Primary hepatic neuroendocrine carcinoma:clinical analysis of 11 cases
2010-12-14YangQingHuangFengXuJiaMeiYangandBinHuang
Yang-Qing Huang, Feng Xu, Jia-Mei Yang and Bin Huang
Shanghai, China
Primary hepatic neuroendocrine carcinoma:clinical analysis of 11 cases
Yang-Qing Huang, Feng Xu, Jia-Mei Yang and Bin Huang
Shanghai, China
(Hepatobiliary Pancreat Dis Int 2010; 9: 44-48)
carcinoma, neuroendocrine;carcinoid tumor;liver neoplasms;liver resection
Introduction
Neuroendocrine carcinoma (NEC) is rarely originated from neuroendocrine cells but is mostly seen in the gastrointestinal tract and pancreas.Oberndorfer fi rst de fi ned it as karzinoid (carcinoid)in 1907; it is described as a tumor that resembles an adenocarcinoma, yet behaves in a more benign fashion.[1]However, further clinical reports showed that some carcinoids still have the characteristics of invasion and metastasis. The liver is more often involved by metastatic NEC. Primary hepatic neuroendocrine carcinoma(PHNEC) is more rare than NEC; therefore it is dif fi cult to reach a proper diagnosis and determine a therapeutic approach. We present 11 cases in this paper.
Methods
The 11 PHNEC patients whose average age was 49.5±8.2 years (34-58 years) were admitted to our hospital between January 1996 and May 2008. B-ultrasonography,CT, MRI scan and digestive endoscopy were performed for diagnosis. All except one patient with the possibility of carcinoid were con fi rmed to have primary liver cancer. All patients were AFP(-) and CEA(-) except one with CA19-9(+) (patient 5). Digestive endoscopy was performed for all patients and no NEC was found in the stomach, duodenum, colon, or rectum. Of these, only 4 patients tested positive for hepatitis B infection.
Hepatectomy was taken in all patients who had been detected during operation to preclude tumors of the stomach, intestine, colon, and pancreas. Patients with a single tumor received radical excisions; others with multiple tumors underwent excision of all tumors including those found outside the liver. Only 2 patients received adjuvant transcatheter arterial chemoemboli-zation (TACE) after liver resection. The others did not receive any treatment until recurrence was found. TACE,percutaneous ethanol injection treatment (PEIT), or octreotide injection was performed in patients with recurrence.
Results
All patients were con fi rmed pathologically to have PHNEC. They were followed up until June 30, 2009.Because serum 5-HT, chromogranin A (CgA), and urinary 5-hydroxyindoleacetic acid (5-HIAA) examinations cannot be conducted at our hospital, B-ultrasonography, CT or/and MRI were used for postoperative examination. The median follow-up time was 33 months (12-107 months).The patients survived, and the longest postoperative survival time was 107 months. The longest disease-free survival time was 98 months, the 1-year survival rate was 100%, and the 1-year recurrence rate was 45.5% (5/11).The Table shows the clinical data of all PHNEC patients.
Discussion
Due to confusion around the terms carcinoid and NEC,the World Health Organization[2]named these species of tumors as NEC in 2000, and classi fi ed them into 3 categories: 1) well-differentiated NEC, i.e., typical carcinoid or carcinoid; 2) moderately-differentiated NEC,i.e., atypical carcinoid; 3) poorly-differentiated NEC,i.e., small cell carcinoma. Based on the combination of typical clinical symptoms, NEC can be determined as functioning or non-functioning. A functioning NEC shares the same symptoms as carcinoid syndrome.[3]
Gastrointestinal tract NEC is often metastized to the liver, but PHNEC is rarely seen. Generally speaking,PHNEC occurs at various ages, especially in young and middle-aged patients. PHNEC is not gender speci fi c. A single tumor is more frequent and there is no signi fi cant difference between the two lobes of the liver.[4,5]Most patients are discovered by health examination with a solid liver mass.[6]Within our series, only 4 patients had abdominal pain and diarrhea.
It is dif fi cult to differentiate PHNEC from other solid tumors, especially hepatocellular carcinoma (HCC), before operation; meanwhile, arguments still occur on the value and risk of liver biopsy; therefore, postoperative pathologic examination is the main method for a fi nal diagnosis.
Laboratory examination
HCC detection indices, such as AFP, CEA, and CA19-9 have almost no diagnostic value.[4,6]Serum 5-HT or 5-HIAA levels in 24-hour urine may be effective diagnostic indices, with a sensitivity of 73% and a speci fi city of over 90%.[7]Research revealed that patients with continuing low urinary 5-HIAA show a higher survival rate than those with high urinary 5-HIAA.[8]Serum CgA[3,9,10]is a sensitive index in diagnosing NEC, with a sensitivity of 87%-100% and a speci fi city of 92%. Moreover, CgA can be used to monitor tumor recurrence.
Imaging detection
Ultrasonography, CT, and MRI lack good speci fi city due to the similarity of PHNEC to hemangioma and HCC.[11]However, we found almost 50% (5/11) of our patients with solid tumor with cystic changes on ultrasonography, CT, and MRI (Figs. 1, 2). It differs from the colliquation necrosis in HCC and is helpful for differential diagnosis. Some reports express the same idea.[11,12]PHNEC often demonstrates high 18F-FDG uptake in PET-CT. It was reported that PET-CT speci fi city and sensitivity are increased with some speci fi c metabolic substrates, even discovering a tumor as small as 2 mm in diameter.[13,14]Octreoscan is also an ideal imaging procedure, with a speci fi city near 83%, and can discover concealed foci.[15]
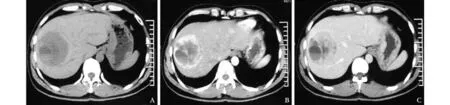
Fig. 1. CT scan images of a PHNEC patient. A: Plain CT scan showing a hypodense liver mass with cystic changes and fl uid level; B:In the arterial phase, the tumor was enhanced except for the cystic area; C: In the late phase, the density of the tumor was lower than the normal liver.
sutatS ecnerrucer,evilA revil-artxe on( )sisatsatem,evilA ecnerruceron ecnerrucer,evilA revil-artxe on( )sisatsatem,evilA ecnerruceron,evilA ecnerruceron,evilA ecnerruceron ecnerrucer,evilA revil-artxe on( )sisatsatem ecnerrucer,evilA revil-artxe on( )sisatsatem,evilA ecnerruceron ecnerrucer,evilA revil-artxe on( sisatsatem ecnerrucer,evilA revil-artxe on( )sisatsatem llarevO lavivrus)shtnom( 70189744342514131*8413321 eerf-esaesiD lavivrus)shtnom( 84895434251519353 evitarepotsoP tnemtaert enoN htnom 1ECAT revil retfa noitceser )eciwt(ECAT ecnerrucerhtiw enoN enoN enoN enoN htiwTIEP ecnerrucer enoN htnom 1ECAT revil retfa dna noitceser ecnerrucer dnaECAT editoertco htiw noitcejni ecnerrucer yrtsime001S+++− − −− −hcotsihon AgC+−ummI ESN++−+++++++st nossaM gniniats+++++++++neitapCENH gniniatsEH yletaredoM,detaitnereffid EVPhtiw llec llamS yletaredoM ,detaitnereffid EVPhtiw -lleW detaitnereffid-lleW detaitnereffid-lleW detaitnereffid-lleW detaitnereffid-lleW detaitnereffid-lleW detaitnereffid-lleW detaitnereffid-lleW detaitnereffid P fo atadlacinilC .elbaT romuT tnemtaertlacigruSrebmun noisicxe romuTelpitluM dnatfelni sebol thgir ymotcetapeh thgiRelpitluM etaduacthgir dna noisicxeebol ,ymotcetapeh thgiRelpitluM hpmyldna EVP noisicxeedon ymotcetapehtfeLelgniS ymotcetapehtfeLelgniS ymotcetapeh thgiRelpitluM noisicxe romuTelpitluM thgirdnatfelni sebol revil noisicxe romuTelpitluM thgirdnatfelni sebol revil ymotcetapehtfeLelgniS noisicxe romuTelpitluM thgirdnatfelni sebol revil ni noisicxe romuTelpitluM thgirdnatfel sebol revil.sulobme niev latrop :Esgnid nif IRMsgnid nifTC ytisned woL ,ssam citapeh nidecnahne yrehpirep citsycytisned woL revildilos dna decnahne ,ssam ,yrehpirepni ytivac lanimodba edon hpmyl EVP ,sisatsatem citapeh dilosdnacitsyC ni decnahne ,ssam yrehpirep citapeh dilosdnacitsyC ni decnahne ,ssam yrehpirep ytisned woL ,ssam citapeh nidecnahne yrehpirep citsyc ytisnedhgiH citapehdilos dna ni decnahne ,ssam yrehpirep citapeh dilosdnacitsyC ni decnahne ,ssam yrehpirep citsycytisned woL citapehdilos dna ni decnahne ,ssam yrehpirep ytisnedhgiH ,ssam citapeh nidecnahne yrehpirep VP .noitarepo erofebromutehtrof sht yhpargonosartlu-B sgnid nif dilosciohceopyH ssam citapeh dilosciohceopyH ssam citapeh citsycciohceopyH revildilos dna ssam dilosciohceopyH ssam revil citsyc ciohcerepyH revildilos dna ssam dilos ciohcerepyH ssam citapeh dilos ciohcerepyH ssam citapeh dilos ciohcerepyH ssam citapeh dnacitsyC dilos ciohcerepyh ssam citapeh dilos ciohcerepyH ssam citapeh dilos ciohcerepyH ssam citapeh nom901rof pu d noitatneserP,redneGt )ry( egA lanimodbA,elaM niap15 lacideM,elaM maxe43 aehrraiD,elameF 25 lacideM,elaM maxe95 lacideM,elaM maxe45 lanimodbA,elaM niap34 lacideM,elameF maxe05 aehrraiD,elaM 73 lacideM,elameF maxe85 lacideM,elameF maxe65 lacideM,elaM maxe05 ewollofsawtneitap sineitaP1234567890111 hT:*
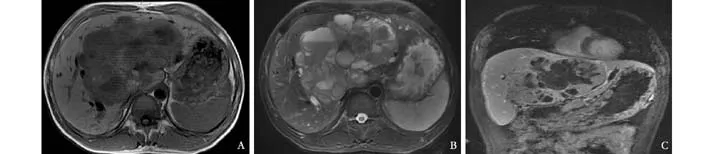
Fig. 2. MRI scan images of a PHNEC patient. A: T1-weighted MRI showing a large low density liver mass with a lower density cystic area in the middle lobe; B: T2-weighed MRI showing a high density liver mass, especially in the cystic area with a fl uid level; C:Coronal view image showing a large cystic-solid mass in the middle lobe.
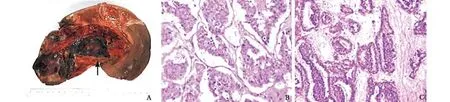
Fig. 3. Pathologic examination of PHNEC. A: The tumor was 16×10 cm, dark red, without peplos, with a capsular space at the center(max diameter 1.9 cm), with hemorrhage at the middle (arrow); B: HE staining showing tumor cells lined up in a nest structure,surrounded by cuboidal cells; cancer nests were encased by blood sinusoids; lumen extended and had hemorrhage; cancer boundary was clear (original magni fi cation ×400); C: immunohistochemistry: NSE(+) (original magni fi cation ×200).
Pathologic diagnosis
Pathologic diagnosis is the most accurate diagnostic method (Fig. 3). Routine HE satining is not speci fi c for diagnosis, but it is helpful in classifying the tumor grade.Some special stains, such as Massons and Grimelius, can raise the diagnosis rate to 80% or above[12]and our result was 100% (9/9). Immunohistochemical analysis also raises the positive rate and accuracy through detecting PHNEC correlative markers, such as neuron-speci fi c enolase (NSE), CgA and neurilemma cell S-100 protein,and synaptic membranes protein (SYP). Among these,the sensitivity of NSE is 80%-90%[16]and our result was 90% (9/10).
Treatment of PHNEC
Until now the most effective therapy for PHNEC is hepatectomy. Massive research reported that the survival rate is satisfactory in spite of recurrence;[12,17]especially for carcinoid, the 5-year recurrence rate is 18% and the 5-year survival rate is 74%-78%.[18,19]Until the end of our study, 54.5% of the patients (6/11) had recurrence,but all patients are still alive, including 4 patients with moderately-differentiated or poorly-differentiated tumors.The longest disease-free survival time was 98 months and the patient had a poorly-differentiated tumor (patient 2).One patient (patient 9) in our series has survived for more than 148 months since the discovery of the tumor and 39 months since hepatectomy, without tumor recurrence.This result suggests that resection of all tumors leads to a higher survival rate in patients with PHNEC.
There is still no report of effective systemic chemotherapy for PHNEC and typical treatment for recurrence.TACE (transcatheter arterial chemoembo-lization), as the common treatment protocol for liver cancer, has an ideal effect for metastatic hepatic NEC according to a report,[20]but for PHNEC, there is no certain result with a large sample set. The effectiveness of other local treatments such as radiofrequency therapy and PEIT have not been reported yet. These methods may be considered for small tumors with diameters ≤3 cm because of direct damaging effect on the tumors. One patient (patient 8)in our series had 3 recurring tumors one month after hepatectomy. He received PEIT three times within 2 weeks and the tumors decreased signi fi cantly without any new recurrence during a 13-month follow-up.
The value of liver transplantation for PHNEC is still a problem. Some research suggests that transplantation can be taken into consideration for patients with multiple liver tumors or bad liver function due to its effectiveness and higher survival rate rather than liver excision.[21]
In summary, a rare liver primary tumor, PHNEC has a unique speci fi city during its occurrence and development.Final diagnosis mainly depends on pathological and immunohistochemical examinations. We need to develop more convenient and effective features in imaging and laboratory detection to differentiate PHNEC from HCC,hemangioma, and other solid liver masses. For patients without a history of chronic liver disease, but with normal serum AFP level and cystic changes in the tumor,combined with diarrhea and abdominal pain, PHNEC should be considered. Serum 5-HT, CgA, or urinary 5-HIAA levels should be assessed for these patients. At present the main therapy for PHNEC is hepatectomy,especially for carcinoid cases. To patients without surgical opportunities, TACE, PEIT, and liver transplantation can be alternatives after evaluation of their effectiveness.
Funding: None.
Ethical approval: Not needed.
Contributors: YJM proposed the study. HYQ wrote the fi rst draft.XF and HB analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. YJM is the guarantor.
Competing interest: No bene fi ts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Sippel RS, Chen H. Carcinoid tumors. Carcinoid tumors. Surg Oncol Clin N Am 2006;15:463-478.
2 Solcia E, Kloeppel G, Sobin LH. Histological typing of endocrine tumors, New York: Springer Verlag;2000.
3 Oberg K. Neuroendocrine gastrointestinal tumors--a condensed overview of diagnosis and treatment. Ann Oncol 1999;10:S3-8.
4 Iimuro Y, Deguchi Y, Ueda Y, Tanaka A, Iwasa Y, Ishihara M, et al. Primary hepatic carcinoid tumor with metachronous lymph node metastasis after long-term follow up. J Gastroenterol Hepatol 2002;17:1119-1124.
5 Abdel Wahab M, Fathy O, Elghwalby N, Sultan A, Mostafa M,El-Baz M, et al. Primary hepatic carcinoid tumor: one Egyptian center experience. Hepatogastroenterology 2006;53:33-38.
6 Donadon M, Torzilli G, Palmisano A, Del Fabbro D, Panizzo V, Maggioni M, et al. Liver resection for primary hepatic neuroendocrine tumours: report of three cases and review of the literature. Eur J Surg Oncol 2006;32:325-328.
7 Lamberts SW, Ho fl and LJ, Nobels FR. Neuroendocrine tumor markers. Front Neuroendocrinol 2001;22:309-339.
8 van der Horst-Schrivers AN, Post WJ, Kema IP, Links TP,Willemse PH, Wymenga AN, et al. Persistent low urinary excretion of 5-HIAA is a marker for favourable survival during follow-up in patients with disseminated midgut carcinoid tumours. Eur J Cancer 2007;43:2651-2657.
9 Eriksson B, Oberg K, Stridsberg M. Tumor markers in neuroendocrine tumors. Digestion 2000;62:33-38.
10 Stridsberg M, Eriksson B, Oberg K, Janson ET. A comparison between three commercial kits for chromogranin A measurements. J Endocrinol 2003;177:337-341.
11 van der Hoef M, Crook DW, Marincek B, Weishaupt D.Primary neuroendocrine tumors of the liver: MRI features in two cases. Abdom Imaging 2004;29:77-81.
12 Bastaki W, Mothaffer F, Varro J, Al-Ghanim M, Malak L,Ayyash E, Asfar S. Primary hepatic carcinoid tumor. Med Princ Pract 2005;14:288-291.
13 Orlefors H, Sundin A, Garske U, Juhlin C, Oberg K, Skogseid B, et al. Whole-body (11)C-5-hydroxytryptophan positron emission tomography as a universal imaging technique for neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and computed tomography. J Clin Endocrinol Metab 2005;90:3392-3400.
14 Hoegerle S, Altehoefer C, Ghanem N, Koehler G, Waller CF,Scheruebl H, et al. Whole-body 18F dopa PET for detection of gastrointestinal carcinoid tumors. Radiology 2001;220:373-380.
15 Oberg K, Eriksson B. Nuclear medicine in the detection,staging and treatment of gastrointestinal carcinoid tumours.Best Pract Res Clin Endocrinol Metab 2005;19:265-276.
16 Zhang LH, Chu Q, Huang ZY. Diagnostic effect of NSE, CgA,SYP in neuroendocrine carcinoma. Shanghai Medical Journal 1997;20:660-661.
17 Iwao M, Nakamuta M, Enjoji M, Kubo H, Fukutomi T, Tanabe Y,et al. Primary hepatic carcinoid tumor: case report and review of 53 cases. Med Sci Monit 2001;7:746-750.
18 Mizuno Y, Ohkohchi N, Fujimori K, Doi H, Orii T, Asakura T, et al. Primary hepatic carcinoid tumor: a case report.Hepatogastroenterology 2000;47:528-530.
19 Knox CD, Anderson CD, Lamps LW, Adkins RB, Pinson CW. Long-term survival after resection for primary hepatic carcinoid tumor. Ann Surg Oncol 2003;10:1171-1175.
20 Bloomston M, Al-Saif O, Klemanski D, Pinzone JJ, Martin EW, Palmer B, et al. Hepatic artery chemoembolization in 122 patients with metastatic carcinoid tumor: lessons learned. J Gastrointest Surg 2007;11:264-271.
21 Fenwick SW, Wyatt JI, Toogood GJ, Lodge JP. Hepatic resection and transplantation for primary carcinoid tumors of the liver.Ann Surg 2004;239:210-219.
BACKGROUND: Primary hepatic neuroendocrine carcinoma(PHNEC) is extremely rare, and fewer than 300 cases have been reported in the English/Chinese-language literature, therefore it is dif fi cult to make a proper diagnosis and determine a therapeutic approach.
METHODS: Eleven PHNEC patients were admitted to our hospital between January 1996 and May 2008. Laboratory examination, digestive endoscopy, B-ultrasonography, CT, MRI,or PET-CT were performed on the patients for preoperative diagnosis. All patients
liver resection. Some patients received transcatheter arterial chemoembolization (TACE),percutaneous ethanol injection treatment (PEIT), or octreotide injection when a recurrence was found. The patients' clinical data were recorded and all patients were followed up.
RESULTS: The patients were con fi rmed pathologically as having PHNEC . Their median follow-up time was 33 months(12-107 months). All patients survived, and the longest postoperative survival time was 107 months, the longest diseasefree survival time was 98 months, the 1-year survival rate was 100%, and the 1-year recurrence rate was 45.5% (5/11).
CONCLUSIONS: Since PHNEC is easy to confuse with hepatocellular carcinoma, careful screening of symptoms is needed to avoid misdiagnosis. Resection is the fi rst choice of treatment for PHNEC and provides the most favorable outcomes including long-term survival. Other treatment such as TACE and PEIT can be considered as well, especially when a tumor recurs.
Author Af fi liations: Department of Special Treatment and Liver Transplantation (Huang YQ, Xu F and Yang JM), and Department of Radiology(Huang B), Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, Shanghai 200438, China
Jia-Mei Yang, MD, Department of Special Treatment and Liver Transplantation, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, Shanghai 200438, China (Tel: 86-21-81875531;Email: jmyang@smmu.edu.cn)
© 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
September 3, 2009
Accepted after revision December 28, 2009
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Critical fl icker frequency for diagnosis and assessment of recovery from minimal hepatic encephalopathy in patients with cirrhosis
- Risk factors for early recurrence of smallhepatocellular carcinoma after curative resection
- Pathological changes at early stage of multiple organ injury in a rat model of severe acute pancreatitis
- Potential etiopathogenesis of seventh day syndrome following living donor liver transplantation: ischemia of the graft?
- Comparatively lower postoperative hepatolithiasis risk with hepaticocholedochostomy versus hepaticojejunostomy
- Effect of sodium salicylate on oxidative stress and insulin resistance induced by free fatty acids
