齐墩果酸衍生物的合成*
2010-11-27李群林李灵芝
李群林, 顾 军, 李灵芝,3
(1. 天津医科大学 药学院,天津 300070; 2. 中国人民武装警察部队医学院, 天津 300162; 3. 天津市职业与环境危害生物标志物重点实验室,天津 300162)
来自天然产物的齐墩果酸(1)其抗HIV活性EC50值为1.7μg·mL-1, TI值为12.8[1],具有进一步改造提升活性的价值。
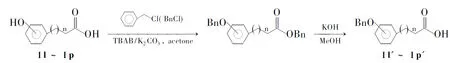
1的3-OH和28-CO2H是抗HIV活性最大的两个潜在部位[2],本文以其为化学修饰对象,通过1的酯化、醚化和酰胺化反应合成了26个齐墩果酸衍生物。1的3-OH与芳香酸(Ⅰl~Ⅰp)在DCC/DMAP缩合成酯(2a~2k, Scheme 1);带有酚羟基的芳香酸可用氯化苄保护后再与1的3-OH成酯(2l~2p, Scheme 1)。1的28-CO2H先与1,2-二氯乙烷在无水碳酸钾作用下成酯得到中间体Ⅱ; Ⅱ再与六水合哌嗪同样在无水碳酸钾作用下形成氮醚4; 4的另一个哌嗪氮与芳香酸(Ⅰ)在EDC·HCl(1-3-二甲氨基丙基-3-乙基碳二亚胺盐酸盐)和HOBT(1-羟基苯并三唑)作用下形成酰胺(3a,3c~3f,3h,3l,3m,3q, Scheme 1)。其结构经1H NMR和MS确认,其中2b~2j,2m~2p, 3和4均未见文献报道。
1 实验部分
1.1 仪器与试剂
XCL-1显微熔点仪(温度未经校正);Varian Inova 500型核磁共振仪(CDCl3为溶剂,TMS为内标);Finnigan FINS 2000型质谱仪。

Scheme 1
1,纯度98%,西安小草植物科技有限责任公司;硅胶,200目~300目,青岛海洋化工厂;其余所用试剂均为分析纯,溶剂经常规干燥处理。
1.2 合成
(1)2a~2k的合成(以2a为例)
在反应瓶中依次加入1 208 mg(0.46 mmol)的CH2Cl2(15 mL)溶液,苯甲酸(Ⅰa) 120 mg(0.99 mmol)和DMAP(对二甲氨基吡啶)54 mg(0.44 mmol),搅拌使其完全溶解,加入DCC(二环己基碳二亚胺)210 mg(1.02 mmol),于室温反应(TLC跟踪)。过滤,滤液经制备薄层分离得白色粉末3-O-苯甲酰基齐墩果酸(2a)。
用类似方法合成白色粉末2b~2k,实验结果见表1。
(2)2l~2p的合成(以2l为例)
在反应瓶中加入对羟基苯甲酸(Ⅰl) 0.5 g(3.6 mmol)的丙酮(15 mL)溶液,无水K2CO31.5 g(10.8 mmol)和TBAB(四丁基溴化铵)115 mg(0.36 mmol),加热至回流,搅拌下滴加氯化苄(BnCl) 0.85 mL(7.4 mmol),滴毕,回流反应(TLC跟踪)。减压蒸除大部分丙酮,残液倒入水(100 mL)中,析出黄白色黏稠物,过滤,滤饼用无水乙醇洗至白色。
将白色滤饼加入0.1 g·mL-1氢氧化钾甲醇溶液(10 mL)中,回流反应2 h。倒入水(200 mL)中,用10%盐酸调至pH 3(溶液中出现大量白色固体),过滤,滤饼真空干燥得对苄氧基苯甲酸(Ⅰl′) 0.726 g,产率88%。
在反应瓶中依次加入1 250 mg(0.548 mmol)的CH2Cl2(10 mL)溶液,Ⅰl′ 250 mg(1.096 mmol)和DMAP 67 mg(0.548 mmol),搅拌至完全溶解,加入DCC 230 mg(1.117 mmol),于室温反应(TLC跟踪)。过滤,滤液经硅胶柱层析[洗脱剂:A=V(石油醚) ∶V(乙酸乙酯)=10 ∶1]分离得白色粉末2l′ 185 mg,产率50%。
用类似方法合成2m′~2p′ 。
在反应瓶加入2l′ 185 mg,混合溶剂CHCl3-CH3OH(等体积比,10 mL),搅拌使其溶解后加入Pd/C 18.5 mg,置中压氢化釜中反应8 h。过滤,滤液浓缩后经制备薄层分离得白色粉末3-O-对羟基苯甲酰基齐墩果酸(2l)。
用类似方法合成2m~2p,实验结果见表1。
(3)3的合成(以3a为例)
在干燥三颈瓶中加入1 10 g(21.9 mmol),二氯乙烷10 mL,丙酮50 mL和无水K2CO34.5 g,搅拌下回流反应(TLC跟踪)。减压蒸除溶剂,浓缩物经硅胶柱层析(A=6 ∶1)分离得白色粉末Ⅱ。在反应瓶子中依次加入Ⅱ,六水合哌嗪20.9 g(107.9 mmol, 5eq)和DMF 50 mL,搅拌使其溶解,加入无水K2CO34.8 g,于80 ℃反应(TLC跟踪)。倾入蒸馏水(200 mL)中,用乙酸乙酯(3×150 mL)萃取,合并有机层,减压浓缩,浓缩物经柱层析[洗脱剂:V(二氯甲烷) ∶V(甲醇)=20 ∶1]分离得白色粉末4。
将4 0.35 mmol溶于适量二氯甲烷中,依次加入Ⅰa65 mg(0.53 mmol), EDC·HCl 102 mg(0.53 mmol)和HOBT 72 mg(0.53 mmol),搅拌下于室温反应(TLC跟踪)。用蒸馏水(2×10 mL)洗涤后经制备薄层分离得白色粉末3a。

表 1 2~4的实验结果Table 1 The experimental results of 2~4
用类似方法合成白色粉末3b,3c,3d(棕色粉末),3e,3f,3h,3l,3m(黄色粉末)和3q,实验结果见表1。
2a:1H NMRδ: 7.41~8.08(m, 5H, PhH), 5.31(s, 1H, 12-H), 4.70(dd,J=10.5 Hz, 5.5 Hz, 1H, 3-H), 2.95(d,J=10.0 Hz, 1H, 18-H), 2.84(d,J=10.0 Hz, 1H, 3-H), 2.18(m, 1H, 2-H),1.02(s, 3H, CH3), 0.99(s, 3H, CH3), 0.97(s, 3H, CH3), 0.95(s, 3H, CH3), 0.92(s, 3H, CH3), 0.88(s, 3H, CH3), 0.79(s, 3H, CH3)。
2b:1H NMRδ: 7.97(d,J=8.5 Hz, 2H, ArH), 7.47(d,J=8.5 Hz, 2H, ArH), 5.39(t,J=3.5 Hz, 1H, 12-H), 3.27(dd,J=11.0 Hz, 5.5 Hz, 1H, 3-H), 2.91(dd,J=13.5 Hz, 4.0 Hz, 1H,18-H), 1.18(s, 3H, CH3), 0.99(s, 3H, CH3), 0.95(s, 3H, CH3), 0.94(s, 3H, CH3), 0.91(s, 3H, CH3), 0.84(s, 3H, CH3), 0.78(s, 3H, CH3)。
2c:1H NMRδ: 8.23(d,J=4.0 Hz, 2H, ArH), 8.20(d, J=4.0 Hz, 2H, ArH), 5.34(t,J=3.3 Hz, 1H, 12-H), 4.79(dd,J=8.5 Hz, 6.0 Hz, 1H, 3-H), 2.85(dd,J=13.5 Hz, 4.0 Hz, 1H, 18-H), 1.18(s, 3H, CH3), 1.04(s, 3H, CH3), 1.00(s, 3H, CH3), 0.96(s, 3H, CH3), 0.95(s, 3H, CH3), 0.93(s, 3H, CH3), 0.84(s, 3H, CH3)。
2d:1H NMRδ: 9.23(t,J=2.0 Hz, 1H, ArH), 9.14(d,J=2.0 Hz, 2H, ArH), 5.31(t,J=3.7 Hz, 1H, 12-H), 4.88(dd,J=11.0 Hz, 5.5 Hz, 1H, 3-H), 2.84(dd,J=14.0 Hz, 3.5 Hz, 1H, 18-H), 1.17(s, 3H, CH3), 1.07(s, 3H, CH3), 1.03(s, 3H, CH3), 0.97(s, 3H, CH3), 0.95(s, 3H, CH3), 0.93(s, 3H, CH3), 0.80(s, 3H, CH3)。
2e:1H NMRδ: 7.83(d,J=8.5 Hz, 2H, ArH), 6.64(d,J=8.5 Hz, 2H, ArH), 5.38(t,J=3.5 Hz, 1H, 12-H), 4.26(s, 2H, NH2), 3.21(dd,J=11.0 Hz, 4.0 Hz, 1H, 3-H), 2.92(dd,J=14.0 Hz, 4.2 Hz, 1H, 18-H), 1.18(s, 3H, CH3), 0.99(s, 3H, CH3), 0.95(s, 3H, CH3), 0.93(s, 3H, CH3), 0.91(s, 3H, CH3), 0.85(s, 3H, CH3), 0.78(s, 3H, CH3)。
2f:1H NMRδ: 8.00(d,J=9.0 Hz, 2H, ArH), 6.92(d,J=9.0 Hz, 2H, ArH), 5.33(s, 1H, 12-H), 4.71(dd,J=10.5 Hz, 5.5 Hz, 1H, 3-H), 3.86(s, 3H, CH3), 2.84(dd,J=13.5 Hz, 3.3 Hz, 18-H), 1.17(s, 3H, CH3), 1.01(s, 3H, CH3), 0.98(s, 3H, CH3), 0.95(s, 3H, CH3), 0.94(s, 3H, CH3), 0.93(s, 3H, CH3), 0.83(s, 3H, CH3)。
2g:1H NMRδ: 7.81(d,J=8.5 Hz, 2H, PhH), 7.53(m, 1H, PhH), 7.45(t,J=8.5 Hz, 2H, PhH), 6.68(t,J=4.8 Hz, 1H, NH), 5.32(t,J=3.5 Hz, 1H, 12-H), 4.62(dd,J=11.0 Hz, 5.5 Hz, 1H, 3-H), 4.25(d,J=5.0 Hz, 2H, CH2), 2.82(dd,J=13.5 Hz, 3.5 Hz, 1H, 18-H), 1.15(s, 3H, CH3), 0.94(s, 3H, CH3), 0.92(s, 3H, CH3), 0.90(s, 3H, CH3), 0.88(s, 3H, CH3), 0.81(s, 3H, CH3), 0.79(s, 3H, CH3)。
2h:1H NMRδ: 9.23(d,J=1.5 Hz, 1H, ArH), 8.78(dd,J=5.0 Hz, 1.5 Hz, 1H, ArH), 8.31(m,J=7.5 Hz, 5.0 Hz, 1.5 Hz, 1H, PyH), 7.41(dd,J=7.5 Hz, 5.0 Hz, 1H, ArH), 5.30(t,J=3.5 Hz, 1H, 12-H), 4.78(dd,J=10.5 Hz, 6.0 Hz, 1H, 3-H), 2.84(dd,J=14.0 Hz, 4.3 Hz, 1H, 18-H), 1.16(s, 3H, CH3), 1.02(s, 3H, CH3), 1.00(s, 3H, CH3), 0.95(s, 3H, CH3), 0.94(s, 3H, CH3), 0.92(s, 3H, CH3), 0.79(s, 3H, CH3)。
2i:1H NMRδ: 7.58(d,J=1.0 Hz, 1H, ArH), 7.14(d,J=3.5 Hz, 1H, ArH), 6.45(dd,J=3.5 Hz, 1.5 Hz, 1H, ArH), 5.32(t,J=3.3 Hz, 1H, 12-H), 4.73(dd,J=9.0 Hz, 7.5 Hz, 1H, 3-H), 2.84(dd,J=13.5 Hz, 4.0 Hz, 1H, 18-H), 1.16(s, 3H, CH3), 0.97(s, 3H, CH3), 0.97(s, 3H, CH3), 0.94(s, 3H, CH3), 0.93(s, 3H, CH3), 0.92(s, 3H, CH3), 0.82(s, 3H, CH3)。
2j:1H NMRδ: 8.13(s, 1H, NH), 7.62(d,J=7.5 Hz, 1H, ArH), 7.35(d,J=7.5 Hz, 1H, ArH), 7.19(dd,J=4.0 Hz, 2.5 Hz, 2H, ArH), 7.14(d,J=7.0 Hz, ArH), 5.26(t,J=3.5 Hz, 1H, 12-H), 4.51(m, 1H, 3-H), 3.77(s, 2H, CH2), 3.09(dd,J=14.0 Hz, 4.5 Hz, 1H, 18-H), 1.12(s, 3H, CH3), 0.93(s, 3H, CH3), 0.91(s, 3H, CH3), 0.87(s, 3H, CH3), 0.79(s, 3H, CH3), 0.77(s, 3H, CH3), 0.75(s, 3H, CH3)。
2k:1H NMRδ: 7.54(m, 2H, PhH), 7.38(m, 3H, PhH), 7.67(d,J=16.0 Hz, 1H, =CH), 6.45(d,J=16.0 Hz, 1H, =CH), 5.33(t,J=3.5 Hz, 1H, 12-H), 4.65(t,J=8.0 Hz, 1H, 3-H), 2.84(dd,J=13.5 Hz, 3.75 Hz, 1H, 18-H), 1.26(s, 3H, CH3), 1.16(s, 3H, CH3), 0.97(s, 3H, CH3), 0.95(s, 3H, CH3), 0.95(s, 3H, CH3), 0.92(s, 3H, CH3), 0.82(s, 3H, CH3)。
2l:1H NMRδ: 7.92(d,J=8.5 Hz, 2H, ArH), 6.89(d,J=9.0 Hz, 2H, ArH), 5.38(t,J=3.5 Hz, 1H, 12-H), 3.26(dd,J=11.0 Hz, 4.5 Hz, 1H, 3-H), 2.92(dd,J=13.5 Hz, 4.0 Hz, 1H, 18-H), 1.17(s, 3H, CH3), 0.99(s, 3H, CH3), 0.95(s, 3H, CH3), 0.93(s, 3H, CH3), 0.90(s, 3H, CH3), 0.83(s, 3H, CH3), 0.79(s, 3H, CH3)。
2m:1H NMRδ: 7.59(s, 1H, ArH), 7.53(d,J=8.0 Hz, 1H, ArH), 6.92(d,J=8.0 Hz, 1H, ArH), 5.39(s, 1H, 12-H), 3.25(dd,J=11.0 Hz, 4.3 Hz, 1H, 3-H), 2.92(dd,J=14.0 Hz, 3.75 Hz, 1H, 18-H), 1.18(s, 3H, CH3), 0.99(s, 3H, CH3), 0.95(s, 3H, CH3), 0.93(s, 3H, CH3), 0.90(s, 3H, CH3), 0.82(s, 3H, CH3), 0.78(s, 3H, CH3)。
2n:1H NMRδ: 7.15(d,J=8.5 Hz, 2H, ArH), 6.78(d,J=8.5 Hz, 2H, ArH), 5.27(t,J=3.8 Hz, 1H, 12-H), 4.48(dd,J=9.0 Hz, 7.0 Hz, 1H, 3-H), 3.54(s, 2H, CH2), 2.81(dd,J=9.0 Hz, 4.3 Hz, 1H, 18-H), 1.12(s, 3H, CH3), 0.92(s, 3H, CH3), 0.91(s, 3H, CH3), 0.90(s, 3H, CH3), 0.78(s, 3H, CH3), 0.75(s, 3H, CH3), 0.74(s, 3H, CH3)。
2o:1H NMRδ: 6.78(d, 2H, ArH),6.69(d, 1H, ArH), 5.27(t,J=3.5 Hz, 1H, 12-H), 4.49(t,J=8.0 Hz, 1H, 3-H), 3.49(s, 2H, CH2), 2.82(dd,J=14.0 Hz, 4.0 Hz, 1H, 18-H), 1.12(s, 3H, CH3), 0.92(s, 3H, CH3), 0.91(s, 3H, CH3), 0.90(s, 3H, CH3), 0.80(s, 3H, CH3), 0.76(s, 3H, CH3), 0.73(s, 3H, CH3)。
2p:1H NMRδ: 6.77(d, 1H, ArH), 6.73(d, 1H, ArH), 6.64(dd,J=8.0 Hz, 2.0 Hz, 1H, ArH), 5.31(t,J=3.5 Hz, 1H, 12-H), 4.49(t,J=8.0 Hz, 1H, 3-H), 2.85(t,J=7.5 Hz, 2H, CH2), 2.59(t,J=7.5 Hz, 2H, CH2), 1.26(s, 3H, CH3), 1.14(s, 3H, CH3), 0.94(s, 3H, CH3), 0.92(s, 3H, CH3), 0.83(s, 3H, CH3), 0.79(s, 3H, CH3), 0.79(s, 3H, CH3)。
3a:1H NMRδ: 7.40(m, 5H, PhH), 5.25(t,J=3.0 Hz, 1H, 12-H), 4.16(m, 2H, CH2), 3.78(s, 2H, H in piperazine), 3.41(s, 2H, H in piperazine), 3.20(dd,J=11.0 Hz, 4.0 Hz, 1H, 3-H), 2.84(dd,J=13.5 Hz, 4.0 Hz, 1H, 18-H), 2.65(t,J=5.5 Hz, 2H, CH2), 2.60(s, 2H, H in piperazine),2.45(s, 2H, H in piperazine), 1.12(s, 6H, CH3), 0.98(s, 6H, CH3), 0.91(s, 3H, CH3), 0.89(s, 3H, CH3), 0.88(s, 3H, CH3), 0.77(s, 3H, CH3), 0.71(s, 3H, CH3)。
3c:1H NMRδ: 8.28(d,J=9.0 Hz, 2H, ArH), 7.57(d,J=9.0 Hz, 2H, ArH), 5.25(t,J=3.5 Hz, 1H, 12-H), 4.16(m, 2H, CH2), 3.79(s, 2H, H in piperazine), 3.35(s, 2H, H in piperazine), 3.21(dd,J=11.0 Hz, 5.0 Hz, 1H, 3-H), 2.84(dd,J=14.0 Hz, 4.3 Hz, 1H, 18-H), 2.66(t,J=5.5 Hz, 2H, CH2), 2.62(s, 2H, H in piperazine), 2.46(s, 2H, H in piperazine), 1.13(s, 3H, CH3), 0.98(s, 3H, CH3), 0.91(s, 3H, CH3), 0.90(s, 3H, CH3), 0.89(s, 3H, CH3), 0.77(s, 3H, CH3), 0.71(s, 3H, CH3)。
3d:1H NMRδ: 9.09(d,J=2.0 Hz, 1H, ArH), 8.59(d,J=2.0 Hz, 2H, ArH), 5.25(t,J=3.0 Hz, 1H, 12-H), 4.16(m, 2H, CH2), 3.82(s, 2H, H in piperazine), 3.41(s, 2H, H in piperazine), 3.19(dd,J=11.0 Hz, 4.0 Hz, 1H, 3-H), 2.84(dd,J=13.5 Hz, 4.0 Hz, 1H, 18-H), 2.68(t,J=5.5 Hz, 2H, CH2), 2.68(s, 2H, H in piperazine), 2.52(s, 2H, H in piperazine), 1.13(s, 3H, CH3), 0.98(s, 3H, CH3), 0.91(s, 3H, CH3), 0.89(s, 3H, CH3), 0.89(s, 3H, CH3), 0.77(s, 3H, CH3), 0.72(s, 3H, CH3)。
3e:1H NMRδ: 7.25(d,J=8.0 Hz, 2H, ArH), 6.65(d,J=8.0 Hz, 2H, ArH), 5.26(t,J=3.3 Hz, 1H, 12-H), 4.16(m, 2H, CH2), 3.88(s, 2H, H in piperazine), 3.61(s, 2H, H in piperazine), 3.21(dd,J=11.0 Hz, 4.0 Hz, 1H, 3-H), 2.84(dd,J=13.5 Hz, 3.8 Hz,1H, 18-H), 2.65(t,J=5.5 Hz, 2H, CH2), 2.52(s, 4H, H in piperazine), 1.13(s, 3H, CH3), 0.98(s, 3H, CH3), 0.91(s, 3H, CH3), 0.90(s, 3H, CH3), 0.89(s, 3H, CH3), 0.78(s, 3H, CH3), 0.72(s, 3H, CH3)。
3f:1H NMRδ: 7.38(d,J=8.5 Hz, 2H, ArH), 6.91(d,J=8.5 Hz, 2H, ArH), 5.26(t,J=3.0 Hz, 1H, 12-H), 4.16(m, 2H, CH2), 3.83(s, 3H, OCH3), 3.68(s, 2H, H in piperazine), 3.51(s, 2H, H in piperazine), 3.20(dd,J=11.0 Hz, 4.0 Hz, 1H, 3-H), 2.84(dd,J=13.5 Hz, 4.0 Hz, 1H, 18-H), 2.65(t,J=5.5 Hz, 2H, CH2), 2.52(s, 4H, H in piperazine), 1.13(s, 3H, CH3), 0.98(s, 3H, CH3), 0.91(s, 3H, CH3),0.90(s, 3H, CH3), 0.89(s, 3H, CH3), 0.77(s, 3H, CH3), 0.72(s, 3H, CH3)。
3h:1H NMRδ: 8.67(d,J=1.5 Hz, 1H, ArH), 8.66(s, 1H, ArH), 7.75(dd,J=6.0 Hz, 2.0 Hz, ArH), 7.37(dd,J=8.0 Hz, 5.0 Hz, ArH), 5.26(s,J=3.0 Hz, 1H, 12-H), 4.16(m, 2H, CH2), 3.79(s, 2H, ArH), 3.43(s, 2H, H in piperazine), 3.21(dd,J=11.0 Hz, 4.0 Hz, 1H, 3-H), 2.84(dd,J=14.0 Hz, 4.0 Hz, 1H, 18-H), 2.66(t,J=5.5 Hz, 2H, CH2), 2.63(d,J=5.0 Hz, 2H, H in piperazine), 2.48(t,J=5.0 Hz, 2H, H in piperazine), 1.13(s, 3H, CH3), 0.98(s, 3H, CH3), 0.91(s, 3H, CH3), 0.90(s, 3H, CH3), 0.89(s, 3H, CH3), 0.78(s, 3H, CH3), 0.72(s, 3H, CH3)。
3l:1H NMRδ: 7.22(d,J=8.0 Hz, 2H, ArH), 6.73(d,J=8.0 Hz, 2H, ArH), 5.25(s, 1H, 12-H), 4.16(m, 2H, CH2), 3.75(s, 2H, ArH), 3.51(s, 2H, ArH), 3.22(dd,J=11.0 Hz, 4.0 Hz, 1H, 3-H), 2.84(dd,J=13.0 Hz, 3.3 Hz, 1H, 18-H), 2.66(t,J=5.3 Hz, 2H, CH2), 2.53(s, 4H, H in piperazine), 1.12(s, 3H, CH3), 0.98(s, 3H, CH3), 0.91(s, 3H, CH3), 0.89(s, 3H, CH3), 0.88(s, 3H, CH3), 0.77(s, 3H, CH3), 0.71(s, 3H, CH3)。
3m:1H NMRδ: 9.12(s, 1H,J=), 6.73(d,J=3.0 Hz, 2H, ArH), 5.26(t,J=3.0 Hz, 1H, 12-H), 4.16(m, 2H, CH2), 3.75(s, 2H, H in piperazine), 3.50(s, 2H, H in piperazine), 3.22(dd,J=11.0 Hz, 4.0 Hz, 1H, 3-H), 2.84(dd,J=13.8 Hz, 3.8 Hz, 1H, 18-H), 2.65(t,J=5.5 Hz, 2H, CH2), 2.58(s, 2H, H in piperazine), 2.47(s, 2H, H in piperazine), 1.13(s, 3H, CH3), 0.98(s, 3H, CH3), 0.91(s, 3H, CH3), 0.90(s, 3H, CH3), 0.89(s, 3H, CH3), 0.77(s, 3H, CH3), 0.72(s, 3H, CH3)。
3q:1H NMRδ: 7.33(t,J=7.0 Hz, 1H, ArH), 7.29(dd,J=7.0 Hz, 1.2 Hz, 1H, ArH), 7.03(dd,J=7.0 Hz, 1.2 Hz, 1H, ArH), 5.28(t,J=3.4 Hz, 1H, 12-H), 4.20(m, 2H, CH2), 3.75(t,J=4.8 Hz, 4H, H in piperazine), 3.22(t, 1H, 3-H),2.897(dd, 1H, 18-H), 2.68(t,J=5.4 Hz, 2H, CH2), 2.59(t,J=4.8 Hz, 4H, H in piperazine), 1.19(s, 3H, CH3), 1.00(s, 3H, CH3), 0.94(s, 3H, CH3), 0.92(s, 3H, CH3), 0.91(s, 3H, CH3), 0.793(s, 3H, CH3), 0.74(s, 3H, CH3)。
4:1H NMRδ: 5.26(t,J=3.5 Hz, 1H, 12-H), 4.16(m, 2H, CH2), 3.21(dd,J=11.0 Hz, 4.0 Hz, 1H, 3-H), 2.91(s, 4H, H in piperazine), 2.85(dd,J=14.0 Hz, 4.5 Hz, 1H, 18-H), 2.61(t,J=6.0 Hz, 2H, CH2), 2.52(s,4H, H in piperazine), 1.13(s, 3H, CH3), 0.98(s, 3H, CH3), 0.91(s, 3H, CH3), 0.90(s, 6H, CH3), 0.78(s, 3H, CH3), 0.72(s, 3H, CH3)。
2 结果与讨论
由于1的3-OH位阻较大,与芳香酸酯化时投料比例、底物结构、反应溶剂等条件对反应收率有一定影响。(1)若按等比例投料,酯化产率较低,当芳香酸的量增加一倍时,羧基与羟基接触几率加大,产物收率也明显提高。(2)含有酚羟基的芳香酸酯化时,TLC检测发现1始终未发生变化,却出现含有芳香酸结构的新点,这说明在DCC/DMAP条件下不易与芳香酸发生酯化反应,过量的芳香酸发生了分子间反应。(3)在酚羟基保护过程中,首先选用了乙酸酐,但乙酰化保护的芳香酸与1反应仅得3-O-乙酰基齐墩果酸。Deng[3]和曲峰等认为采用羟基乙酰化保护糖基进行糖苷化反应时,在反应过程中乙酰基易发生转移[4]。推测本实验中生成3-O-乙酰基齐墩果酸的原因可能是乙酰基与酚羟基形成的酯不稳定,在DCC/DMAP弱碱性反应条件下易裂解,产生的活性中间体乙酰基易与1的3-OH成酯。我们改用苄基保护后产率提高,催化氢化脱保护也较容易且不会破坏酯键。文献[5]报道先将芳香酸的羧基与甲醇在对甲基苯磺酸作用下形成甲酯,用苄基保护羟基,然后在氢氧化钾的甲醇溶液中水解甲酯得苄基保护酚羟基的芳香酸,但该法酯化反应需较长时,且产率只有75%。本文直接用芳香酸(Ⅰl~Ⅰp)与过量氯化苄反应,在氢氧化钾的甲醇溶液中水解脱苄酯得苄基保护酚羟基的芳香酸(Ⅰl′~Ⅰp′, Scheme 2),反应时间缩短,产率提高到85%。
在1的28-CO2H改造过程中,(1)与1,2-二氯乙烷反应时,首先以丙酮作为溶剂,由于丙酮沸点较低,反应需要很长时间,且反应不完全;后改用DMF为溶剂,但DMF沸点较高,反应后不易除去;1,2-二氯乙烷沸点是83.5 ℃,在回流状态下对1有较好的溶解性,因此以1,2-二氯乙烷既作反应物又作溶剂,不仅缩短了反应时间,同时提高了产率。(2)哌嗪的另一个氮与芳香酸形成酰胺后,哌嗪上靠近酰胺的碳上的两个氢发生裂分,且偶合常数非常大(J=180 Hz)。肖志会等[6]对大量的苯基哌嗪类衍生物进行NMR研究时,发现所有化合物都出现这种情况。本文也证实该现象,且认为靠近酰胺的碳上的两个氢由于在芳香酰基的共轭作用影响下发生化学不等价,导致出现偶合常数非常大的裂分。
本实验合成反应条件温和,产率较高,能够得到较多类型的齐墩果酸衍生物,为测试抗HIV活性和研究构效关系提供了较为可靠的实验依据。
Scheme 2
[1] Yoshiki Kashiwada, Hui-Kang Wang, Tsuneatsu Nagao,etal.anti-AIDS agents.30.anti-HIV activity of oleanolic acid,pomolic acid,and structurally related triterpenoids[J].Journal of Natural Products,1998,61(9):1090-1095.
[2] Chaomei Ma, Norio Nakamura, Hirotsugu Miyashiro,etal. Inhibitory effects of constituents from cynomorium songaricum and related triterpene derivatives on HIV-1 protease[J].Chemical & Pharmaceutical Bulletin,1999,47(2):141-145.
[3] Shaojiang Deng, Biao Yu, Jianming Xie,etal. Highly efficient glycosylation of sapogenins[J].J Org Chem,1999,64:7265-7266.
[4] 曲峰,李英霞,张一纯,等. 几种齐墩果酸糖缀合物的合成[J].有机化学,2003,23(2):249-257.
[5] 段新方,张站斌,段新红. 5,3′,4′-三羟基-6,7-二甲氧基黄酮的另法全合成[J].有机化学,2003,23(4):353-355.
[6] 肖志会,袁牧,黄建设,等. 苯基哌嗪衍生物的NMR波谱研究[J].波谱学杂志,2004,21(3):365-369.
