一种新型细梗胡枝子黄酮类提取物的结构和抗氧化活性
2010-11-30李敏杰李亚军彭淳容陆文聪
李敏杰 李亚军 彭淳容 陆文聪
(上海大学化学系,上海 200444)
一种新型细梗胡枝子黄酮类提取物的结构和抗氧化活性
李敏杰*李亚军 彭淳容 陆文聪
(上海大学化学系,上海 200444)
利用密度泛函理论的B3LYP交换相关泛函对从细梗胡枝子中提取的一种新型黄酮类化合物的分子结构和抗氧化活性进行了研究,获得了该化合物的中性分子、阴离子、自由基和自由基阳离子的稳定几何构型和能量.通过分析前线分子轨道特征,确定了与实验结果一致的现象:A环是参加化学反应的活性部位,并发现A′环也是重要的抗氧化活性部位.为判断其抗氧化活性,预测其水溶液中,中性和阴离子的电离势分别为509.0和432.2 kJ·mol-1,均裂O—H键解离能为347.3 kJ·mol-1,羟基自由基电子亲和势和氢原子亲和势分别为-620.6和-487.5 kJ·mol-1.通过理论分析比较,该黄酮类化合物清除羟基自由基的三种机理即H原子转移、电子转移-质子转移和质子丢失-电子转移在热力学上并存,其中质子丢失-电子转移是热力学最有利的机理.本文为设计新型高效黄酮类抗氧化剂,研究黄酮类化合物的构效关系和抗氧化机理提供了理论依据.
密度泛函理论;细梗胡枝子黄酮类提取物;键解离能;电离势;抗氧化活性
Lespedeza species(Leguminosae),which distributed in East Asia and North America,have been used as folk medicine for the treatment of nephritis,azothemia,and diuresis[1].Many low-toxic antioxidants have been isolated,including stilbenoids,flavonoids,and otherphenolic compounds[2-6].Lespedeza virgata(Thunb.) DC.,a Chinese herb,is used in treatment of various nephropathies in central China.Potent antioxidants with low toxicity are desired to be used as food additives and medical substances. Thus interests in search for potent antioxidants from Lespedeza virgata have start up since 2007[6-10].
Recently,a novel flavonoid-type compound 5-hydroxy-6-methyl-2′-methoxy-[6,6″-dimethylpyrano(2″,3″:7,8)][6‴,6‴-dimethylpyrano(2‴,3‴:4′,5′)]-(2S)flavonone(lespedezaflavon-one, Fig.1)from Lespedeza virgata was isolated and identified by Liu′s group[6];and lespedezaflavonone is found experimentally to have the strongest antioxidant activity among the flavoniods extracted from Lespedeza virgata.
The previous experimental studies have contributed positively to the search for potent antioxidants from Lespedeza virgata[6-10]. Nevertheless,it is worth mentioning that no study has provided structure information,the antioxidant activity mechanism,and structure-activity relationship of the new flavonoid-type compound.
Flavonoid is a group of polyphenolic compounds which can be found in seeds,fruits,tea,vegetables,and green leaves[11-14]. They execute a wide range of biological effects,such as antibacterial,antiviral,anti-inflammatory,antiallergenic,and vasodilatory actions[15-17].Based on the predicted physico-chemical properties,some theoretical studies on the antioxidant activities of flavonoids were done[11,18-23].
This paper focuses on the molecular structures and antioxidant activities of lespedezaflavonone.The character of the frontier molecular orbital was analyzed;and BDE and IP values for lespedezaflavonone and EA and HA values for the scavenged hydroxyl radical in aqueous solution were predicted.On the basis of the values,discussion was then made about the hydroxyl radical scavenging mechanisms of lespedezaflavonone.With the current work we hope to highlight the potent antioxidative activities of compounds from Lespedeza virgata and stimulated the interest for further studies and exploitation in food and pharmaceutical industries of extracts in Lespedeza virgata.
1 Computational details

Fig.1 Structure of lespedezaflavonone from Lespedeza virgata
All calculations were performed by the Gausssian 03 suite of programs[24].Conformation analyses and geometry optimizations were carried out using the B3LYP hybrid density functional[25-26]with the 6-31g(d)basis set.The optimized structures were confirmed as true minima by vibrational analysis at the same level. Frequency calculations were carried out and performed on all of the species to confirm convergence to appropriate local minima of the energy surface and to extract zero-point,enthalpy and entropy corrections.Single-point electronic energies(SPEs)were then calculated at the B3LYP/6-311++g(2df,2p)levels to the most stable conformer.Next SPEs were converted to the enthalpy values at 298 K,101329 Pa,by adding the thermal contributions to enthalpy(TCE,in which the vibrational contributions include zero-point vibrational energy).The B3LYP/6-311++g(2df,2p)//B3LYP/6-31g(d)method was successfully used for the study on the radical scavenging activity of maritimetin and related aurones by Nenadis′s group[27].
Finally,the O—H BDE[28-29],IP[30-31],EA,and HA can be computed using Eqs.(1-4),respectively.

where,BDE is homolytic bond dissociation enthalpy.HA●,HAHand HH●
are the enthalpies of radical,neutral of an antioxidant, and hydrogen radical in Eq.(1),respectively.In Eq.(2),IP is the adiabatic ionization potential;EAH,EAH+●,EA-,and EA●
are the energies of neutral,cation free radical,anion,and radical of an antioxidant,respectively.In Eq.(3),EA is adiabatic electron affinity;ERO-and ERO●are the energies of anion and radical of a scavenged radical,respectively.In Eq.(4),HA means H-atom affinity;HROHand HRO●are the enthalpies of neutral and radical of a scavenged radical.Solvent effects were computed on the single-point level by using the integral equation formulation-polarized continuum model IEF-PCM[32]and the united atom for Hartree-Fock(UAHF)cavitymodelin aqueoussolution(ε=78.39).
2 Results and discussion
2.1 Conformational analysis and molecular geometries
The behavior of the hydroxyl group is largely influenced by the neighboring groups and the geometry.Thus,the conformation can be regarded as the first important parameter to analyze the antioxidant capacity of lespedezaflavonone.The optimized structures of the most stable conformers for neutral,radical cation,radical,and anion forms generated from lespedezaflavonone are shown in Fig.2.
The most stable conformers of neutral and radical cation are those possessing intramolecular hydrogen bonds(IHBs).In global minimum structures of neutral and radical cation molecules, the hydroxyl group in ring A can form a hydrogen bond with the keto group in ring C via a six-membered ring.The rings A,A′, and C are planar in the global minimum structures of four molecules.The rings B and B′are planar in the most stable con-formers of four molecules.The two planes are almost perpendicular.The coplanarity of the rings A and C could increase the antioxidant activity of lespedezaflavonone which is consistent with the previous studies[11,18].

Fig.2 The most stable conformers of neutral,radical cation,radical,and anion forms of lespedezaflavonone
2.2 Character of the frontier molecular orbital
According to Fukui′s frontier molecular orbital theory[33-34],the highestoccupied molecularorbital(HOMO)and the lowest unoccupied molecular orbital(LUMO)play fundamental roles in the interpretation of chemical reactivity.The contours of HOMO and LUMO for neutral and anion forms of lespedezaflavonone, are displayed in Fig.3.

Fig.3 HOMO and LUMO for neutral and anion forms of lespedezaflavonone
From Fig.3,it is observed that the HOMO electron density concentrated in rings A and A′and the LUMO electron density focused on rings A,A′,and C for neutral molecule of lespedezaflavonone.These results show that ring A is responsible for the high activity for flavonoid-type compound,which is consistent with what is observed in previous studies[18,35-36].Furthermore,it is noteworthy that ring A′is firstly found to be the important part for the potent antioxidant activity of flavonoid-type compound.The atomic orbital distributions of ring A′in HOMO and LUMO are 51.7%and 20.5%,respectively,which also show that ring A′is the significant part for the potent radical scavenging activity.
The HOMO electron density concentrated in rings A and A′and the LUMO electron density focused on rings B,and B′for anion form of lespedezaflavonone(see Fig.3).It can be inferred that rings A,A′,B and B′are responsible for the antioxidant activity of lespedezaflavonone in polar solvent,which is in agreement with the previous results[18,35-36].In view of the obtained results it is of interest to design new flavonoid-type compound with enhanced activity.
2.3 IP and O—H BDE for lespedezaflavonone,EA and HA for hydroxyl radical
As we know,there are at least three mechanisms reported for the radical scavenging processes of antioxidants(AH)by donating H-atom in physiological systems[37-41]:(1)one-step H-atom transfer(HAT)(Eq.(5));(2)stepwise electron-transfer-protontransfer(ET-PT)(Eq.(6));(3)sequential-proton-loss-electrontransfer(SPLET)(Eq.(7)).
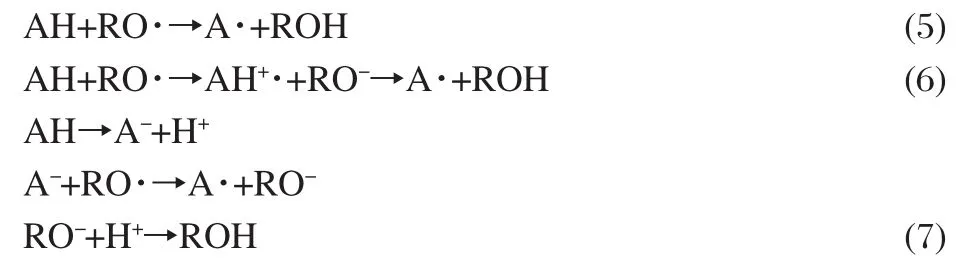
All of them may occur in parallel,but with different rates in a certain chemical or biological system[38,41].In mechanism(1),the reactivity of an AH can be estimated by calculating the O—H BDE[38,42],where the lower the BDE value,the higher the expected activity.IP of AH is the controlling parameter in mechanisms (2)and(3)[38].Molecules with the low IP values are expected to have high activity.Furthermore,the antioxidant activity is also governed by solvents,structure characteristics of the scavenged radicals,and the capacity of electron affinity and H-atom affinity[38,43].For radicals with high HA,the former mechanism is preferred,while for radicals with high EA,the latter two mechanisms are favored.In addition,the radicals are scavenged by antioxidant in biological system.The solvation effect of aqueous solution can not be ignored.Thus,in the present study BDE and IP values for lespedezaflavonone,EA and HA values for the scavenged radicals(hydroxyl radical)in aqueous solution are used to elucidate the radical scavenging activity of lespedezaflavonone.
The homolytic O—H BDE,IP for lespedezaflavonone in aqueous solution were calculated.The calculated BDE is 347.3 kJ·mol-1.The BDE is 23.7 kJ·mol-1lower than the experimental dataofphenol[44].The lowerthe BDE values,the easierthe H atom abstraction.It is obviously that the H atom in the hydroxyl group of lespedezaflavonone is easier to be abstracted than that of phenol.
The adiabatic IP for neutral and anion form are 509.0 and 432.2 kJ·mol-1,respectively.HA and adiabatic EA for hydroxyl radical in aqueous solution were also computed.The data are -487.5 and-620.6 kJ·mol-1.The IP of lespedezaflavonone is lower than those widely used food synthetic additives such as butylated hydroxyanisole,nordihydroguaiaretic acid,and propyl gallate(638.3,668.8,701.4 kJ·mol-1,respectively)[37],and the natural polyphenoic flavonoid epigallocatechin-3-gallate(617.4 kJ·mol-1)[45],one of the most active antioxidant obtained from green tee.Apparently lespedezaflavonone seems to donate an electron easily,comparable to the activity of some food additives.
2.4 Antioxidant mechanisms
According to the results of Zhang et al.[38],the mechanism dominating the antioxidant activity of an antioxidant can be inferred by the sum value of its BDE and HA of scavenged radicals and the sum value of its IP and EA of with scavenged radicals.If the sum value of the former is positive,HAT is thermodynamically forbidden.Otherwise,HAT is thermodynamically permitted.Likewise,if the sum value of the latter is positive, SPLET or ET-PT is permitted.Otherwise,SPLET or ET-PT is not permitted.
From the above results,it is observed that the O—H BDE of lespedezaflavonone,is 140.2 kJ·mol-1lower than the absolute HA of hydroxyl radical,which suggests that HAT is thermodynamically permitted in the scavenging of hydroxyl radical by lespedezaflavonone.The adiabatic IP of lespedezaflavonone and its anion are 111.6,188.4 kJ·mol-1lower than absolute EA of hydroxyl radical.The sum values are both negative.It can be inferred that the electron transfer between neutral,anion,and hydroxyl radical are both thermodynamically permitted.The thermodynamically favored hydroxyl radical scavenging mechanisms for lespedezaflavonone are in the order of:ET-PT<HAT<SPLET since the sum data are 111.6,140.2,188.4 kJ·mol-1,respectively.
Overall,three antioxidant mechanisms for lespedezaflavonone to scavenge hydroxyl radical may occur thermodynamically in parallel.Therein,SPLET is the most preferred one for lespedezaflavonone to scavenge hydroxyl radical in thermodynamics.
3 Conclusions
Using the B3LYP/6-311++g(2df,2p)//B3LYP/6-31g(d)method,the molecular structures and antioxidant activities of lespedezaflavonone were studied.The optimized geometries and energies of neutral,radical cation,radical,and anion forms from lespedezaflavonone were obtained.The ring A and A′are responsible for the high activity for flavonoid-type compound through the analysis of the character of the frontier molecular or-bital.Then,IP(509.0,432.2 kJ·mol-1for neutral and anion forms) and homolytic O—H BDE(347.3 kJ·mol-1)for lespedezaflavonone and EA(-620.6 kJ·mol-1)and HA(-487.5 kJ·mol-1)for hydroxyl radical in aqueous solution were determined.Our theoretical analysis indicates that three antioxidant mechanisms for lespedezaflavonone to scavenge hydroxyl radical may occur thermodynamically in parallel and SPLET is the most favorable one.These findings are helpful in the further study on the design of novel efficient flavonoid-type antioxidants,the structureactivity relationship and the antioxidant mechanism of flavonoidtype compounds.
1 Yarnell,E.World J.Urol.,2002,20:285
2 Miyase,T.;Sano,M.;Nakai,H.;Muraoka,M.;Nakazawa,M.; Suzuki,M.;Yoshino,K.;Nishihara,Y.;Tanai,J.Phytochemistry, 1999,52:303
3 Miyase,T.;Sano,M.;Yoshino,K.;Nonaka,K.Phytochemistry, 1999,52:311
4 Deng,F.;Chang,J.;Zhang,J.S.J.Asian Nat.Prod.Res.,2007,9: 655
5 Maximov,O.B.;Kulesh,N.I.;Stepanenko,L.S.Fitoterapia, 2004,75:96
6 Li,T.;Zhang,X.F.;Yan,B.Z.;Shi,H.M.;Du,L.B.;Zhang,Y. Z.;Wang,L.F.;Tang,Y.L.;Liu,Y.Bioorg.Med.Chem.Lett., 2007,17:6311
7 Yan,C.;Xie,H.H.;Wei,X.Y.;Deng,H.Z.J.Nat.Prod.,2008, 71:929
8 Chen,Y.;Deng,H.Z.;Zhou,Y.;Chen,S.W.;Chen,B.China J. Chin.Mater.Med.,2008,33:1024 [陈 艳,邓虹珠,周 毅,陈少伟,陈 彬.中国中药杂志,2008,33:1024]
9 Chen,Y.;Deng,H.Z.;Liang,L.J.South.Med.Univ.,2008,28: 858 [陈 艳,邓虹珠,梁 磊.南方医科大学学报,2008,28: 858]
10 Tan,L.;Zhang,X.F.;Liu,Y.;Yan,B.Z.Chinese Traditional Herb Drugs,2008,39:189 [谭 莉,张秀凤,刘 扬,严宝珍.中草药,2008,39:189]
11 Marchand,L.L.Biomed.Pharmacother.,2002,56:296
12 Zhou,L.;Xie,J.C.;Ge,Y.F.;Xu,X.J.Acta Phys.-Chim.Sin., 2002,18:808 [周 力,谢建春,戈育芳,徐筱杰.物理化学学报,2002,18:808]
13 Lu,J.;Li,X.W.;Gui,M.Y.;Liu,G.Y.;Zhu,N.;Yu,A.M.; Okuyama,T.;Masaki,B.;Jin,Y.R.Chem.J.Chin.Univ.,2009, 30:468 [陆 娟,李绪文,桂明玉,刘桂英,朱 娜,于爱民,马场正树,奥山徹,金永日.高等学校化学学报,2009,30:468]
14 Yuan,S.;Yao,S.J.;Geng,Y.;Cai,P.;Zhang,W.J.;Guo,Y.L. Acta.Chim.Sin.,2009,67:318 [袁 帅,姚胜军,耿 昱,蔡澎,章文峻,郭寅龙.化学学报,2009,67:318]
15 Yokozawa,T.;Nakagawa,T.;Kitani,K.J.Agric.Food Chem., 2002,50:3549
16 Chen,Z.F.;Peng,Y.;Tan,M.X.;Liu,Y.C.;Wang,H.S.;Liang, H.Prog.Chem.,2009,21:923 [陈振锋,彭 艳,谭明雄,刘延成,王恒山,梁 宏.化学进展,2009,21:923]
17 Zheng,M.H.;Gan,Y.;Xie,S.Q.;Wang,C.J.;Zhao,J.Chin.J. Org.Chem.,2009,29:1445 [郑梅花,甘 莹,谢松强,王超杰,赵 瑾.有机化学,2009,29:1445]
18 Li,M.J.;Liu,L.;Fu,Y.;Guo,Q.X.J.Mol.Struct.-Theochem, 2007,815:1
19 Aaby,J.;Hvattum,E.;Skrede,G.J.Agric.Food Chem.,2004,52, 4595
20 Yan,X.;Xia,L.L.;Yu,J.;Ding,W.J.Acta.Chim.Sin.,2008,66: 39 [延 玺,夏玲玲,于 静,丁万见.化学学报,2008,66:39]
21 Wu,H.;Gao,P.Z.J.Math.Med.,2009,22:434 [吴 洪,高平章.数理医药学杂志,2009,22:434]
22 Amat,A.;Angelis,F.D.;Sgamellotti,A.;Fantacci,S.J.Mol. Struct.-Theochem,2008,868:12
23 Lekka,C.E.;Ren,J.;Meng,S.;Kaxiras,E.J.Phys.Chem.B, 2009,113:6478
24 Frisch,M.J.;Trucks,G.W.;Schlegel,H.B.;et al.Gaussian 03. Revision C.02.Wallingford,CT:Gaussian Inc.,2004
25 Becke,A.D.J.Chem.Phys.,1993,98:5648
26 Lee,C.;Yang,W.;Parr,R.G.Phys.Rev.B,1988,37:785
27 Nenadis,N.;Sigalas,M.P.J.Phys.Chem.A,2008,112:12196
28 Feng,Y.;Liu,L.;Wang,J.T.;Huang,H.;Guo,Q.X.J.Chem. Inform.Comput.Sci.,2003,43:2005
29 Fu,Y.;Liu,L.;Mou,Y.;Lin,B.L.;Guo,Q.X.J.Mol.Struct.-Theochem,2004,674:241
30 Fang,Y.;Liu,L.;Feng,Y.;Li,X.S.;Guo,Q.X.J.Phys.Chem.A, 2002,106:4669
31 Fu,Y.;Liu,L.;Yu,H.Z.;Wang,Y.M.;Guo,Q.X.J.Am.Chem. Soc.,2005,127:7227
32 Tomasi,J.;Mennucci,B.;Cammi,R.Chem.Rev.,2005,105:2999 33 Fukui,K.;Yonezawa,T.;Shingu,H.J.Chem.Phys.,1952,20:722
34 Fukui,K.;Yonezawa,T.;Nagata,C.;Shingu,H.J.Chem.Phys., 1954,22:1433
35 Tsimogiannis,D.;Oreopoulou,V.J.Am.Chem.Soc.,2007,84: 129
36 Kevin,H.;Karsten,O.;Leif,H.S.J.Org.Chem.,2009,74,7283
37 Zhang,H.Y.;Sun,Y.M.;Wang,X.L.Chem.-Eur.J.,2003,9: 502 and references therein
38 Zhang,H.Y.;Ji,H.F.New J.Chem.,2006,30:503
39 Nakanishi,I.;Kawashima,T.;Ohkubo,K.;Kanazawa,H.;Inami, K.;Mochizuki,M.;Fukuzumi,S.;Ikota,N.Org.Biomol.Chem., 2005,3:626
40 Musialik,M.;Litwinienko,G.Org.Lett.,2005,7:4951
41 Zhang,H.Y.;Wang,L.F.J.Phys.Chem.A,2003,107:11258
42 Wright,J.S.;Carpenter,D.J.;McKay,D.J.;Ingold,K.U.J.Am. Chem.Soc.,1997,119:4245
43 Shen,L.;Zhang,H.Y.;Ji,H.F.Org.Lett.,2005,7:243
44 Luo,Y.R.Handbook of bond dissociation energies in organic compounds.Boca Raton:CRC Press,2003
45 Lien,E.J.;Ren,S.;Bui,H.H.;Wang,R.Free Radic.Biol.Med., 1999,26:285
November 25,2009;Revised:December 7,2009;Published on Web:December 31,2009.
Structures and Antioxidant Activities of Lespedezaflavonone from Lespedeza Virgata
LI Min-Jie*LI Ya-Jun PENG Chun-Rong LU Wen-Cong
(Department of Chemistry,Shanghai University,Shanghai200444,P.R.China)
The molecular structures and antioxidant activities of a novel flavonoid-type compound(lespedezaflavonone) from Lespedeza virgata were studied using density functional theory(DFT)with the B3LYP exchange correlation functional. The optimized geometries of neutral,radical cation,radical,and anion forms of lespedezaflavonone were obtained.Ring A was found to be responsible for the high activity of the flavonoids by an analysis of the character of the frontier molecular orbital,which was consistent with what was observed experimentally.Furthermore,it was noteworthy that ring A′was firstly found to be the important part for the potent antioxidant activity of lespedezaflavonone.To quantify the antioxidant activities, we determined the adiabatic ionization potential(IP,509.0,432.2 kJ·mol-1for the neutral and anion forms,respectively),the homolytic O—H bond dissociation enthalpy(BDE,347.3 kJ·mol-1)for lespedezaflavonone,the adiabatic electron affinity (EA,-620.6 kJ·mol-1)and the H-atom affinity(HA,-487.5 kJ·mol-1)for hydroxyl radical in aqueous solution.Our theoreticalanalysis shows thatH-atomtransfer,stepwise electron-transfer-proton-transfer,and sequentialproton-loss-electrontransfer mechanisms for lespedezaflavonone to scavenge hydroxylradicalmay occur thermodynamically in parallel,while the last process is the most favorable.These findings are helpful for further study on the design of novel efficient flavonoid-type antioxidants,the structure-activity relationship and the antioxidantmechanismof flavonoid-type compounds.
Density functional theory; Lespedezaflavonone; Bond dissociation enthalpy; Ionization potential; Antioxidant activity
O641
*Corresponding author.Email:minjieli@shu.edu.cn;Tel:+86-21-66133513.
The project was supported by the National Natural Science Foundation of China(20902056),Science Foundation of Shanghai for Excellent Young Teachers,China(B.37-0101-07-716),Innovation Foundation of Shanghai University,China(A.10-0101-08-423),and Leading Academic Discipline
Project of Shanghai Municipal Education Commission,China(J50101).
国家自然科学青年基金(20902056)、上海高校选拨培养优秀青年教师科研专项基金((B.37-0101-07-716))、上海大学创新基金(A.10-0101-08-423)和上海市教委第五期重点学科建设项目(J50101)资助
