Effect of sodium salicylate on oxidative stress and insulin resistance induced by free fatty acids
2010-12-14BingHeShengZhaoWeiZhangYanLiandPingHan
Bing He, Sheng Zhao, Wei Zhang, Yan Li and Ping Han
Shenyang, China
Effect of sodium salicylate on oxidative stress and insulin resistance induced by free fatty acids
Bing He, Sheng Zhao, Wei Zhang, Yan Li and Ping Han
Shenyang, China
(Hepatobiliary Pancreat Dis Int 2010; 9: 49-53)
free fatty acids;sodium salicylate;oxidative stress;insulin resistance;hepatic glucose production
Introduction
The association among obesity, insulin resistance,and type 2 diabetes mellitus is well documented,[1]and free fatty acids (FFAs) have been implicated as an important causative link among them.[2]An elevation of plasma FFAs has been reported to impair insulin action, to accelerate β-cell apoptosis, and might be a major risk factor for type 2 diabetes.[2,3]
Almost 100 years ago, Williamson[4]showed that high-dose salicylate treatment reduces the severity of glycosuria in diabetic patients, and in 1957, Reid et al[5]further demonstrated that 10-14 days of aspirin treatment improves the results of oral glucose tolerance tests in diabetic patients. It has been reported recently that high-dose salicylate improves FFA-induced insulin resistance and β-cell dysfunction,[6,7]but the mechanism remains uncertain. Previously we found that in insulinresistant rats, the supplementation of sodium salicylate is associated with a reduction of plasma malondialdehyde(MDA), a marker of oxidative stress. To date, few studies have investigated the impact of salicylates on oxidative stress levels in animal models. While oxidative stress is associated with a wide variety of pathologies, including diabetes, cardiovascular disease, and cancer, diabetes mellitus is particularly strongly associated.[8]Thus, the objective of this study was to assess the impact of theanti-in fl ammatory drug sodium salicylate on insulin sensitivity and to explore the potential mechanism by which it improves hepatic and peripheral insulin resistance.
MethodsAnimal models
Forty-eight normal male Wistar rats, weighing 230-260 g,were housed in the Department of Laboratory Animals,China Medical University (Shenyang, China). The rats were housed under controlled temperature (23 ℃) and were exposed to a 12∶12-hour light-dark cycle with ad libitum access to water and standard rat chow. After 3-5 days of adaptation to the facility, the rats were anesthetized, and indwelling catheters were inserted as described previously.[9]The rats were allowed 3-4 days of postsurgical recovery before experiments.
Experimental design
The rats were fasted overnight for 14 hours and randomized to three groups, one of which received intralipid (20% intralipid+20 U/ml heparin, 5.5 μl/min;IH group, n=16), one was a saline-treated control (equal volume; SAL group, n=16), while another group received sodium salicylate (20% intralipid+20 U/ml heparin, 5.5 μl/min+sodium salicylate, 0.117 mg/kg per minute; IHS group, n=16). The duration of infusion in each group was 7 hours, and [6-3H] glucose (20 μCi, bolus+0.4 μCi/min infusion) was given during the last 2 hours of the experiment to assess endogenous glucose production(EGP) and glucose utilization (GU). Further, the rats were divided into 2 groups of 8 each: a basal infusion group and a hyperinsulinemic-euglycemic clamping group. Clamping was made to maintain blood glucose concentrations at 5.0 mmol/L during the last 2 hours, while steady-state human insulin infusion (5 mU/kg per minute) was given by infusing 20% glucose at a variable rate. Blood samples for testing glucose, insulin, FFAs, C-peptide, and [6-3H]glucose-speci fi c activity were taken during the last 30 minutes. The total blood volume withdrawn was 3.0-3.3 ml during the basal experiment and 3.5-3.8 ml during the clamping experiment. After plasma separation, red blood cells diluted 1∶1 in heparinized saline (4 U/ml)were reinfused into the rats. At the end of the experiment,liver and gastrocnemius samples were removed within 45 seconds of anesthetic injection while infusion was maintained through the jugular vein.
Laboratory methods
Plasma glucose was measured with the glucose oxygenase method (BIOSEN5030, Germany). Plasma radioactivity from [6-3H] glucose was determined after deproteinization with Ba(OH)2and ZnSO4. The intraassay coef fi cient of variation was 6.5%. Insulin and C-peptide levels were determined by radioimmunoassay(Beijing Furui Biological Engineering Co., China).The coef fi cients of variation were <8% and 10.5%respectively. Plasma FFA levels were measured using a colorimetric kit, MDA levels and glutathione peroxidase(GSH-PX) activity in the liver and muscle were also measured using colorimetric kits (Nanjing Jiancheng Institute of Bio-engineering, China).
Calculations
Glucose turnover (rate of appearance of glucose determined with [6-3H] glucose) was calculated using the steady-state formula.[10]Data were presented as average values in samples taken in the last 30 minutes of the experiment.
Statistical analysis
The data were expressed as mean±SD. All calculations were performed using the SPSS12.0 software package.Experimental results were analyzed using one-way ANOVA with a probability for type 1 error set at P<0.05.
ResultsPlasma levels of FFAs, glucose, insulin, and C-peptide
IH elevated plasma FFA levels by 2-fold, and increased basal plasma insulin and C-peptide levels by 0.6 and 0.7-fold respectively. Plasma glucose levels were higher with IH vs. SAL infusion in the basal experiments but were maintained at 5.0 mmol/L during the clamping(Table). Sodium salicylate decreased FFAs slightly,signi fi cantly decreased basal plasma glucose level by 70%, and reduced basal plasma insulin and C-peptide levels by 39% and 32%, respectively (Table).
Hepatic glucose production (HGP)
In the basal state, IH increased HGP by 1.5-fold,while sodium salicylate decreased HGP by 16%. During the hyperinsulinemic-euglycemic clamping, HGP in the IH group was 2-fold that in the SAL group and the infusion of sodium salicylate resulted in a decrease of 20% in HGP.
Glucose utilization (GU)
Under basal state conditions, IH increased GU by 1.6-fold. GU was reduced by 20% with intralipidinfusion during the clamping, as compared with that with SAL infusion. Sodium salicylate decreased GU by 18% in the basal state, and increased GU by 14% in clamping conditions, compared with the IH group.
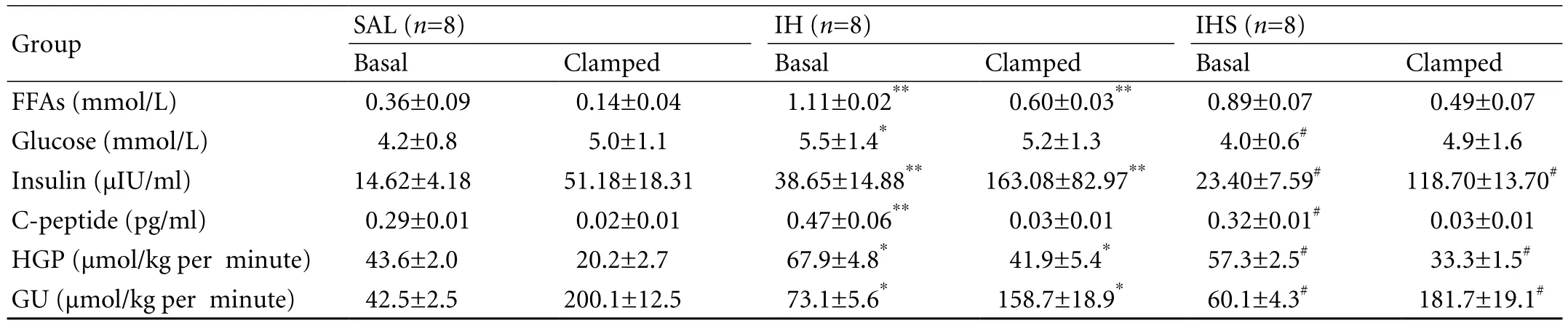
Table. Plasma levels of FFAs, glucose, insulin, and C-peptide in basal fasting state and in the clamped state (insulin infusion rate: 5 mU/kg per minute)
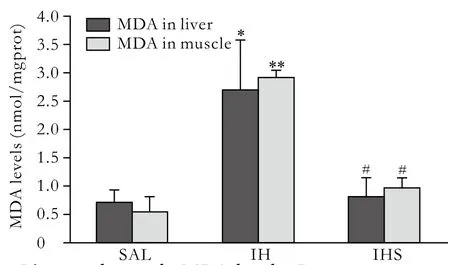
Fig. 1. Liver and muscle MDA levels. Data were expressed as mean±SD; SAL: saline; IH: intralipid + heparin; IHS: intralipid+heparin+sodium salicylate; *: P<0.05, vs. SAL; **: P<0.01, vs.SAL; #: P<0.01, vs. IH.
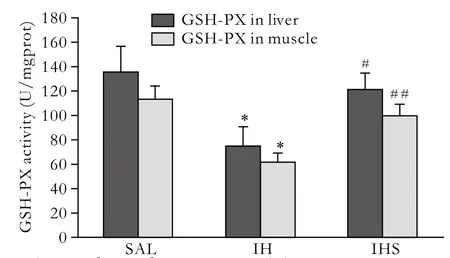
Fig. 2. Liver and muscle GSH-PX activity. Data were expressed as mean±SD; SAL: saline; IH: intralipid+heparin; IHS: intralipid+heparin+sodium salicylate; *: P<0.01, vs. SAL; #: P<0.05, vs. IH;##: P<0.01, vs. IH.
MDA levels and GSH-PX activity in the liver and muscle
After intralipid infusion, MDA levels in the liver and muscle were increased by 2- and 4-fold, while GSHPX activity decreased by 45% and 46%, respectively.Compared to the IH group, sodium salicylate treatment reduced MDA content in the liver and muscle by 63%and 66%, and elevated the GSH-PX activity by 35% and 37%, respectively (Figs. 1, 2).
Discussion
The elevation of plasma FFAs has been shown to impair insulin action and cause insulin resistance. Insulin resistance is a key etiological factor for type 2 diabetes mellitus. Additional 41 million people are prediabetic with a constellation of insulin resistance, hypertension,and dyslipidemia, which puts them at increased risk for cardiovascular morbidity and mortality.[11]Thus,there is an urgent need for effective interventions to prevent diabetes in insulin-resistant populations. In recent studies, the improvement of insulin resistance by anti-in fl ammatory salicylates has been investigated,but the molecular target remains uncertain. A better understanding of the mechanisms will be required to combat the epidemics of type 2 diabetes and cardiovascular diseases that are fueled by obesityassociated insulin resistance. In this study, the effects of FFAs on hepatic and skeletal muscle glucose metabolism were tested. In addition, we determined whether highdose anti-in fl ammatory salicylates prevent FFA-induced alterations of insulin action and the biochemical mechanisms that underlie these effects.
In our animal model, IH elevated basal plasma FFAs to above the physiological range but within the FFA elevation seen in uncontrolled diabetes. The FFA levels in the clamping were lower than the basal FFA levels,which are consistent with the antilipolytic and FFA reesteri fi cation effects of insulin.[12]IH increased insulin and C-peptide levels in all groups, because of increased insulin secretion in the basal state and a decreased insulin clearance during the clamping.[13]Sodium salicylate down-regulated high FFA-induced endogenous insulin secretion, and decreased plasma glucose levels accordingly. Acetylsalicylic acid was reported to promote fatty acid oxidation and reduce the plasma FFA level.[14]However, we did not fi nd a signi fi cant decrease of FFAs after sodium salicylate infusion, which may be due to the short infusion time.
Previous studies have reported that FFAs cause insulin resistance by increasing gluconeogenesis in the liver,[15]impairing the insulin-mediated suppression of HGP, and inhibiting insulin-stimulated glucose uptake in skeletal muscle.[16]The skeletal muscle is the major site for insulin-stimulated glucose disposal, and is the major target for peripheral insulin resistance.[17]Our study showed that FFAs induced hepatic insulin resistance by elevating HGP levels and induced peripheral insulin resistance by decreasing GU and metabolism. A 7-hour infusion of sodium salicylate resulted in signi fi cant improvements in insulin sensitivity, including a 20%decrease in HGP and a 15% increase in GU.
We found that IH increased MDA levels in the liver and skeletal muscle by 2- and 4-fold, and reduced the GSH-PX activity by 45% and 46%, respectively. MDA is a marker of oxidative stress, while GSH-PX re fl ects the capacity for elimination of free radicals. These results showed that FFAs are strongly associated with a persistent imbalance between the production of highly reactive molecular species and antioxidant defense.[18]It has been reported that the increased production of these active molecules or a reduced capacity for elimination causes abnormal changes in intracellular signaling and gene expression, ultimately resulting in a pathological situation that includes insulin resistance.[19]After administration of sodium salicylate, MDA levels in the liver and muscle decreased by 63% and 64%,and the GSH-PX activity increased by 35% and 37%,respectively. These results indicated that sodium salicylate signi fi cantly relieves the degree of oxidative stress in the liver and skeletal muscle. At the same time,it improved hepatic and peripheral insulin resistance by decreasing HGP and increasing GU. High doses of salicylate (4-10 g/d), including sodium salicylate and aspirin, have been used to treat in fl ammatory conditions such as rheumatic fever and rheumatoid arthritis. These high doses are thought to inhibit nuclear factor kappa B (NF-κB) and its upstream activator IκB kinase β (IKK-β), as opposed to working through cyclooxygenases, the classical targets of non-steroidal anti-in fl ammatory drugs.[20,21]High doses of salicylates also lower blood glucose concentrations although their potential for treating diabetes has been all but forgotten by modern biomedical science. In this study, we found that the anti-in fl ammatory drug, sodium salicylate,relieved oxidative damage in the liver and skeletal muscle, and improved FFA-induced insulin resistance.Thus we presumed that sodium salicylate might inhibit IKK-β- and NF-κB-mediated transcription, which in certain cells would enhance the production of low-level in fl ammatory cytokines, such as TNF-α and IL-6. It has been demonstrated that in fl ammatory cytokines increase the transcription and translation of reactive molecular species and activate some reactive molecular species.[22,23]Ultimately, the anti-in fl ammatory drug sodium salicylate may improve insulin resistance through abating the degree of oxidative stress in the liver and skeletal muscle.
In summary, our data demonstrate that the shortterm elevation of fatty acids induces insulin resistance by enhancing oxidative stress levels in the liver and skeletal muscle. Also, in this study we preliminarily assessed the ef fi cacy of the anti-in fl ammatory drug sodium salicylate as a new treatment for insulin resistance.This effect was associated with at least one mechanism:Sodium salicylate reduced the degree of oxidative stress in the liver and skeletal muscle, and therefore improved hepatic and peripheral insulin resistance. IKK-β and NF-κB might provide a potential pathogenic link to oxidative stress.
Funding: This study was supported by a grant from the Bureau of Education of Liaoning Province, China (No. 20060999).
Ethical approval: Not needed.
Contributors: HB proposed the study and wrote the fi rst draft.ZS analyzed the data. HP carried out the experiments. All authors contributed to the design and interpretation of the study and to further drafts. HP is the guarantor.
Competing interest: No bene fi ts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Westphal SA. Obesity, abdominal obesity, and insulin resistance.Clin Cornerstone 2008;9:23-31.
2 Wilding JP. The importance of free fatty acids in the development of Type 2 diabetes. Diabet Med 2007;24:934-945.
3 Oprescu AI, Bikopoulos G, Naassan A, Allister EM, Tang C,Park E, et al. Free fatty acid-induced reduction in glucosestimulated insulin secretion: evidence for a role of oxidative stress in vitro and in vivo. Diabetes 2007;56:2927-2937.
4 Williamson RT. On the treatment of glycosuria and diabetes mellitus with sodium salicylate. Br Med J 1901;1:760-762.
5 Reid J, MacDougall AI, Andrews MM. Aspirin and diabetes mellitus. Br Med J 1957;2:1071-1074.
6 Manrique C, Lastra G, Palmer J, Gardner M, Sowers JR.Aspirin and Diabetes Mellitus: revisiting an old player. Ther Adv Cardiovasc Dis 2008;2:37-42.
7 Zeender E, Maedler K, Bosco D, Berney T, Donath MY, Halban PA. Pioglitazone and sodium salicylate protect human betacells against apoptosis and impaired function induced by glucose and interleukin-1beta. J Clin Endocrinol Metab 2004;89:5059-5066.
8 Rees MD, Kennett EC, Whitelock JM, Davies MJ. Oxidative damage to extracellular matrix and its role in human pathologies. Free Radic Biol Med 2008;44:1973-2001.
9 Han P, Zhang YY, Lu Y, He B, Zhang W, Xia F. Effects of different free fatty acids on insulin resistance in rats.Hepatobiliary Pancreat Dis Int 2008;7:91-96.
10 Lam TK, van de Werve G, Giacca A. Free fatty acids increase basal hepatic glucose production and induce hepatic insulin resistance at different sites. Am J Physiol Endocrinol Metab 2003;284:E281-290.
11 Misra A, Khurana L. Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab 2008;93:S9-30.
12 Wiesenthal SR, Sandhu H, McCall RH, Tchipashvili V, Yoshii H, Polonsky K, et al. Free fatty acids impair hepatic insulin extraction in vivo. Diabetes 1999;48:766-774.
13 Lam TK, Yoshii H, Haber CA, Bogdanovic E, Lam L, Fantus IG, et al. Free fatty acid-induced hepatic insulin resistance:a potential role for protein kinase C-delta. Am J Physiol Endocrinol Metab 2002;283:E682-691.
14 van der Crabben SN, Allick G, Ackermans MT, Endert E, Romijn JA, Sauerwein HP. Prolonged fasting induces peripheral insulin resistance, which is not ameliorated by highdose salicylate. J Clin Endocrinol Metab 2008;93:638-641.
15 Li L, Yang GY. Effect of hepatic glucose production on acute insulin resistance induced by lipid-infusion in awake rats.World J Gastroenterol 2004;10:3208-3211.
16 Lam TK, Carpentier A, Lewis GF, van de Werve G, Fantus IG,Giacca A. Mechanisms of the free fatty acid-induced increase in hepatic glucose production. Am J Physiol Endocrinol Metab 2003;284:E863-873.
17 Abdul-Ghani MA, Matsuda M, DeFronzo RA. Strong association between insulin resistance in liver and skeletal muscle in non-diabetic subjects. Diabet Med 2008;25:1289-1294.
18 Yang R, Shi Y, Li W, Yue P. Effect of lipoic acid on gene expression related to oxidative stress, lipid and glucose metabolism of mice fed with high fat diet. Wei Sheng Yan Jiu 2008;37:560-562, 565.
19 Evans JL, Maddux BA, Gold fi ne ID. The molecular basis for oxidative stress-induced insulin resistance. Antioxid Redox Signal 2005;7:1040-1052.
20 Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, et al.Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med 2005;11:183-190.
21 Hundal RS, Petersen KF, Mayerson AB, Randhawa PS,Inzucchi S, Shoelson SE, et al. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest 2002;109:1321-1326.
22 Martínez JA. Mitochondrial oxidative stress and in fl ammation:an slalom to obesity and insulin resistance. J Physiol Biochem 2006;62:303-306.
23 Tilg H, Moschen AR. In fl ammatory mechanisms in the regulation of insulin resistance. Mol Med 2008;14:222-231.
BACKGROUND: It has been reported that high-dose salicylates improve free fatty acids (FFAs)-induced insulin resistance and β-cell dysfunction in vitro, but the mechanism remains uncertain. In insulin-resistant rats, we found that the supplementation of sodium salicylate is associated with a reduction of plasma malondialdehyde (MDA), a marker of oxidative stress. Few studies have investigated the effects of salicylates on oxidative stress levels in insulin-resistant animal models. This study aimed to assess the effect of sodium salicylate on insulin sensitivity and to explore the potential mechanism by which it improves hepatic and peripheral insulin resistance.
METHODS: Intralipid+heparin (IH), saline (SAL), or intralipid+heparin+sodium salicylate (IHS) were separately infused for 7 hours in normal Wistar rats. During the last 2 hours of the infusion, hyperinsulinemic-euglycemic clamping was performed with [6-3H] glucose tracer. Plasma glucose was measured using the glucose oxygenase method. Plasma insulin and C-peptide were determined by radioimmunoassay. MDA levels and glutathione peroxidase (GSH-PX) activity in the liver and skeletal muscle were measured with colorimetric kits.RESULTS: Compared with infusion of SAL, IH infusion increased hepatic glucose production (HGP), and decreased glucose utilization (GU) (P<0.05). The elevation of plasma free fatty acids increased the MDA levels and decreased the GSH-PX activity in the liver and muscle (P<0.01). Sodium salicylate treatment decreased HGP, elevated GU (P<0.05),reduced MDA content by 60% (P<0.01), and increased the GSH-PX activity by 35% (P<0.05).CONCLUSIONS: Short-term elevation of fatty acids induces insulin resistance by enhancing oxidative stress levels in the liver and muscle. The administration of the anti-in fl ammatory drug sodium salicylate reduces the degree of oxidative stress,therefore improving hepatic and peripheral insulin resistance.IKK-β and NF-κB provide potential pathogenic links to oxidative stress.
Author Af fi liations: Department of Endocrinology, Shengjing Af fi liated Hospital, China Medical University, Shenyang 110004, China (He B,Zhao S, Li Y and Han P); and Department of Internal Medicine, Fourth Af fi liated Hospital, China Medical University, Shenyang 110032, China(Zhang W)
Ping Han, MD, Department of Endocrinology,Shengjing Af fi liated Hospital, China Medical University, Shenyang 110004,China (Tel: 86-24-83955273; Fax: 86-24-83955273; Email: hanping85@hotmail.com)
© 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
February 2, 2009
Accepted after revision November 7, 2009
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Critical fl icker frequency for diagnosis and assessment of recovery from minimal hepatic encephalopathy in patients with cirrhosis
- Risk factors for early recurrence of smallhepatocellular carcinoma after curative resection
- Pathological changes at early stage of multiple organ injury in a rat model of severe acute pancreatitis
- Potential etiopathogenesis of seventh day syndrome following living donor liver transplantation: ischemia of the graft?
- Comparatively lower postoperative hepatolithiasis risk with hepaticocholedochostomy versus hepaticojejunostomy
- Endoscopic nasojejunal feeding tube placement in patients with severe hepatopancreatobiliary diseases:a retrospective study of 184 patients
