Critical fl icker frequency for diagnosis and assessment of recovery from minimal hepatic encephalopathy in patients with cirrhosis
2010-12-14PraveenSharmaBarjeshChanderSharmaandShivKumarSarin
Praveen Sharma, Barjesh Chander Sharma and Shiv Kumar Sarin
New Delhi, India
Critical fl icker frequency for diagnosis and assessment of recovery from minimal hepatic encephalopathy in patients with cirrhosis
Praveen Sharma, Barjesh Chander Sharma and Shiv Kumar Sarin
New Delhi, India
(Hepatobiliary Pancreat Dis Int 2010; 9: 27-32)
minimal hepatic encephalopathy;critical fl icker frequency;lactulose
Introduction
Minimal hepatic encephalopathy (MHE) impairs the ability to carry out daily activities and the health-related quality of life, and produces an increased risk of traf fi c accident.[1-9]
Some studies have used computerized tests for the diagnosis of MHE and the assessment of the ability to drive.[4,6,8]MHE may be a marker for future episodes of clinical hepatic encephalopathy (HE).[10-12]Treatment with lactulose is of bene fi t as it decreases ammonia production and absorption by modulating the gut microecology.[9,10,13-15]Psychometric and electrophysiologic tests are currently used to detect MHE.[1-19]However,psychometric tests are in fl uenced by variables like age, educational status, and learning effect, and may over-diagnose MHE if corrections are not applied.[1]Neurophysiological tests (P300 event related potential)provide more objective results but cannot be done at the bedside.[1,12,15-17]In an AASLD survey, only a minority of hepatologists were able to test for MHE >50% of the time and 38% of respondents never tested for it. The main barriers to MHE testing are adding time to clinic visits and lack of standardized norms.[20]Diagnostic tools for MHE to be used in a clinical routine must be easy to use and should ful fi ll several preconditions, i.e.,they must be sensitive, valid, objective, and reliable.Recently, critical fl icker frequency (CFF) has been used for the diagnosis of MHE as many of the problems can be circumvented.[21-23]Its utility for the assessment of recovery of patients who were treated for MHE has never been evaluated.
Methods
Patient population
One hundred and fi fty consecutive cirrhotic patients without HE were screened for MHE. Cirrhosis was diagnosed on a clinical basis involving laboratory tests,endoscopic evidence, sonographic fi ndings, and liver histology if available. The exclusion criteria were the presence of overt HE or a history of HE at enrollment,a history of taking lactulose or any antibiotics, alcohol intake, gastrointestinal hemorrhage or spontaneous bacterial peritonitis during the past 12 weeks, previous TIPS or shunt surgery, signi fi cant co-morbid illness such as heart, respiratory or renal failure, and any neurologic diseases such as Alzheimer's disease, Parkinson's disease and nonhepatic metabolic encephalopathies. Patients with color blindness, mature cataract or diabetic retinopathy and patients on psychoactive drugs such as antidepressants or sedatives and antileptic drugs were also excluded. Forty patients were excluded with a history of recent bleeding (n=12), lactulose intake (n=15),a history of HE (n=10) or hepatocellular carcinoma(n=3). Fifty healthy controls without evidence of acute or chronic liver disease served as age-matched controls for P300 auditory event related potential (P300ERP) and CFF.All patients who were enrolled in the study underwent psychometric tests, P300ERP, and CFF at the time of enrollment. Patients who were diagnosed with MHE and treated with lactulose underwent repeat psychometric tests, P300ERP and CFF one month after starting the treatment (4 weeks treatment and measurement directly after the last lactulose administration). All patients underwent ultrasound and Doppler/CT abdomen studies to look for spontaneous shunts. Informed consent was obtained and the research protocol was approved by the ethics committee of the hospital in accordance with the ethical guidelines of the 1975 Declaration of Helsinki.
Psychometric testing
All patients underwent a series of psychometric tests including number connection tests (NCT-A, NCT-B)if literate, and fi gure connection tests (FCT-A, FCT-B)if illiterate. Four parallel forms are available for both NCT and FCT, and we used 2 of them to avoid learning effects, one at the start, the other at the end. In the NCT-A, which measures cognitive motor abilities,patients connected numbers from 1 to 25 printed on paper as quickly as possible. In the NCT B, letters were also included and the patients connected alternating numbers and letters (1-A-2-B-3...L-13). In principle, the FCT was similar to the NCT, except that numbers were replaced by fi gures. Each circle had one to fi ve motifs,thus giving the required 25 fi gures. FCT is a universally applicable test for the assessment of mental status which transcends the barriers of illiteracy and linguistic difference. The test score is the time required to complete the test, including the time needed to correct any errors.
The results of tests were considered abnormal when test scores were more than the mean +2SD from ageand education-matched controls.[24]The result of a psychometric test was considered abnormal when the results of both NCT-A and NCT-B or FCT-A and FCT-B were abnormal.
P300 auditory event related potential
The P300 response was elicited by the standard"auditory odd-ball paradigm". The fi rst major positive peak at 250-500 msec for the rare tone was identi fi ed as the P300 response and was marked. Latency was measured from the point of stimulus to the peak of the P300 waveform in msec. A P300ERP latency was considered abnormal if it was above +2SD of the mean latency measured in age-matched controls.
Measurement of CFF threshold
CFF threshold was used to measure visual discrimination and general arousal. CFF was determined with a HEPAtonorm analyzer at the bedside. Patients were fi rst instructed and trained about the procedure.Flicker frequencies were measured 8 times and the mean value was calculated. The whole procedure took about 15 minutes. CFF threshold was measured by intrafoveal stimulation with a luminous diode. Decreasing the frequency of the light pulses from 60 Hz downward, the CFF threshold was determined as the frequency when the impression of fused light turned to a fl ickering one.The CFF thresholds and psychometric measurements were determined on the same day. CFF was considered abnormal when the value was <39 Hz.[23]It was measured at the time of enrollment and after one month of treatment (4 weeks treatment and measurement directly after the last lactulose administration)
Dietary habits
Protein intake was not restricted in the enrolled patients. As most of our patients were strict vegetarians(85%), they took vegetable-based protein (1 gm/kg body weight) in supplementation with casein-based protein.Salt was restricted to <2 gm/day in every patient. The diets were routinely supplemented with vitamins. The patients who were on minimal diuretics or on beta blockers continued their medication.
Assessment of MHE
Patients without evidence of manifesting HE according to the mental status (West-Haven criteria) were de fi ned as having no HE. MHE was de fi ned by abnormal results(>2SD) of psychometric tests (NCT-A and NCT-B or FCT-A and FCT-B) and abnormal P300 ERP. Response to treatment was de fi ned by normalization (<2SD of age-matched controls) of the abnormal parameters of tests. Patients with abnormalities (>2SD) in either psychometry or P300ERP after therapy were classi fi ed as non-responders.
Blood tests and biochemical examinations
After overnight fasting, venous blood was taken for routine liver function and hematologic tests. Venous ammonia was measured within 3 minutes after blood sampling by the ammonia checker Ⅱ (Daiichi Kagaku Co. Ltd., Kyoto, Japan) before and after treatment for one month.
Treatment
Patients were given 30-60 ml lactulose per day so that they passed 2-3 semi-formed stools every day.Compliance with the therapy was assured primarily by ensuring increased stool frequency and a change to a softer consistency and by counting the number of bottles of lactulose consumed.
Statistical analysis
Data were given as mean±SD. Statistical analysis was made using Student's paired t test and Fisher's exact test. Correlations between different tests were calculated by Spearman's rank-order correlation coef fi cient. A signi fi cance level of 0.05 was used in all analyses.Statistical analyses were made using SPSS version 10.0(SPSS, Chicago, IL) in this study.
Results
In the 110 patients (Child Turcott Pugh score A∶B∶C 39∶42∶29; age 41.6±11.6 years; M∶F 82∶28) (Table 1), 60(54.5%) were diagnosed with MHE. Baseline characteristics of MHE and non-MHE patients were compared(Table 2). Among the patients with MHE, 17/39 (44%)were in Child A, 21/42 (50%) in Child B, and 22/29 (76%)in Child C. There was a signi fi cant difference between Child C vs. Child A and B, but no difference between Child A and B. These 60 patients were followed up for one month, and repeat psychometry, P300ERP and CFF were determined immediately after 4 weeks of treatment.Of the 60 patients treated with lactulose, 34 (57%)recovered, but 26 (43%) continued to show abnormal results of psychometric or P300ERP tests.

Table 1. Demographic, clinical, and biochemical characteristics of the study group (n=110)
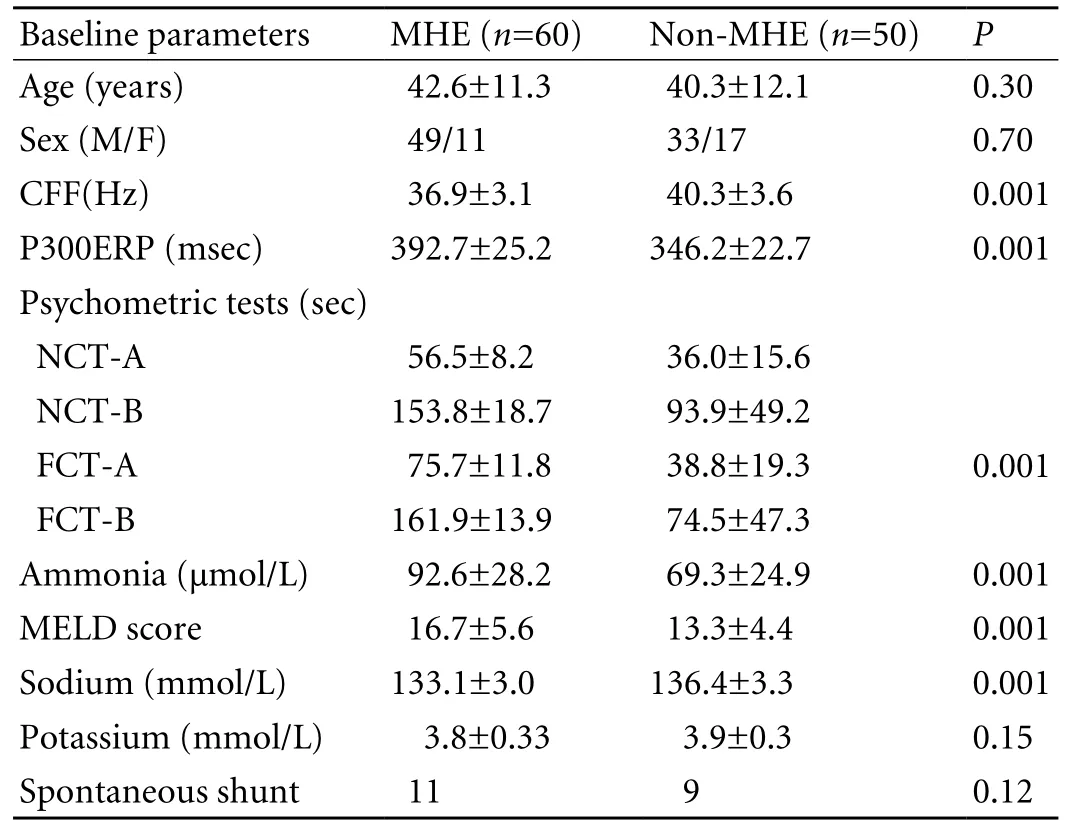
Table 2. Demographic, clinical, and biochemical characteristics of patients with and without MHE
Psychometric tests
Out of the 110 patients, 21 were illiterate (unable to read and write), 69 were sub-graduates (≤12 years of formal education), and 20 were graduates (holder of bachelor degree with 15 years of formal education). Of these 110 patients, 89 (81%) could have NCT and 21(19%) FCT due to illiteracy. Seventy- fi ve patients (68%)had abnormal results of psychometric tests, and 15 (20%)with abnormal results of psychometric tests had normal P300ERP. After one-month treatment of MHE, the results of psychometry was abnormal in 22 patients (36.6%).
Determination of P300ERP in controls and in cirrhotics with and without MHE
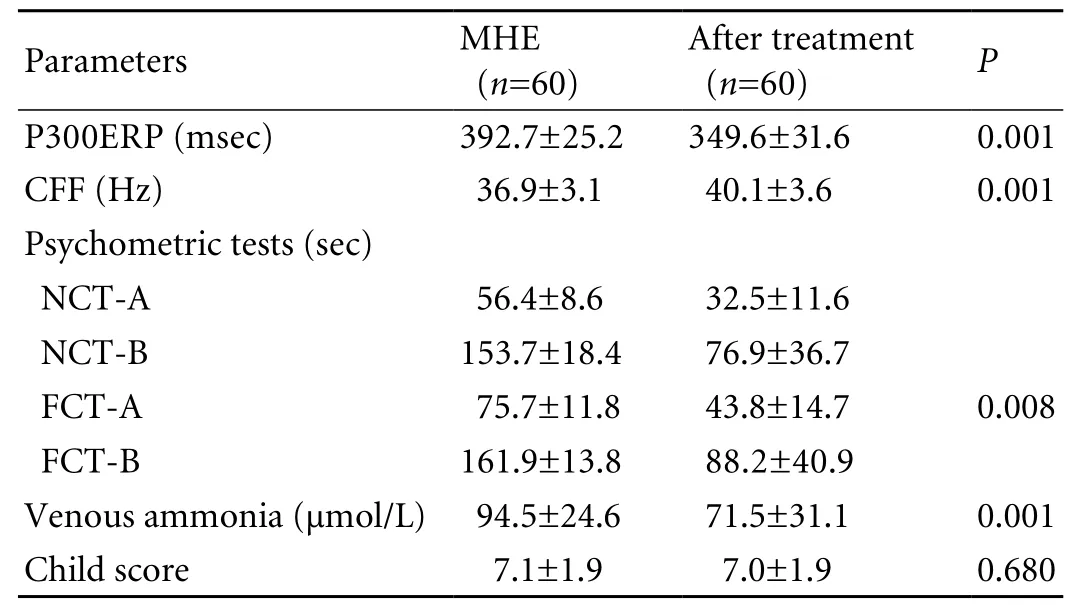
Table 3. Treatment response in MHE patients
The latency of P300ERP in controls (n=50) was 326.8±12.5 msec (range 298-351). The value of P300ERP was considered abnormal when it was above +2 SD in controls (>352 msec)
Of the 110 patients, 74 (67%) had a delayed P300ERP.Patients with abnormal results of psychometry had a more delayed P300ERP (383.5±29.5 msec) than those with normal results of psychometry (347.1±7.3 msec;P<0.005). Fourteen patients (19%) with an abnormal P300ERP had normal results of psychometric tests.There was a signi fi cant reduction in the P300ERP latency after treatment (392.7±25.2 vs. 349.6±31.6 msec,P=0.001), and P300ERP was abnormal in 21 patients(35%) after treatment (Table 3).
CFF in MHE patients before and after treatment
In the control group (n=50), mean CFF was 42.8±1.4 Hz (40.2-46.0) and these patients were not given a repeat CFF estimation after one month. CFF was <39 Hz in 72 patients (65.4%) before treatment. Patients with CFF <39 Hz had a signi fi cantly lower P300ERP than those with CFF >39 Hz (382.5±30.7 vs. 350.7±28.4 msec,P=0.001). CFF sensitivity, speci fi city, positive predictive value, negative predictive value, and diagnostic accuracy for the diagnosis of MHE and assessment of its recovery are shown in Table 4.
Correlation of CFF with P300ERP, venous ammonia level, psychometry parameters, and Child score before and after treatment in cirrhotic patients
CFF was correlated signi fi cantly with psychometric tests (NCT-A), venous ammonia, and P300ERP before and after treatment (Table 5).
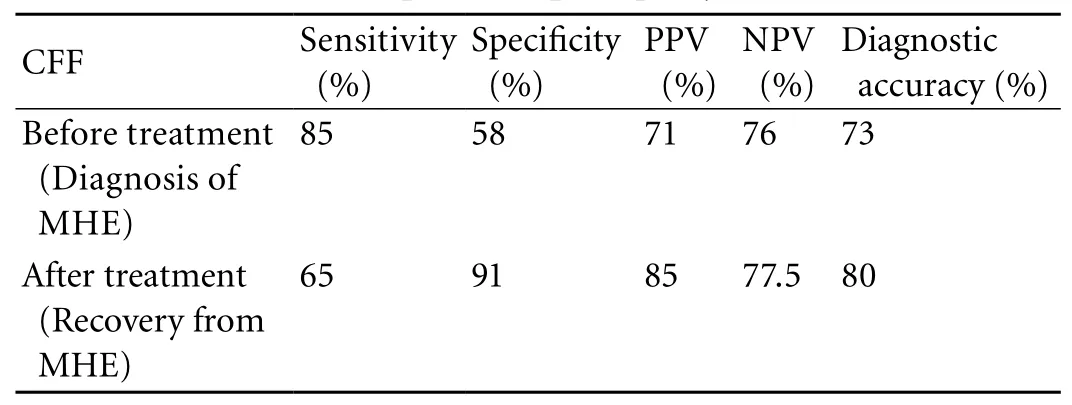
Table 4. CFF sensitivity, speci fi city, positive predictive value, negative predictive value, and diagnostic accuracy for the diagnosis and assessment of minimal hepatic encephalopathy
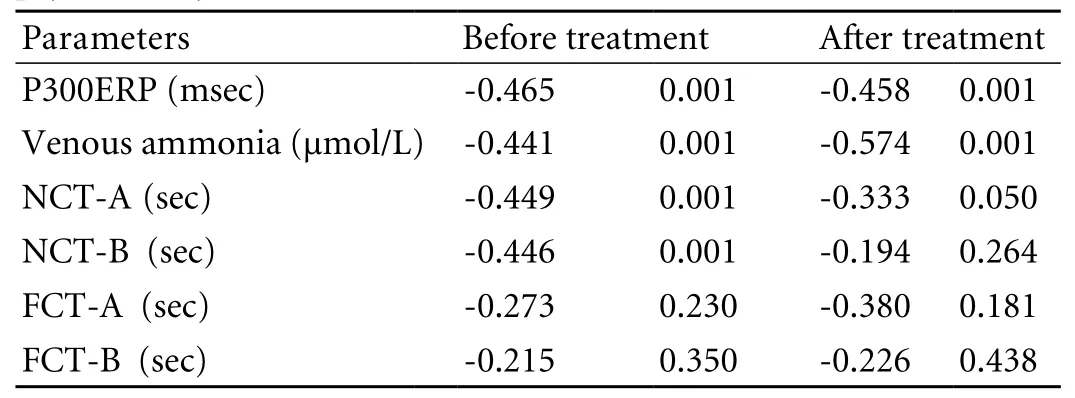
Table 5. Correlation (r) of CFF to P300ERP, venous ammonia, and psychometry (before and after treatment)
Discussion
In this study, the prevalence of MHE was 54.5% and lactulose improved MHE in 57% of cirrhotics patients.CFF alone diagnosed MHE in 73% of the patients, and CFF had a diagnostic accuracy of 80% in assessing the recovery of MHE.
Various methods, including neurophysiological or psychometric test or in combination, have been used in the diagnosis of MHE.[5-15]Among these methods NCT (A, B) is commonly used for detecting MHE, but this test may over-diagnose if corrections for age and educational status are not applied.[1,9,14,24]NCT cannot be performed by illiterate people and those unfamiliar with roman alphanumeric notations, especially in developing countries. FCT is based on the subject's identi fi cation of fi gures rather than the alphabet or numerals. FCT is as useful as NCT in detecting psychomotor performance defects in a large cohort of cirrhotic patients without overt encephalopathy.[15,24]In this study, 21 patients could not perform NCT due to illiteracy, hence FCT is an important test in these patients. Using the same variant for follow-up of MHE patients may give a false impression of improvement,but this can be circumvented if a different variant is used, as we did in our study. In addition, no single psychometric test can pick up MHE reliably, and because of the diversity of symptoms, test batteries have to be used.[11,14,24,25]Although the majority of surveyed hepatologists in Spain and the United States agree that MHE is a signi fi cant problem requiring testing, the minority are able to actually test for MHE as part of their clinical practice because of lack of standardization of normal values, insurance problems, and the time needed to do a battery of tests.[20,25]
The advantage of using computerized tests over pencil and paper tests is that there is less reliance on the considerable motor activity required to perform certain psychometric tests. To evaluate cortical and subcortical cognitive function, complicating factors such as movement disorders may give a false impression of signi fi cant cognitive de fi cits in patients with reduced peripheral motor skills needed for psychometric tests.
CFF is a well-established neurophysiological technique that measures the ability of the central nervous system to detect fl ickering light, which is directly in fl uenced by cortical activity.[26]CFF appears to detect a broad spectrum of neuro-psychological abnormalities ranging from visual signal processing (retinal gliopathy) to cognitive functions.[27]In recent years, various studies have shown CFF in the diagnosis of MHE.[21-23]In this study, CFF correlated well with the psychometry tests NCT-A and P300ERP (P=0.001) in the diagnosis of MHE and assessment of its recovery. We did not fi nd any correlation of CFF with FCT, and this might be due to the small number of patients who were illiterate and had MHE. We did not exclude these patients from the analysis as that would create selection bias in the study.These relationships suggest that CFF measurements can pick up components of cognitive motor abilities in cirrhotic patients.
Lactulose is an effective treatment for MHE. In this study, lactulose also improved MHE in 57% of the patients, and all patients tolerated it well. Ten patients(6%) complained of excessive abdominal distension and sweet taste but did not discontinue the therapy. Most patients took lactulose between 30 and 60 ml per day.After treatment with lactulose, recovery from MHE was assessed by psychometric tests in a few studies.[9,10,14]A methodologic reservation about psychometry tests is that there are no data validating their use in a serial longitudinal manner, and age, education, and learning effects may adversely affect the results. Similarly,some studies used methods like brain stem auditory evoked potential and P300ERP to assess recovery from MHE.[13,15]Though these tests are more objective and do not show learning effects, they are expensive, dif fi cult to perform, cannot be done at the bedside without trained personnel and standardization.[12,13,15,17]On the other hand, in this study, we found CFF was a simple,reliable tool for the diagnosis of MHE and assessment of its recovery. CFF had a diagnostic accuracy of 73%, as reported.[21,22]The accuracy of CFF in diagnosis of MHE and recovery was 80% in this study with a sensitivity and speci fi city of 65% and 91%, respectively. It is economical, can be administered easily with relatively little training to the patient, and does not show a learning effect. Thus CFF satis fi es many requirements of an ideal assessment tool for the diagnosis and assessment of recovery from MHE. However, diagnosing MHE by CFF has limitations. We took CFF <39 Hz as the cutoff for diagnosis of MHE, but in the paper by Kircheis et al[23]39 Hz represents the borderline between HE0 and overt HE, not the cutoff for MHE diagnosis. Similarly,Romero-Gomez et al[22]took CFF <38 Hz as an arti fi cial borderline for prediction of further bouts of overt HE. So the optimum CFF cutoff value for the diagnosis of MHE represents only the borderline with the best sensitivity and speci fi city, not an absolute cut-off. Hence, using 38 Hz or 39 Hz one should take account of relative bias.Ammonia has been shown to be an important etiological parameter in the pathogenesis of MHE.[15,23,28,29]In this study, venous ammonia was signi fi cantly higher in MHE patients than non-MHE patients (94.5±24.6. vs. 71.5±31.1 μmol/l, P=0.001). Previous studies have suggested that the neurological abnormality in cirrhotic patients has little or no relationship with the degree of liver failure, but is correlated with disturbances in nitrogen metabolism.[28,29]Previously, we also demonstrated a signi fi cant correlation of venous ammonia level with CFF,in accord with Kircheis et al.[15,23]In the present study, we found a signi fi cant correlation between CFF and plasma ammonia concentration both before and after treatment.
A limitation of this study is that we used psychometric tests and P300ERP to diagnose MHE. The event-related P300 wave represents endogenous mechanisms of stimulus processing, is the most consistent wave, and can be considered the electrophysiological counterpart of psychometric tests as both involve active use of cognitive faculties. P300ERP as a diagnostic method for MHE has limitations, since it has been shown to be in fl uenced by signi fi cant changes in stimulus intensity and age. The use of a fi xed cut-off overestimates the prevalence of changes in old patients, and underestimates that in young patients. However repeated P300ERP for follow-up of MHE patients eliminates the learning effect.Another limitation is the lack of a placebo limb for the management of MHE and evaluating CFF for the same group. In summary, CFF is a simple, relatively reliable tool that is suitable for use as one of the methods for the diagnosis of MHE and assessment of its recovery.
Funding: None.
Ethical approval: Not needed.
Contributors: SP and SBC proposed the study. SP wrote the fi rst
draft and analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. SBC is the guarantor.
Competing interest: No bene fi ts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K,Blei AT. Hepatic encephalopathy--de fi nition, nomenclature,diagnosis, and quanti fi cation: fi nal report of the working party at the 11th World Congresses of Gastroenterology,Vienna, 1998. Hepatology 2002;35:716-721.
2 Weissenborn K, Giewekemeyer K, Heidenreich S, Bokemeyer M, Berding G, Ahl B. Attention, memory, and cognitive function in hepatic encephalopathy. Metab Brain Dis 2005;20:359-367.
3 Schomerus H, Hamster W. Quality of life in cirrhotics with minimal hepatic encephalopathy. Metab Brain Dis 2001;16:37-41.
4 Schomerus H, Hamster W, Blunck H, Reinhard U, Mayer K,Dölle W. Latent portasystemic encephalopathy. I. Nature of cerebral functional defects and their effect on fi tness to drive.Dig Dis Sci 1981;26:622-630.
5 Groeneweg M, Quero JC, De Bruijn I, Hartmann IJ, Essinkbot ML, Hop WC, et al. Subclinical hepatic encephalopathy impairs daily functioning. Hepatology 1998;28:45-49.
6 Kircheis G, Knoche A, Hilger N, Manhart F, Schnitzler A,Schulze H, et al. Hepatic encephalopathy and fi tness to drive.Gastroenterology 2009;137:1706-1715.
7 Wein C, Koch H, Popp B, Oehler G, Schauder P. Minimal hepatic encephalopathy impairs fi tness to drive. Hepatology 2004;39:739-745.
8 Bajaj JS, Saeian K, Schubert CM, Hafeezullah M, Franco J, Varma RR, et al. Minimal hepatic encephalopathy is associated with motor vehicle crashes: the reality beyond the driving test. Hepatology 2009;50:1175-1183.
9 Prasad S, Dhiman RK, Duseja A, Chawla YK, Sharma A,Agarwal R. Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology 2007;45:549-559.
10 Watanabe A, Sakai T, Sato S, Imai F, Ohto M, Arakawa Y, et al. Clinical ef fi cacy of lactulose in cirrhotic patients with and without subclinical hepatic encephalopathy. Hepatology 1997;26:1410-1414.
11 Das A, Dhiman RK, Saraswat VA, Verma M, Naik SR.Prevalence and natural history of subclinical hepatic encephalopathy in cirrhosis. J Gastroenterol Hepatol 2001;16:531-535.
12 Saxena N, Bhatia M, Joshi YK, Garg PK, Tandon RK. Auditory P300 event-related potentials and number connection test for evaluation of subclinical hepatic encephalopathy in patients with cirrhosis of the liver: a follow-up study. J Gastroenterol Hepatol 2001;16:322-327.
13 Liu Q, Duan ZP, Ha DK, Bengmark S, Kurtovic J, Riordan SM. Synbiotic modulation of gut fl ora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology 2004;39:1441-1449.
14 Dhiman RK, Sawhney MS, Chawla YK, Das G, Ram S, Dilawari JB. Ef fi cacy of lactulose in cirrhotic patients with subclinical hepatic encephalopathy. Dig Dis Sci 2000;45:1549-1552.
15 Sharma P, Sharma BC, Puri V, Sarin SK. An open-label randomized controlled trial of lactulose and probiotics in the treatment of minimal hepatic encephalopathy. Eur J Gastroenterol Hepatol 2008;20:506-511.
16 Kullmann F, Hollerbach S, Holstege A, Schölmerich J.Subclinical hepatic encephalopathy: the diagnostic value of evoked potentials. J Hepatol 1995;22:101-110.
17 Mehndiratta MM, Sood GK, Sarin SK, Gupta M. Comparative evaluation of visual, somatosensory, and auditory evoked potentials in the detection of subclinical hepatic encephalopathy in patients with nonalcoholic cirrhosis. Am J Gastroenterol 1990;85:799-803.
18 Yen CL, Liaw YF. Somatosensory evoked potentials and number connection test in the detection of subclinical hepatic encephalopathy. Hepatogastroenterology 1990;37:332-334.
19 Mechtcheriakov S, Schocke M, Kugener A, Graziadei IW,Mattedi M, Hinterhuber H, et al. Chemical shift magnetic resonance spectroscopy of cingulate grey matter in patients with minimal hepatic encephalopathy. Neuroradiology 2005;47:27-34.
20 Bajaj JS, Etemadian A, Hafeezullah M, Saeian K. Testing for minimal hepatic encephalopathy in the United States: An AASLD survey. Hepatology 2007;45:833-834.
21 Sharma P, Sharma BC, Puri V, Sarin SK. Critical fl icker frequency: diagnostic tool for minimal hepatic encephalopathy. J Hepatol 2007;47:67-73.
22 Romero-Gómez M, Córdoba J, Jover R, del Olmo JA, Ramírez M, Rey R, et al. Value of the critical fl icker frequency in patients with minimal hepatic encephalopathy. Hepatology 2007;45:879-885.
23 Kircheis G, Wettstein M, Timmermann L, Schnitzler A,Häussinger D. Critical fl icker frequency for quanti fi cation of low-grade hepatic encephalopathy. Hepatology 2002;35:357-366.
24 Dhiman RK, Saraswat VA, Verma M, Naik SR. Figure connection test: a universal test for assessment of mental state. J Gastroenterol Hepatol 1995;10:14-23.
25 Vergara-Gómez M, Flavià-Olivella M, Gil-Prades M,Dalmau-Obrador B, Córdoba-Cardona J. Diagnosis and treatment of hepatic encephalopathy in Spain: results of a survey of hepatologists. Gastroenterol Hepatol 2006;29:1-6.
26 Za fi ris O, Kircheis G, Rood HA, Boers F, Häussinger D, Zilles K. Neural mechanism underlying impaired visual judgement in the dysmetabolic brain: an fMRI study. Neuroimage 2004;22:541-552.
27 Curran S, Wattis J. Critical fl icker fusion threshold: a potentially useful measure for the early detection of Alzheimer's disease. Hum Psychopharmacol 2000;15:103-112.28 Rikkers L, Jenko P, Rudman D, Freides D. Subclinical hepatic encephalopathy: detection, prevalence, and relationship to nitrogen metabolism. Gastroenterology 1978;75:462-469.
29 Lockwood AH, Yap EW, Wong WH. Cerebral ammonia metabolism in patients with severe liver disease and minimal hepatic encephalopathy. J Cereb Blood Flow Metab 1991;11:337-341.
BACKGROUND: Minimal hepatic encephalopathy (MHE)impairs quality of life and predicts overt hepatic encephalopathy (HE) in cirrhotic patients. Diagnosis of MHE requires cumbersome tests. Lactulose is effective in the treatment of MHE. This study aimed to evaluate the use of critical fl icker frequency (CFF) for the diagnosis of MHE in cirrhotic patients after treatment.
METHODS: One hundred and ten patients were evaluated by psychometry (number connection tests A, B or fi gure connection tests A, B), P300 auditory event related potential(P300ERP), venous ammonia, and CFF for MHE. MHE was diagnosed by abnormal psychometry (>2SD age matched controls) and P300ERP. MHE patients were treated with lactulose for one month. Response was de fi ned by normalization(<2SD of matched controls) of both psychometry and P300ERP.RESULTS: Of the 110 patients [Child Turcott Pugh score A∶B∶C 39∶42∶29, (age 41.6±11.6 years, M∶F 82∶28)], 75(68%) had abnormal results of psychometric tests, and 74(67%) had prolonged P300ERP. Fifteen (20%) patients with abnormal results of psychometric tests had normal P300ERP.Thus sixty (54.5%) patients were diagnosed as having MHE.After treatment for one month, 34 (57%) recovered while 26(43%) continued to have abnormal resents of psychometric or P300ERP tests. CFF was <39 Hz in 72 (65.4%) patients before treatment and in 20 (33.3%) after treatment. CFF sensitivity,speci fi city, positive predictive value, negative predictive value, and diagnostic accuracy for the assessment of recovery of MHE were 65%, 91%, 85%, 77% and 80%, respectively.
CONCLUSION: CFF is a simple, relatively reliable, and accurate test without any dependence on age or literacy in the diagnosis and assessment of recovery of patients with MHE.
Author Af fi liations: Department of Gastroenterology, G. B. Pant Hospital,New Delhi, India (Sharma P, Sharma BC and Sarin SK)
Barjesh Chander Sharma, MD, Department of Gastroenterology, Room 203, Academic Block, G. B. Pant Hospital, New Delhi-110002, India (Fax: +91-11-23219222; Email: drbcsharma@hotmail.com)
© 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
July 22, 2009
Accepted after revision December 11, 2009
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Risk factors for early recurrence of smallhepatocellular carcinoma after curative resection
- Pathological changes at early stage of multiple organ injury in a rat model of severe acute pancreatitis
- Potential etiopathogenesis of seventh day syndrome following living donor liver transplantation: ischemia of the graft?
- Comparatively lower postoperative hepatolithiasis risk with hepaticocholedochostomy versus hepaticojejunostomy
- Effect of sodium salicylate on oxidative stress and insulin resistance induced by free fatty acids
- Endoscopic nasojejunal feeding tube placement in patients with severe hepatopancreatobiliary diseases:a retrospective study of 184 patients
