焦炉煤气甲烷重整制氢热力学分析和实验研究
2010-11-30杨志彬张玉文张云妍丁伟中沈培俊
杨志彬 张玉文 张云妍 丁伟中 沈培俊
刘 勇 周宇鼎 黄少卿
(上海大学上海市现代冶金与材料制备重点实验室,上海 200072)
焦炉煤气甲烷重整制氢热力学分析和实验研究
杨志彬 张玉文 张云妍 丁伟中*沈培俊
刘 勇 周宇鼎 黄少卿
(上海大学上海市现代冶金与材料制备重点实验室,上海 200072)
对焦炉煤气甲烷部分氧化重整热力学进行分析,考察反应温度、CH4/O2摩尔比及水蒸气加入量等因素对重整性能的影响,并分析焦炉煤气原始氢含量对其部分氧化重整性能的影响.分析结果表明甲烷转化率均随CH4/O2摩尔比和水蒸气加入量的增大以及反应温度的升高而增大.在CH4/O2摩尔比1.7-2.1,温度825-900℃及压力1.01×105Pa的反应条件下,可得较好重整性能;甲烷转化率,氢及一氧化碳的选择性分别为91.0%-99.9%,87.0%-93.4%和100%-107%,重整后得到的氢量增大到原始氢量的1.95-2.05倍,每摩尔焦炉煤气消耗的热量仅为2.94 J,同时得出在CH4/O2摩尔比2,温度825-900℃及1.01×105Pa条件下,往焦炉煤气内添加体积分数为2%-4%的水蒸气时重整性能得到较大提高;重整后甲烷转化率、氢及一氧化碳选择性分别由92.6%、87.2%、104%增大到98.6%、96.4%、107%.并在BaCo0.7Fe0.2Nb0.1O3-δ透氧膜反应器上研究NiO/MgO固溶体催化剂焦炉煤气部分氧化重整性能.结果表明该重整反应效果较好,于875℃下获得16.3 mL·cm-2·min-1透氧量,95%甲烷转化率及80.5%氢和106%一氧化碳选择性.且所得实验结果与热力学分析结果符合较好,表明NiO/MgO固溶体催化剂有较好的催化重整性能.
焦炉煤气;氢;热力学分析;混合导体透氧膜反应器;NiO/MgO固溶体催化剂
The next-generation recycling processes and equipments for iron&steel production are one of the major construction projects of the 11th five-year plan in China.The goal is to achieve high-quality steel production,high-efficiency energy transformation,and better waste utilization of society at the same time[1]by forming an industrial chain of circular economy with steel plants as its core.Producing syngas from coke oven gas(COG) in the proceeding of metallurgy is important for energy transformation[2-5]and is receiving growing attention.The typical compositions of COG are shown in Table 1.
The syngas from COG can be used as the reduction gas of metallurgy or transformed to liquid fuels such as methanol and dimethyl.The reforming of CH4in COG is the key to produce syngas from COG.Thermodynamics analysis and technology of syngas production from COG by partial oxidation reforming, carbon dioxide reforming,and steam reforming of CH4[6-8]have been disclosed by some articles and patents.Zhang et al.[9-10]reported thermodynamics analysis on producing metallurgical reduction gas from COG.The equilibrium composition for nonequal phase system consisting of carbon,hydrogen,oxygen,and inert gas from COG was calculated and the optimal reaction condition was achieved at the conversion temperature of 950℃andpressureof1.01×105Pa with O2addition of 15%.Tang et al.[11]studied the influence factors such as the molar ratio of(CO2+ H2O)/CH4,CH4conversion temperature,and the molar ratio of O2on partial oxidation reforming of COG.The results showed that CH4conversion larger than 95%can be achieved using the following optimal reaction conditions:(n(CO2)+n(H2O))/n(CH4)= 1.1-1.3,temperature in the range of 950-1050℃,and the molar ratio of O2about 6%.Li et al.[12]reported that the best condition of operation temperature should be 1100℃ and oxygen to methane molar ratio should be 0.39 for the reforming of COG with gasfication gas to syngas.Zhang et al.[13]suggested that an optimal hydrogen yield of 1.04-1.10 mol per mol of the consumed COG would be achieved when the initial molar ratio of O2and CH4is 0.57-0.46 in the temperature range of 900-1000℃and the corresponding amplification of original hydrogen in COG could be 1.8-1.9 times higher.However,thermodynamic equilibrium analysis on the effects of the original H2and the steam addition on partial oxidation of methane(POM)in COG have not been reported,and the majority of the reports are based on the fix-bed reactor.
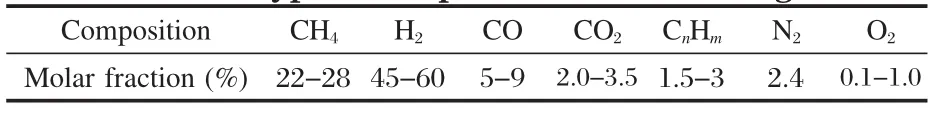
Table 1 Typical composition of coke oven gas
Among the reforming technology,many researches,both in the academia and industry,have paid attention to the syngas production from CH4by catalytic partial oxidation(CPO)because it has a low energy requirement.Oxygen permeable membrane made from mixed ionic and electronic conductors has potential applications in the separation of pure oxygen from air. Membranes also make it possible to integrate oxygen separation, steam reforming,and partial oxidation in a single reactor[14-19]and 30%of the potential capital cost could be saved with this technology[20].In addition,the hot-spot problem of the conventional co-fed reactor could also be avoided by the separate feeding of methane and oxygen by using mixed ionic and electronic conducting(MIEC)membrane reactors[20].
This paper presents the profound thermodynamic analysis of reforming technology of POM in COG.The effect of the original hydrogen,steam addition on POM in COG,energy consumption of steam addition,and the molar ratio of CH4/O2on conversion of CH4,the selectivity of H2or CO for the reforming of COG are carefully examined.The NiO/MgO solid solution catalyst packed on the mixed-conducting oxygen-permeable membrane reactor was used for the POM reaction in COG.The dense ceramic BaCo0.7Fe0.2Nb0.1O3-δmembrane used in the experiment was developed for partial oxidation of methane to syngas by Harada and co-workers in 2006[21].
1 Thermodynamic analysis methods
The thermodynamic equilibrium analysis is performed by means of Gibbs free energy minimization method[22].The possible species formed in the final product gas are assumed to be CH4, CO2,CO,H2O,H2,O2,N2,C(s),H,O,OH,HO2,HCO,CH,and CH2. C(s)refers to solid carbon and H,O,OH,HO2,HCO,CH,CH2are radicals that could be produced in the reforming reaction.In the equilibrium calculation,the concentrations of radicals are found to be negligible compared with those of other products.
The primary reactions:
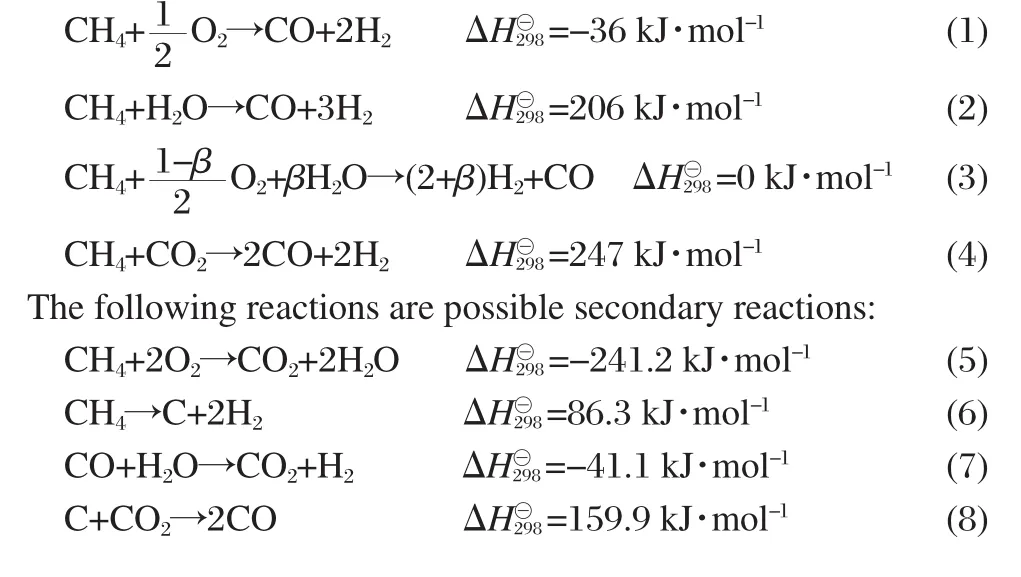

As a general goal,it is desirable to maximize the H2yield within allowable operating conditions.Prediction for the consumption of energy and the formation of solid carbon are other main tasks in this thermodynamic analysis,because the presence of the solid carbon can deactivate the catalyst and degrade the performance of the reforming system and the amount of whole energy consumption will lead to the variation of production costs.The operating regime,in which the solid carbon appears,could be avoided in practical reforming of CH4in COG.The compositions (volume fraction)of model COG for thermodynamic equilibrium calculation are 57.9%H2,31.6%CH4,7.4%CO,and 3.1%CO2.
The thermodynamic equilibrium in a reforming reactor of coke oven gas can be calculated by minimizing the Gibbs free energy,which has been adopted in this study.For given operating conditions,the equilibrium compositions and equilibrium temperature have been calculated.To analysis the reforming reactor effectively,these parameters:the selectivity of H2(SH2)or CO(SCO)and the conversion of CH4(XCH4)are used here.These parameters can be written as following. where n,n(),and n)denote the mole numbers of hydrogen,carbon monoxide,and methane in the product; n(),n(COin),and n()denote mole numbers of the hydrogen,carbon monoxide,and methane in the reactant;if SCOis larger than 100%,it denotes that the carbon dioxide in COG converted into carbon monoxide.
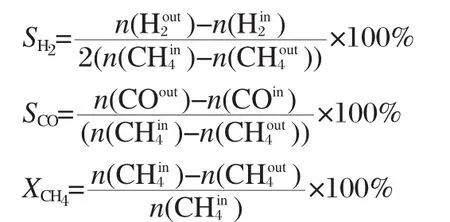
2 Experimental
2.1 Catalyst preparation
NiO/MgO solid solution catalysts were prepared by impregnating MgO(≥98.5%,purchased from Sinopharm Chemical Reagent Co.,Ltd.)with aqueous Ni(NO3)2·6H2O(≥98.0%,purchased from Shanghai Hengxin Chemical Reagent Co.,Ltd.)solution,followed by drying at 110℃overnight.The dried solid was calcined in air at 800℃for 5 h to obtain the nickel magnesium solid solution catalyst.The mass fraction of Ni loading on the catalysts was controlled to be 35%.These catalysts were pressed into tablets and crushed to 20-40 mesh particles
2.2 Reforming of COG in the oxygen-permeable membrane reactor
BaCo0.7Fe0.2Nb0.1O3-δ(BCFNO)powder was prepared by a solidstate reaction method[13].The as-prepared oxide was pressed into a disk pellet in a stainless steel mold under a hydraulic pressure of 15-20 MPa.The green disk was sintered at 1010℃for 3-6 h at static air,and then cooled to ambient temperature.The experimental apparatus and the membrane reactor configuration were presented in Fig.1.The membranes had a diameter of 16 mm and a thickness of 1.0 mm.Only membranes with relative densities higher than 90%were used to construct membrane reactors.The discoid membrane was sealed to the reactor with a silver seal.The effective inner surface area of the discoid membrane was around 1.3 cm2.one gram of 20-40 mesh NiO/MgO catalyst was directly placed on the membrane.One side of the membrane was exposed to compressed air,the other side to the model COG.The oxygen permeability of the discoid sample was determined from the content of CO and CO2in the reacted gas and the yield of H2O evaluated from the balance of hydrogen before and after the reaction.The flow rate of outlet gas was measured by a soap flow meter.Products were analyzed with a gas chromatograph(VARIAN,CP3800)equipped with a thermal conductivity detector.
3 Results and discussion
3.1 Thermodynamic analysis of reforming of COG
3.1.1 Effect of hydrogen on partial oxidation of CH4in COG
Compared with the reforming of pure CH4,the compositions of COG have 58%H2and 32%CH4.The original hydrogen in COG may have a significant influence on the partial oxidation of CH4.To examine the effect of the original hydrogen on the reforming of CH4in COG,the volume fraction of H2in model COG is varied from 0%to 57.9%.The initial volume fraction of the rest compositions are composed of the following 31.6%CH4, 7.4%CO,and 3.1%CO2.N2is added to keep the total amount constant(100%)and the amount of O2is 15.8%.
From Fig.2 it could be seen that the conversion of CH4and the selectivity of H2or CO increase steadily with increasing the reaction temperature,while decrease with increasing the amount of the original hydrogen at a given temperature.The conversion of CH4and the selectivity of H2or CO are strongly affected by the amount of the original H2in COG at low temperature(700-800℃),and the effect on the reforming of CH4becomes slight at high temperature(800-900℃).The conversion of CH4and the selectivity of H2and CO vary from 93.5%,89.1%,95.7%to 83.7%,74.7%,80.4%,respectively,when the original of hydrogen in COG changes from 0 to 50%at 750℃.However,the conversion of CH4,selectivity of H2and CO are vary from 99.4%,96%,105%to 97.5%,92.7%,104%,respectively,when the original of hydrogen in COG changes from 0 to 50%at 875℃.The reason of the selectivity of CO over 100%is the original of CO2of COG converted into CO in the processor of reforming. The equilibrium amount of solid carbon decreases with the increase of the original of hydrogen(see Fig.2(d)).And no solid carbon is generated in the temperature range of 825-900℃.
3.1.2 Effect of CH4/O2molar ratio on partial oxidation of CH4in COG
Fig.3 exhibits conversion of CH4,selectivity of H2and CO and formation of C(s)as a function of CH4/O2molar ratio at different temperature and a pressure of 1.01×105Pa.The initial amount of COG compositions are composed of 57.9%H2,31.6%CH4, 7.4%CO,and 3.1%CO2.
The formation of C(s)is strongly affected by the n(CH4)/n(O2) ratio.It is generated at ratio of n(CH4)/n(O2)more than 2.0 in the temperature range of 700-900℃at 1.01×105Pa,because the CH4decomposition and Boudouard reaction(2CO=C+CO2, ΔH⊖00298K=-159.9 kJ·mol-1)mainly contribute to formation of C(s)in the range of 700-900℃.With increasing reaction temperature, thecokingboundarymovestowardthehigherinitial n(CH4)/n(O2) ratio.In the reaction temperature of 700-800℃and a n(CH4)/ n(O2)ratio of 1.9-2.4,the formation of C(s)can be suppressed or avoided by increasing the reaction temperature or decreasing the ratio of n(CH4)/n(O2).The behavior of C(s)formation in partial oxidation of CH4in COG is similar to the reforming process for nature gas[23-24].From Fig.3,in order to avoid formation of C(s), the ratio of n(CH4)/n(O2)should be in the range of 0.4-2.1 and reaction temperature in the range of 750-900℃.
Fig.3(b,c)show that if sufficient air is added,the CH4will oxidize almost completely,yielding mainly CO2and H2O,thus resulting in the reduction of H2and CO yield,even in low temperature(less than 800℃).With decreasing the ratio of n(CH4)/ n(O2)from 4 to 0.4 at 800℃,the conversion of CH4increases from 87.1%to 100%,while with increasing temperature from 700 to 900℃with a n(CH4)/n(O2)ratio of 0.8,the conversion of CH4increases from 95.9%to 100%.Make sure to get a high conversion of CH4,selectivity of H2and CO,the best condition of both the n(CH4)/n(O2)ratio and reaction temperature should be 1.7-2.1 and 825-900℃,respectively.

Fig.2 Plotofthethermodynamicequilibriumof(a)theconversionofCH4,(b)theselectivityofH2,(c)theselectivityofCO,(d)the amountofCasafunctionoftheoriginalH2fortheprocessofthepartialoxidationofcokeovengasatapressureof1.01×105Pa
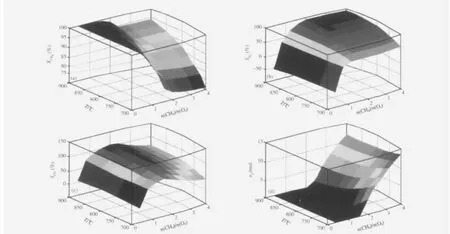
Fig.3 Plot of the thermodynamic equilibrium of(a)the conversion of CH4,(b)the selectivity of H2,(c)the selectivity of CO, (d)the amount of C as a function of the ratio of n(CH4)/n(O2)for the process of the POM in COG at a pressure of 1.01×105Pa
Thewholeprocessisanexothermicprocess(seeFig.5(a))when ratio of n(CH4)/n(O2)is lower than 2 at the temperature range of 700-900℃,but when the ratio of n(CH4)/n(O2)is higher than 2,the whole reaction is a endothermic process and the consumption enthalpy increases with increasing the ratio of n(CH4)/ n(O2).At the temperature range of 700-900℃with a n(CH4)/ n(O2)ratio of 0.8-2,the whole enthalpy per mol of COG is in the range of-2.46-31.5 J.
3.1.3 Effect of steam on partial oxidation of CH4in COG

Fig.4 Plot of the thermodynamic equilibrium(a)the conversion of CH4,(b)the selectivity of H2,(c)the selectivity of CO,(d)C molar amount as a function of steam addition for the process of the POM in COG with n(CH4)/n(O2)=2 at a pressure of 1.01×105Pa

Fig.5 Plot of the thermodynamic equilibrium energy(a)n(CH4)/n(O2)ratio and(b)steam addition for the process of the POM in COG at a pressure of 1.01×105Pa
Fig.4 show the results of reforming of coke oven gas as a function of the steam addition and temperature.The initial compositions were 57.9%H2,31.6%CH4,7.4%CO,3.1%CO2, and 15.8%O2.The percent of steam addition varies in the range of 0-16%in the processes.Fig.4(d)shows that the formation of C(s)is strongly affected by the value of the steam addition.The formation of C(s)decreases steadily with increasing steam addition at a given temperature.The amount of C(s)decreases from 3.81 to 0 mol when the steam addition is increased from 0 to 6%at 700℃.There may be the reason that the steam can easily react with solid carbon even in low temperature owing to the equilibrium formation(9)and the formation of C(s)decreases with the increase of temperature.All these results demonstrate that the formation of C(s)can be avoided by increasing the steam addition or reactor temperature.
Fig.4(a,b)illustrates the conversion of CH4and selectivity of H2increase with increasing temperature or steam addition.The conversion of CH4increases sharply with increasing steam addition especially in the low temperature range(less than 800℃), while the conversion of CH4and selectivity of H2increase slowly with increasing the steam addition in the high temperature range(higher than 825℃)almost up to 100%.The reason may be that steam reforming of methane is a high endothermic reaction.The higher temperature is benefited to the reforming reaction and the conversion of CH4almost reaches to 100% when the steam addition is enough even in high temperature. Thus,the conversion of CH4increases slightly with increasing the steam addition in high temperature.
The consumption of enthalpy increases with the increase of reaction temperature and amount of steam addition(Fig.5(b)). Such as the enthalpy increases from-1.77 to 3.03 J per mol of COG with the increase of steam addition from 0 to 16%at 700℃.At the same time,the enthalpy increases from-1.77 to 31.8 J per mol of COG with increasing the temperature from 700 to 900℃.
The optimal steam addition should be in the region between 2%and 4%at the reaction temperature ranging 800-875℃with n(CH4)/n(O2)ratio of 2.Under these conditions,it will get a high conversion of CH4,selectivity of H2and CO more than 91%, 84%,93%respectively,and no C(s)generation.The consumption of enthalpy per mol of COG is from 21.3 to 30.2 J.
From the above analysis,the conversion of CH4and the selectivity of H2and CO slightly decrease with increasing the amount of the original H2on POM in COG at a different temperature,and the formation of solid carbon decreases with the increase of the original of hydrogen.The favorable operating conditions for the POM in COG without steam addition are at an initial n(CH4)/n(O2)ratio of 1.7-2.1,a reaction temperature of 825-900℃.Under these operating conditions,the conversion of methane is 91.0%-99.9%,the selectivity of H2and CO are 87.0%-93.4%,100%-107%,respectively.Solid carbon is effectively suppressed and the enthalpy per mol of COG is in the range of 7.83-31.5 J per mol of COG.The corresponding of H2yields per mol of COG is 1.09-1.17 mol.The amplification of the original of hydrogen in COG is 1.9-2.02 times.If we add small amount of H2O(2%-4%)to the former conditions,the conversion of CH4,selectivity of H2or CO become better than before and solid carbon is effectively suppressed while the enthalpy per mol of COG is only from 21.3 to 30.2 J.The corresponding of H2yields per mol of COG consumed is 1.13-1.19 mol.The corresponding amplification of raw hydrogen in COG is 1.95-2.05 times.
3.2 Reforming of COG using NiO/MgO solid solution catalyst in MIEC membrane reactor
3.2.1 Properties of NiO/MgO composite oxides
From Table 2 it can be seen that the BET area of pure MgO was 196.1 m2·g-1.The surface area of the 30%(molar ratio)NiO/ MgO catalyst slightly decreased after reaction from 15.39 to 13.62 m2·g-1,The average grain size for the fresh,reduced and used catalyst calculated from XRD by Scherrer equation are 64.7,56.4,and 59.7 nm,respectively.The size of Ni metal particle slightly increased after experiment.The result indicated that NiO/MgO catalyst showed high stability in high temperature.
3.2.2 X-ray diffraction characterization of 30%NiO/MgO
As shown in Fig.6,the XRD patterns of MgO are very similarto those of NiO.For MgO,the peak positions corresponding to the(220),(311),and(222)plane are at 2θ=62.64°,75.00°,and 79.00°,respectively.For NiO,the corresponding peaks are at 2θ=62.94°,75.43°and 79.37°,respectively.The above three diffraction lines can be used to identify the formation of a solid solution[25].The phase analysis was carried out by XRD(Fig. 6)for both the mechanical mixtures,fresh,reduced and used 30%NiO/MgO catalyst.

Table 2 Characterization of system 30%(molar ratio) NiO/MgO
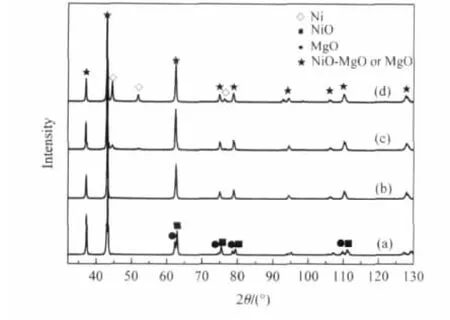
Fig.6 X-ray diffraction profiles of the 30%NiO/MgO catalyst(a)mechanical mixture,(b)fresh catalyst,(c) reduced catalyst,and(d)used catalyst
As shown in Fig.6,three double peaks near 2θ=62.64°,75.00°, and 79.00°,which correspond to the(220),(311),and(222)plane, are present for the mechanical mixtures.In contrast,the fresh 30%NiO/MgO catalyst has only three single peaks near the above 2θ values.This indicates that a solid solution was indeed formed.The 30%NiO/MgO catalyst reduced in a 10%H2only has a small Ni peak while mostly remains the fresh 30%NiO/ MgO solid solution catalyst peak.The spectrum of 30%NiO/ MgO catalyst after experiment was similar to that of 30%NiO/ MgO catalyst after reduction,only the intensity of nickel peak increased.No solid carbon and other peaks were found.This demonstrated that the 30%NiO/MgO catalyst has excellent carbon-deposition resistance for the POM in COG on the BCFNO membrane reactor.Yamazaki et al.[26]found the similar results on NiO-MgO solid solution catalyst for the partial oxidation of natural gas owing to the strong anti sintering of active metal andstable crystal phase structure during high temperature reaction.

Table 3 Comparison of the experimental values in membrane reactor and the thermodynamically predicted values at 875℃
3.2.3 Membrane reactor performance for POM
The CPO reaction of CH4in COG was studied in a discoid BCFNO membrane reactor packed with unreduced 30%NiO/ MgO catalysts.Fig.7 shows the COG flow rate dependence of the oxygen permeation flux(JO2)under an air/COG gradient in the BCFNO membrane reactor.It can be seen that the oxygen permeation flux increases rapidly with increasing the COG flow rate while the CH4conversion decreases slowly in contrast.For example,the oxygen permeation flux increased from 8.9 to 17.2 mL·cm-2·min-1,while the conversion of methane decreased from 100%to 94.1%with the flow rate of COG increasing from 38 to 108 mL·min-1at 875℃.At the same time,the selectivity of H2and CO increased from 56.6%,91.3%to 75.1%,108.2%,respectively.
The changes of the H2or CO selectivity with the methane flux could be interpreted by the POM reaction performance under different ratios of n(CH4)/n(O2).The low ratio of n(CH4)/n(O2) was responsible for the deep oxidation of methane and the low H2or CO selectivity and high methane conversion.
The experimental values of the CH4conversion,the H2or CO selectivity were according to the thermodynamically predicated one under the different n(CH4)/n(O2)molar ratio with a deviation of less than 8%(see Table 3).
Under the experimental condition,compared with the amount of original hydrogen in COG,the amplification of hydrogen after reforming amounted to 1.8-1.9 times,which was in the range of the previous thermodynamic calculated values.
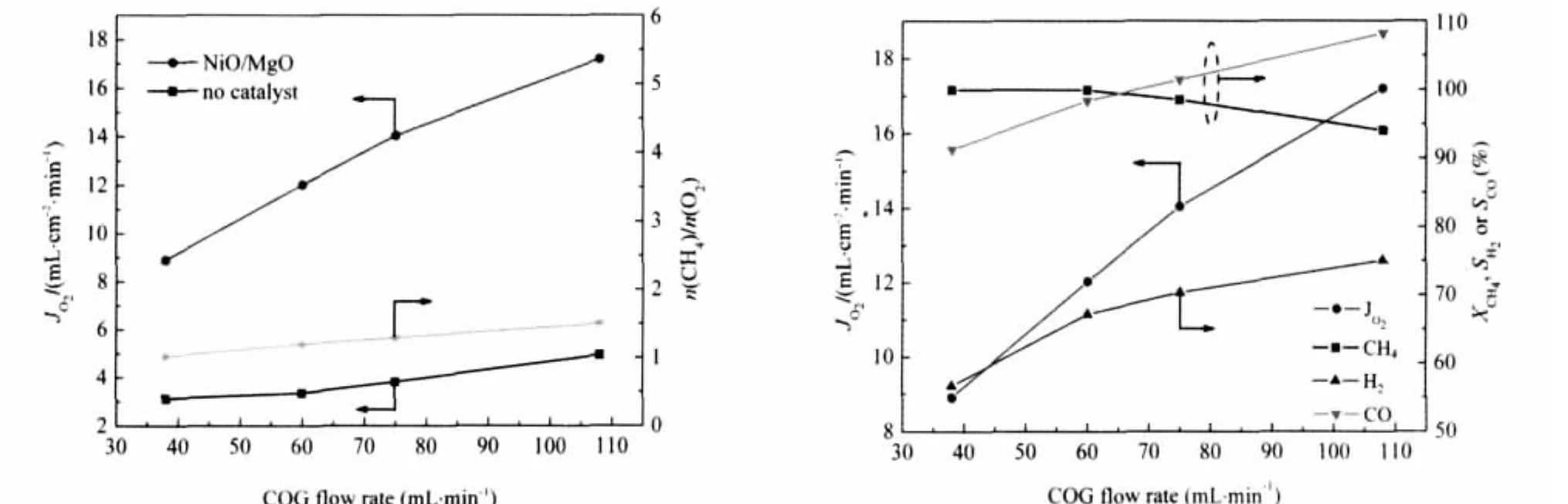
Fig.7 Oxygen permeation of BCFNO membrane at different fluxes of COG at 875℃under 172 mL·min-1air
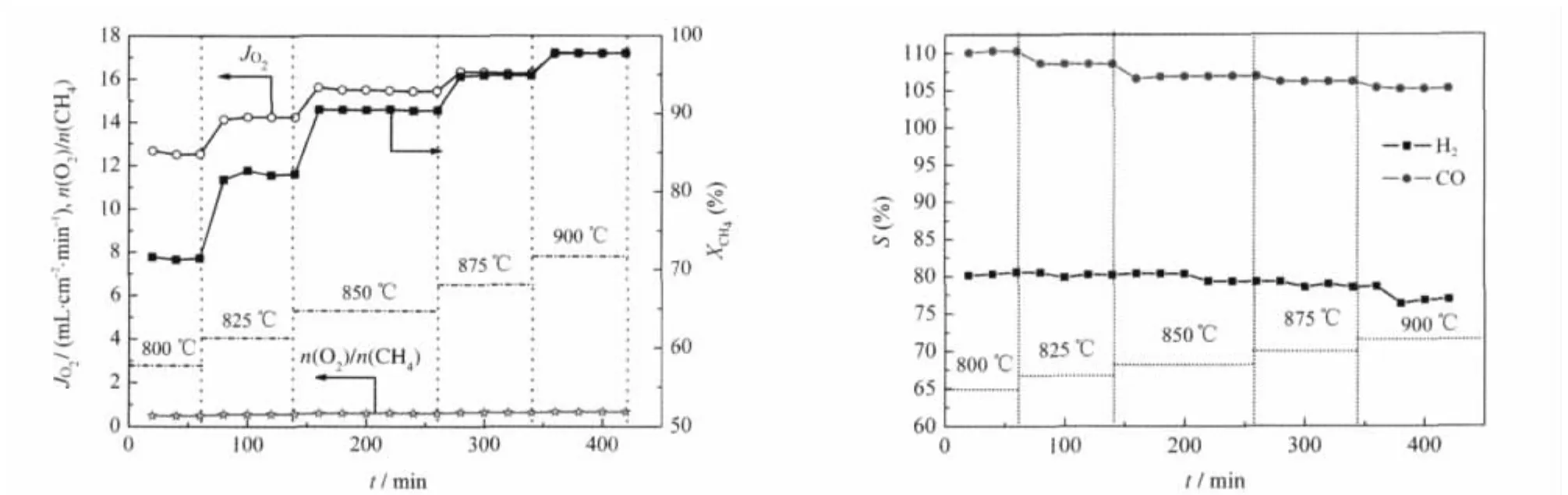
Fig.8 Effect of temperature on the oxygen permeation of the membrane reaction under COG of 108 mL min-1and air of 172 mL·min-1

Fig.9 Variation of components in COG before and after reforming at different temperatures
The effect of temperature on oxygen permeation flux,the CH4conversion,H2and CO selectivity with 108 mL·min-1COG and 172 mL·min-1air are shown in Fig.8.The CH4conversion and oxygen permeation flux increase rapidly while the H2or CO selectivity decrease slowly with the increase of temperature from 800 to 900℃.With the increasing of oxygen permeation flux,the oxygen was in excess and deep oxidation of H2and CO took place as a result of the H2or CO selectivity decreased slowly.For example the oxygen permeation flux increases from 12.5 to 17.2 mL·cm-2·min-1and the CH4conversion increases from 71.4%to 97.8%while the H2and CO selectivity decrease from 80.5%,110.3%to 76.9%,105.2%,respectively,when the temperature increases from 800 to 900℃.
The variation in the components of COG before and after reforming in membrane reactor at different temperatures are shown in Fig.9,from which it can be seen that at different reaction temperatures,the average yields of H2and CO are 101.2-119.2 mL·min-1and 34.6-43.8 mL·min-1,respectively.With temperature decreasing,the amounts of H2,CO or CO2after reforming decrease gradually and the amount of CH4behaves a contrary trend.The amplification of the original of hydrogen in COG is 1.6-1.9 times.
4 Conclusions
The favorable operating conditions for the POM in COG without steam addition are an initial n(CH4)/n(O2)ratio of 1.7-2.1 and a reaction temperature of 825-900℃at 1.01×105Pa. The conversion of CH4,the selectivity of H2and CO are varied from 91.0%-99.9%,87.0%-93.4%and 100%-107%,respectively.Solid carbon is effectively suppressed.The consumption enthalpy per mol of COG is in the range of 7.83 to 31.5 J per mol of COG.The corresponding amplification of raw hydrogen per mol of COG is 1.9-2.02 times.
After adding a few amount of steam in the initial of the COG, the range of formation of solid carbon becomes smaller than before.The favorable operating conditions are the H2O ratio of 2%-4%,reaction temperature 825-900℃under 1.01×105Pa and the CH4/O2molar ratio of 2;the conversion of CH4in COG is 92.6%-98.6%,the selectivity of H2and CO are 87.2%-96.4%,104%-107%,respectively.The corresponding H2yield per mol of COG consumed is 1.13-1.19 mol.However,the corresponding amplification of raw hydrogen in COG is 1.95-2.05 times.These results also show that the solid carbon is effectively suppressed under the former conditions.The enthalpy per mol of COG is only from 21.3 to 30.2 J.
The 30% NiO/MgO catalyst reforming reaction in the BCFNO membrane reactor not only confirms the equilibrium analysis results but also shows very good activity,which possesses a widespread available prospect of POM in COG.16.3 mL·cm-2·min-1of oxygen permeation flux,94.9%CH4conversion,80.5%H2selectivity and 106.2%CO selectivity are achieved at a temperature of 875℃,108 mL·min-1COG and 172 mL·min-1air.The average yields of H2and CO are 117.2 mL·min-1and 42.1 mL·min-1respectively,which the production of hydrogen is 1.8 times of the initial gas.
1 Xu,K.D.Iron and Steel,2008,43:1 [徐匡迪.钢铁,2008,43: 1]
2 Yin,R.Y.;Zhang,C.X.Iron and Steel,2005,40:1 [殷瑞钰,张春霞.钢铁,2005,40:1]
3 Yin,R.Y.Shanghai Metals,2006,28:1 [殷瑞钰.上海金属, 2006,28:1]
4 Yin,R.Y.Iron and Steel,2002,37:1 [殷瑞钰.钢铁,2002,37: 1]
5 Maruoka,N.;Akiyama,T.Energy,2006,31:1632
6 Andrew,E.L.;Robert,W.B.;Leslie,B.;Alex,R.Int.J.Hydrogen Energy,2004,29:809
7 Amin,N.A.S.;Yaw,T.C.Int.J.Hydrogen Energy,2007,32: 1789
8 Andrew,E.L.;Robert,W.;Bradshaw,J.O.;Keller,D.W.Int.J. Hydrogen Energy,2003,28:159
9 Zhang,J.Y.;Yin,Z.Q.Gas&Heat,2004,24:183 [张家元,殷志群.煤气与热力,2004,24:183]
10 Zhang,J.Y.;Yin,Z.Q.Energy for Metallurgical Industry,2004, 23:37 [张家元,殷志群.冶金能源,2004,23:37]
11 Tang,W.W.;Zhou,J.M.Metal Materials and Metallurgy Engineering,2008,36:22 [唐文武,周孑民.金属材料与冶金工程,2008,36:22]
12 Li,Y.B.;Xiao,R.;Jin,B.S.Chem.Eng.Technol.,2007,30:91
13 Zhang,Y.W.;Li,Q.;Shen,P.J.;Liu,Y.;Yang,Z.B.;Ding,W.Z.; Lu,X.G.Int.J.Hydrogen Energy,2008,33:3311
14 Lu,Y.;Dixon,A.G.;Moser,W.R.;Ma,Y.H.;Balachandran,U. Catal.Today,2000,56:297
15 Balachandran,U.;Dusek,J.T.;Mieville,R.L.;Poeppel,R.B.; Kleefish,M.S.;Pei,S.;Kobykinsky,T.P.;Udovich,C.A.;Bose, A.C.Appl.Catal.A,1995,133:19
16 Ten Elshof,J.E.;Bouwmeester,H.J.M.;Verweij,H.Appl.Catal. A,1995,130:195
17 Dyer,P.N.;Richards,R.E.;Russek,S.L.;Taylor,D.M.Solid State Ionics,2000,134:21
18 Shao,Z.P.;Dong,H.;Xiong,G.X.;Cong,Y.;Yang,W.S. J.Membr.Sci.,2001,183:181
19 Dong,H.;Shao,Z.P.;Xiong,G.X.;Tong,J.H.;Sheng,S.S.; Yang,W.S.Catal.Today,2001,67:3
20 Chen,C.M.;Dyer,P.N.;Gerdes,K.F.;Lowe,C.M.;Akhave,S. R.;Rowley,D.R.;Sen,K.I.;Eriksen,E.H.Stud.Surf.Sci.Catal., 2001,136:45
21 Harada,M.;Domen,K.;Hara,M.;Tatsumi,T.Chem.Lett.,2006, 35:1326
22 Roine,A.Outokumpu HSC chemistry.Version 5.1.Pori: Outokumpu Research Oy(ORC),2002
23 Lutz,A.E.;Bradshaw,R.W.;Keller,J.O.;Witmer,D.E.Int.J. Hydrogen Energy,2003,28:159
24 Seo,Y.S.;Shirley,A.;Kolaczkowski,S.T.J.Power Sources, 2002,108:213
25 Ruckenstein,E.;Hu,Y.H.Appl.Catal.A,1999,183:85
26 Yamazaki,O.;Tomishige,K.;Fujimoto,K.Appl.Catal.A,1996, 136:49
May 5,2009;Revised:November 24,2009;Published on Web:December 23,2009.
Hydrogen Production from Coke Oven Gas by Methane Reforming: Thermodynamic Analysis and Experimental Study
YANG Zhi-Bin ZHANG Yu-Wen ZHANG Yun-Yan DING Wei-Zhong*SHEN Pei-Jun LIU Yong ZHOU Yu-Ding HUANG Shao-Qing
(Shanghai Key Laboratory of Modern Metallurgy and Materials Processing,Shanghai University,Shanghai 200072,P.R.China)
A thermodynamic analysis of the partial oxidation of methane(POM)in coke oven gas(COG)was carried out.The optimized conditions were CH4/O2molar ratios of 1.7-2.1 and reaction temperatures of 825-900℃. We obtained CH4conversions of 91.0%-99.9%,H2selectivity of 87.0%-93.4%,and CO selectivity of 100%-107%at 1.01×105Pa.The effect of H2in the COG on the performance of POM was also investigated between 825 and 900℃. The optimized volume ratio of steam addition was 2%-4%and the molar ratio of CH4/O2was 2 at 1.01×105Pa and 825-900℃.A maximum conversion rate of 98.6%was achieved for CH4using COG,while the maximum selectivities of H2and CO were 96.4%and 107%,respectively.The amount of hydrogen obtained after reforming was doubled despite a thermal consumption of only 2.94 J·mol-1for the COG.The performance of a NiO/MgO solid solution catalyst packed on a BaCo0.7Fe0.2Nb0.1O3-δ(BCFNO)membrane reactor was also investigated for the POM in COG.The reforming process was successfully performed.At 875℃,95%CH4conversion,80.5%H2selectivity,and 106%CO selectivity at an oxygen permeation flux of 16.3 mL·cm-2·min-1were achieved.The results for POM reforming in COG on the membrane reactor were consistent with the thermodynamic analysis.The NiO/MgO solid solution catalyst, therefore,has good activity and is suitable for application in hydrogen production.
Coke oven gas;Hydrogen;Thermodynamic analysis;Mixed-conducting membrane reactor; NiO/MgO solid solution catalyst
O641;O643
*Corresponding author.Email:wzhding@shu.edu.cn;Tel/Fax:+86-21-56331618.
The project was supported by the National High Technology Research and Development Program of China(863)(2006AA11A189),Science and Technology Commission of Shanghai Municipality,China(06DZ12212),National Engineering Research Center of Advanced Steel Technology (NERCAST),China(050209),and Innovation Fund for Graduate Student of Shanghai University,China.
国家高技术研究发展计划(863)(2006AA11A189),上海市科委重点科研项目(06DZ12212),先进钢铁材料技术国家工程研究中心(050209)及上海大学研究生创新基金资助
