, directly injures the cells of multiple organs, and causes ischemia, hemorrhage, necrosis,in fl ammation and edema. As the secondary chemotactic factor of in fl ammatory factors, TNF-α initiates a cascade reaction, accumulates neutrophilic leucocytes,increases ICAM and VCAM levels, and stimulates the production of NO, ROS and other pro-in fl ammatory factors such as IL-6 and IL-1β.[16]IL-6 has extensive proin fl ammatory effects to cause tissue damage. IL-6 also in fl uences the coagulation and fi brinolytic systems, and causes thrombosis of fi brin in blood vessels.[17]PLA2 is an important mediator of multiple organ injury.When SAP occurs, a great amount of PLA2 is released by polymorphonuclear leukocytes and mononuclear macrophages, stimulated by endotoxin. This enters the blood, attacks and degrades phospholipids in membranes, damages membrane stability and causes massive leakage of lysosomal enzymes. On the other hand, PLA2 generates bioactive free fatty acids and lytic lecithin to destroy the functions and structures of cells and organs.[18,19]As a fi nal common in fl ammatory mediator of the cascade reaction in the in fl ammatory reaction,[20]NO is regarded as an index for predicting the pathological severity of SAP. Some researchers believe that a low concentration of endogenous NO protects against ischemic reperfusion injury, prevents the increase of intestinal vasopermeability, and obviates endotoxemia and bacteria translocation. MDA is a stable metabolite of oxygen free radicals. Because oxygen free radicals attack biological structures and biochemical compounds, MDA indirectly re fl ects the severity of their effects. ET causes continuous spasm of pancreatic capillaries and promotes the in fl ow of calcium ions to directly injure pancreatic cells. ET also causes contraction of coronary arteries and in fl uences heart function to injure pancreatic tissue indirectly.[21]
As shown in the experimental results, the levels of in fl ammatory mediators were higher in the model control than in the sham-operated group in this study.In fl ammatory cell in fi ltration was found in the pancreas,liver, kidney, lung, ileum, heart, lymph nodes and other tissues, and pathological changes such as edema,hemorrhage and necrosis were found in multiple organs of the model control group. All these changes indicate that the interaction of in fl ammatory mediators causes the pathological changes in multiple organs at the early stage of SAP.
Funding: This study was supported by grants from the Intensive Foundation Project for Technology of Hangzhou (2004Z006).
Ethical approval: This study was approved by the Ethics Committee of the hospital.
Contributors: ZXP, ZJ and MML wrote the fi rst draft. All authors contributed to the intellectual context and approved the fi nal version. ZXP is the guarantor.
Competing interest: No bene fi ts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Rau BM, Krüger CM, Hasel C, Oliveira V, Rubie C, Beger HG,et al. Effects of immunosuppressive and immunostimulative treatment on pancreatic injury and mortality in severe acute experimental pancreatitis. Pancreas 2006;33:174-183.
2 Rau BM, Bothe A, Kron M, Beger HG. Role of early multisystem organ failure as major risk factor for pancreatic infections and death in severe acute pancreatitis. Clin Gastroenterol Hepatol 2006;4:1053-1061.
3 Makhija R, Kingsnorth AN. Cytokine storm in acute pancreatitis. J Hepatobiliary Pancreat Surg 2002;9:401-410.
4 Pereda J, Sabater L, Cassinello N, Gómez-Cambronero L, Closa D, Folch-Puy E, et al. Effect of simultaneous inhibition of TNF-alpha production and xanthine oxidase in experimental acute pancreatitis: the role of mitogen activated protein kinases. Ann Surg 2004;240:108-116.
5 Gómez-Cambronero LG, Sabater L, Pereda J, Cassinello N,Camps B, Vina J, et al. Role of cytokines and oxidative stress in the pathophysiology of acute pancreatitis: therapeutical implications. Curr Drug Targets In fl amm Allergy 2002;1:393-403.
6 Aho HJ, Koskensalo SM, Nevalainen TJ. Experimental pancreatitis in the rat. Sodium taurocholate-induced acute haemorrhagic pancreatitis. Scand J Gastroenterol 1980;15:411-416.
7 Zhang XP, Zhang L, Chen LJ, Cheng QH, Wang JM, Cai W, et al. In fl uence of dexamethasone on in fl ammatory mediators and NF-kappaB expression in multiple organs of rats with severe acute pancreatitis. World J Gastroenterol 2007;13:548-556.
8 Zhang XP, Zhang L, Wang Y, Cheng QH, Wang JM, Cai W,et al. Study of the protective effects of dexamethasone on multiple organ injury in rats with severe acute pancreatitis.JOP 2007;8:400-412.
9 Zhang XP, Tian H, Wu DJ, Feng GH, Chen L, Zhang J, et al. Pathological changes in multiple organs of rats with severe acute pancreatitis treated by baicalin and octreotide.Hepatobiliary Pancreat Dis Int 2009;8:85-92.
10 Xu GF, Lu Z, Gao J, Li ZS, Gong YF. Effect of ecoimmunonutrition supports on maintenance of integrity of intestinal mucosal barrier in severe acute pancreatitis in dogs. Chin Med J (Engl) 2006;119:656-661.
11 Kimura Y, Hirota M, Okabe A, Inoue K, Kuwata K, Ohmuraya M, et al. Dynamic aspects of granulocyte activation in rat severe acute pancreatitis. Pancreas 2003; 27:127-132.
12 Mikami Y, Takeda K, Shibuya K, Qiu-Feng H, Egawa S,Sunamura M, et al. Peritoneal in fl ammatory cells in acute pancreatitis: Relationship of in fi ltration dynamics and cytokine production with severity of illness. Surgery 2002;132:86-92.
13 Rahman SH, Ammori BJ, Holm fi eld J, Larvin M, McMahon MJ. Intestinal hypoperfusion contributes to gut barrier failure in severe acute pancreatitis. J Gastrointest Surg 2003;7:26-36.
14 Ammori BJ, Fitzgerald P, Hawkey P, McMahon MJ. The early increase in intestinal permeability and systemic endotoxin exposure in patients with severe acute pancreatitis is not associated with systemic bacterial translocation: molecular investigation of microbial DNA in the blood. Pancreas 2003;26:18-22.
15 Zhao YF, Zhai WL, Zhang SJ, Chen XP. Protection effect of triptolide to liver injury in rats with severe acute pancreatitis.Hepatobiliary Pancreat Dis Int 2005;4:604-608.
16 Lundberg AH, Granger N, Russell J, Callicutt S, Gaber LW,Kotb M, et al. Temporal correlation of tumor necrosis factoralpha release, upregulation of pulmonary ICAM-1 and VCAM-1, neutrophil sequestration, and lung injury in dietinduced pancreatitis. J Gastrointest Surg 2000;4:248- 257.
17 Pooran N, Indaram A, Singh P, Bank S. Cytokines (IL-6,IL-8, TNF): early and reliable predictors of severe acute pancreatitis. J Clin Gastroenterol 2003;37:263-236.
18 Kihara Y, Yoshikawa H, Honda H, Fukumitsu K, Yamaguchi T, Otsuki M. Natural disruption of group 2 phospholipase A2 gene protects against choline-de fi cient ethionine-supplemented diet-induced acute pancreatitis and lung injury.Pancreas 2005;31:48-53.
19 Tomita Y, Kuwabara K, Furue S, Tanaka K, Yamada K,Ueno M, et al. Effect of a selective inhibitor of secretory phospholipase A2, S-5920/LY315920Na, on experimental acute pancreatitis in rats. J Pharmacol Sci 2004;96:144-154.
20 Um SH, Kwon YD, Kim CD, Lee HS, Jeen YT, Chun HJ, et al. The role of nitric oxide in experimental cerulein induced pancreatitis. J Korean Med Sci 2003;18:520-526.
21 Inoue K, Hirota M, Kimura Y, Kuwata K, Ohmuraya M,Ogawa M. Further evidence for endothelin as an important mediator of pancreatic and intestinal ischemia in severe acute pancreatitis. Pancreas 2003;26:218-223.
BACKGROUND: Severe acute pancreatitis (SAP) is a commonly seen acute abdominal syndrome characterized by sudden onset, rapid progression and high mortality rate.The damage in peripheral organs may be more severe than that in the pancreas, and can even lead to multiple organ dysfunction. It is critical to recognize early pathological changes in multiple organs. This study aimed to assess the early pathological features of damaged organs in a rat model of SAP.
METHODS: Thirty clean grade healthy male Sprague-Dawley rats weighing 250-300 g were randomly divided into a model control group (n=15) and a sham-operated group (n=15). The SAP rat model was induced by sodium taurocholate. Samples of blood and from multiple organs were collected 3 hours after operation. We assessed the levels of IL-6, TNF-α, PLA2, NO,ET-1, MDA, amylases and endotoxin in blood and observed the early pathological changes in multiple damaged organs.
RESULTS: Levels of IL-6, TNF-α, PLA2, NO, ET-1 and MDA in serum and of amylase and endotoxin in plasma of the model control group rats were signi fi cantly higher than those of the sham-operated group (P<0.01). Different degrees of pathological change were observed in multiple damaged organs.
CONCLUSION: Multiple organ injury may occur at the early stage of SAP in rats.
Author Af fi liations: Department of General Surgery (Zhang XP, Cai Y and Xie Q), and Department of Pathology (Xu RJ), Hangzhou First People's Hospital, Hangzhou 310006, China; Zhejiang University of Traditional Chinese Medicine, Hangzhou 310053, China (Zhang J, Ma ML, Jiang XG and Ye Q)
Xi-Ping Zhang, MD, Department of General Surgery, Hangzhou First People's Hospital, Hangzhou 310006, China (Tel:86-571-87065701; Fax: 86-571-87914773; Email: zxp99688@vip.163.com)
© 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Critical fl icker frequency for diagnosis and assessment of recovery from minimal hepatic encephalopathy in patients with cirrhosis
- Risk factors for early recurrence of smallhepatocellular carcinoma after curative resection
- Potential etiopathogenesis of seventh day syndrome following living donor liver transplantation: ischemia of the graft?
- Comparatively lower postoperative hepatolithiasis risk with hepaticocholedochostomy versus hepaticojejunostomy
- Effect of sodium salicylate on oxidative stress and insulin resistance induced by free fatty acids
- Endoscopic nasojejunal feeding tube placement in patients with severe hepatopancreatobiliary diseases:a retrospective study of 184 patients
