Glioma-conditioned Medium Blocks Endothelial Cells’ Apoptosis Induced by Hypoxia and Promotes Its Angiogenesis via Up-regulation of u-PA/u-PAR
2010-07-18XizhenXuZhiqiangLiZhihuaWen
Xi-zhen Xu, Zhi-qiang Li,*, Zhi-hua Wen
1Department of Neurosurgery,2Laboratory of Neuro-oncology, Zhongnan Hospital, Wuhan University Wuhan 430071, China
Glioma-conditioned Medium Blocks Endothelial Cells’ Apoptosis Induced by Hypoxia and Promotes Its Angiogenesis via Up-regulation of u-PA/u-PAR
Xi-zhen Xu1, Zhi-qiang Li1,2*, Zhi-hua Wen2
1Department of Neurosurgery,2Laboratory of Neuro-oncology, Zhongnan Hospital, Wuhan University Wuhan 430071, China
Objective:To investigate the role of urokinase-type plasminogen activator/urokinase-type plasminogen receptor(u-PA/u-PAR) system in glioma angiogenesis under hypoxic conditions, we studied the effect of glioma-conditioned medium on the hypoxia induced changes in human endothelial-like ECV304 cells proliferation, apoptosis, cord formationin vitroand u-PA/u-PAR expression.
Methods:MTT assay was used to examine the changes in cell proliferation. Cell apoptosis was analyzed by Hoechst 33258 staining. Matrigel cord-like formation assay was used to evaluate the angiogenesis ability of ECV304 cellsin vitro. Expressions of u-PA/u-PAR mRNA were detected by quantitative real-time RT-PCR.
Results:Hypoxia inhibited ECV304 cells proliferation and induced cell apoptosis. Hypoxic conditioned medium(H-CM) while not normoxic conditioned medium(N-CM) of U251 glioma cells partially blocked the effect of hypoxia on ECV304 cells proliferation and apoptosis. H-CM of U251 glioma cells also promoted the cord formation of ECV304 cells seeded on matrigel. When u-PA or u-PAR monoclonal antibodies were added into ECV304 cells culturing medium, cord formation ability was partially inhibited. H-CM of U251 glioma cells induced uPA and uPAR expression in ECV304 cells.
Conclusion:These suggest that u-PA/u-PAR system is involved in glioma angiogenesis trigged by hypoxic microenviroment.
Endothelia; Glioma; Hypoxia; u-PA; u-PAR
INTRODUCTION
Malignant glioma, the most common primary tumor in the central nervous system and the most aggressive brain tumor with very poor prognosis, is characterized by rapid growth, intense angiogenesis, vascular malformations and recurrent tendency. Except for these widely used treatment including operative resection, chemotherapy and radiotherapy, many new therapeutic strategies are exploring. The translation of antiangiogenic therapy from laboratoryto the clinic is underway and is robust after more than three decades of research on angiogenesis[1].
Angiogenesis is one of essential components which can provide oxygen and nutrients for the growth of malignant gliomas. However, regional necrosis, a very often described pathological feature existed in glioma tissue, implicates that a few tumor cells and vascular endothelial cells survive in regional hypoxic conditions and form new vessels. During this process, many molecular changes in endothelial cells and glioma cells contribute to the angiogenic response, such as hypoxia-inducible factor-1α (HIF-1α), vascular endothelial growth factor (VEGF) and its receptor, matrix metalloproteinases (MMPs), and so on. Exposure to hypoxia causes an increase in gene expression of VEGF, VEGFR2 and activities ofMMP-2 and MMP-9, which induce an increase in capillary-like structure formation in endothelial cells seeded into matrigel[2,3]. However, antiangiogenic agents targeting VEGF, HIF-1α or MMPs, which have been shown strong efficacy in experimental models, did not demonstrate exciting results in clinical trial[1], implicating that other factors may also be involved in the hypoxia-induced angiogenesis.
The urokinase-type plasminogen activator (u-PA) is secreted as a single-chain inactive zymogen form pro-u-PA, which is activated by a surface-bound plasmin to a two-chain u-PA. The uPA molecule binds to its cellular receptor u-PAR (CD87), a member of the glycosylphosphatidylinositol (GPI) anchored proteins, with high affinity[4,5]. It has been shown that in a number ofin vitroas well asin vivostudies, the binding of u-PA to u-PAR is essential for its action in tumor cell migration, invasion and tumor angiogenesis[6,7]. Nevertheless both u-PA and u-PAR are often expressed at high levels in malignant brain tumors[8,9], little was done to observe the effect of hypoxia on u-PA/u-PAR expression in vascular endothelial cells and on following angiogenesis. In this study we observed the formation of capillary-like tubular structuresin vitroby human endothelial-like ECV304 cells under hypoxic glioma cells conditioned medium, and evaluated the role of u-PA/u-PAR in this process.
MATERIALS AND METHODS
Cell Lines and Chemicals
Cell culture was performed as described[10]. Briefly, human glioblastoma U251 cells and human endothelial-like ECV304 cells, obtained from Central Chinese Type Culture Collection (Wuhan, China), were maintained in RPMI-1640 medium(Invitrogen, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin G, 0.1 mg/ml streptomycin at 37°C in 5% CO2, and 10 ng/ml human basic fibroblast growth factor (bFGF; Sigma, St. Louis, MO, USA) was added in the medium of ECV304 cells. Cells used in this study were passaged 4 times or less. For culturing in hypoxic conditions, cells were placed in a humidified chamber maintained at 1% O2, 5% CO2and balanced in N2 for the indicated times.
Preparation of glioma conditioned medium. When reaching 70–80% confluence, U251 glioma cells were washed twice with serum-free medium and then cultered in fresh medium containing 1% FBS at 37°C under normoxic or hypoxic conditions. After 24 h incubation, the medium was collected and centrifuged at 2000×g at 4°C for 10 min. The supernatant was stored at -20°C until use.
Cell Viability Assay
MTT assay was performed to assess the viability of ECV304 cells under hypoxic conditions or treated with conditioned medium. Briefly, ECV304 cells were plated in 96-well flat-bottom plates at a concentration of 1×104cells/well in 100μl of medium. After indicated treatments, 20µl 3-[4,5-dimethyhlthizaol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT, Sigma, USA) at a final concentration of 5mg/ml were added to each well. Four hours later, cell medium was replaced with 100 µl dimethyl sulfoxide (DMSO) and the optical density of each well was measured by microplate reader (Hua Dong Electronic Co., Nanjing, China) at 490 nm.
Hoechst 33258 Staining
ECV304 cells were cultured in 12-well plates under normal or hypoxic conditions in the presence or absence of U251 conditioned medium for 48 h. Then, cells were washed with PBS and fixed with 4% paraformaldehyde prepared in PBS. After fixation, cells were stained with 1μg/ml of Hoechst 33258 (Molecular Probes, Eugene, OR) for 10 min at 37°C in the dark. The proportion of apoptotic nuclei was determined by counting three random fields in duplicate wells per group using a fluorescent microscope (Olympus, Melville, NY) with apoptosis defined as the presence of two or more condensed bodies per nucleus.
Matrigel Cord-like Formation Assay
Matrigel cord-like formation assay was performed as described[11]. 250 μl of Matrigel (BD Biosciences, Palo Alto, CA) was placed in each well of an ice-cold 24-well plate. The plate was allowed to sit at room temperature for 15 min, and at 37°C for 30 min for Matrigel to polymerize. ECV304 cells in normoxic or hypoxic U251 conditioned medium were plated at a concentration of 1×105cells/well. After 24h incubation under hypoxic condition, pictures were taken under phase-contrast microscopy and capillary cord structure was defined as cellular extension linking cell masses or branch points. Network formation was measured by counting the number of cord angles/field (each field is defined as the area visualized by a 4×magnification lens). Each experimental condition was tested in 8 separate experiments.
Quantitative Real-time RT-PCR Assay
Total RNA was extracted from ECV304 cells treated with U251 conditioned medium, according to the manufacturer's instructions for Trizol reagent (Invitrogen, Carlsbad, CA, USA). Tow μg of total RNA were converted to first-strand cDNA using a Reverse Transcription System (Promega, Madison, WI). Quantitative Real-time PCR was carried out using qPCR SYBR Green kit (ABgene, Rochester, NY) and analyzed on a Bio-Bad iCycler system (Bio-Bad, Hercules, CA). The PCR conditions were∶95°C for 5 min, followed by 40 cycles of 95°C for 15 s, 55°C for 30 s and 72°C for 30 s with fluorescence measurements read during each cycle. The expression levels of targer gene mRNAs were normalized to those of GAPDH mRNA. The series of measurements was performed four times. Specific primers are as follows[12]∶ uPA forward, 5’-TGC GTC CTG GTC GTG AGC GA-3’, and reverse, 5’-CAA GCG TGT CAG CGC TGT AG-3’; uPAR forward, 5’-CAT GCA GTG TAA GAC CCA ACG GGG A-3’, and reverse, 5’-AAT AGG TGA CAG CCC GGC CAG AGT-3’; GAPDH forward, 5’-CGG AGT CAA CGG ATT TGG TCG TAT-3’, and reverse, 5’-AGC CTT CTC CAT GGT GGT GAA GAC- 3’.
Statistical Analysis
The data are expressed as the ¯x±s. Statistical analysis was performed by SPSS11.0 using the unpaired Student t-test for the comparison between two sample groups and one-way ANOVA test for the multiple comparisons.P<0.05 were considered to be statistically significant.
RESULTS
Hypoxia Inhibited the Proliferation of ECV304 Cells and Induced Apoptosis
To investigate the viability of endothelial cells under hypoxic condition, we detected the proliferation of endothelial cells, which is prerequisite during the angiogenic process. ECV304 cells were cultured to 90% confluence, then exposed to normoxia or hypoxia for 24 h and 48 h. Cell viabilities under hypoxic condition for 24 h reduced by 25% compared with normoxic condition (P=0.0083), and by 55% for 48 h (P=0.0002)(Figure 1A). The data suggested that hypoxia resulted in a significant reduction in cell proliferation of ECV304 cells. To determine whether the reduction of cell numbers might result from hypoxia-induced apoptosis, we exposed ECV304 cells to hypoxic conditions for 48 h to analyze the cells by fluorescence microscopy following Hoechst 33258 staining. Within 48 h of hypoxic exposure, apoptotic cells indicated by two or more condensed bodies per nucleus significantly increased (69.16±19.93) in ECV304 cells. However, few stained apoptotic cells were observed under normoxic conditions (13.83±4.69) (P=0.0002) (Figure 1B, 1C). It was suggested that hypoxia-induced apoptosis was one of possible mechanisms for decreased cell proliferation in ECV304 cells cultured under hypoxic condition.
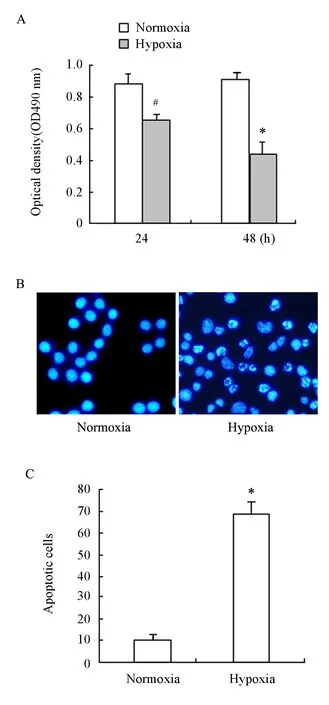
Figure 1. Effect of hypoxia on ECV304 cells proliferation (A) and apoptosis (B, C). 1× 104ECV304 cells were cultured to 90% confluence, then exposed to normoxia or hypoxia for 24 h and 48 h, and cell viability was measured by MTT assay.#P<0.05, and*P<0.01 vs normoxia by studentt-test; cell apoptosis was assessed by Hoechst 332581 staining following exposure to normoxia or hypoxia for 48 h (B,C).*P<0.01vs. normoxia by Studentt-test.
Conditioned Medium of Hypoxic Glioma Cells Promoted ECV304 Proliferation
Since hypoxia reduced the proliferation rate and induced apoptosis of ECV304 cells, we further exposed ECV304 cells to conditioned medium collected from cultured U251, a human glioblastoma cell line, to investigate the effect of glioma cells on the survival of ECV304 cells under hypoxic condition. After 90% confluence of ECV304 cells, medium was replaced by U251 normoxic conditioned medium(N-CM) or hypoxic conditioned medium (H-CM) accompanying with exposure to hypoxia for 48 h. ECV304 cells treated by H-CM (0.56±0.05) were partially corrected in reduction of cell proliferation compared with N-CM (0.45±0.05) (P=0.0304) (Figure 2A). Presence of H-CM significantly prevented reduction in cell numbers in cultures of ECV304 cells grown in hypoxia. Furthermore, compared to ECV304 cells cultured with normal medium(NM) under hypoxic condition, a marked reduction in apoptotic ECV304 cells in cultures containing H-CM was observed, while there was no significant change in apoptotic cells in cultures containing N-CM (Figure 2B). These results suggested that H-CM, but not N-CM, increased the survival of ECV304 cells.

Figure 2. Effect of U251 H-CM on ECV304 cells proliferation (A) and apoptosis (B). ECV304 cells (1×104/well) were cultured in normal medium (NM) to 90% confluence, then medium was replaced by NM, N-CM, H-CM and the cells were exposed to hypoxia or normoxia for 48 h. Cell proliferation was measured by MTT assay. H-CM but not N-CM promoted ECV304 cells proliferation under either hypoxia or normoxia (A)*P<0.05 hypoxiavs. normoxia under different culture medium condition;#P<0.05)vs. NM under hypoxia;▲P<0.05 vs. N-CM under hypoxia. Apototic cells were assesed by Hoechst 332581 staining following 48 h exposure to hypoxia (B).*P<0.01 hypoxic vs normoxic group under different culture medium condition;#P<0.05 vs. NM under hypoxia;▲P<0.05 vs. N-CM under hypoxia. Student t-test was used for the comparison between 2 sample groups and ANOVA test for the multiple comparisons.
Hypoxic U251 Conditioned Medium Promoted Cord Formation of ECV304 Cells
In order to investigate the angiogenic function of ECV304 cells survived from hypoxia by H-CM, we further studied the effect of H-CM on cord formation ability of ECV304 cells seeded on polymerized matrigel. Few cords were formed by ECV304 cells in NM under normoxia. Hypoxia itself induced cord formation of ECV304 cells in all three different medium, while no significant difference of this induction was observed between N-CM and NM. However, more abundant cord structures were observed with the addition of U251 H-CM under either normoxia or hypoxia. Quantitative analysis showed significant difference between N-CM and H-CM groups (Figure 3).
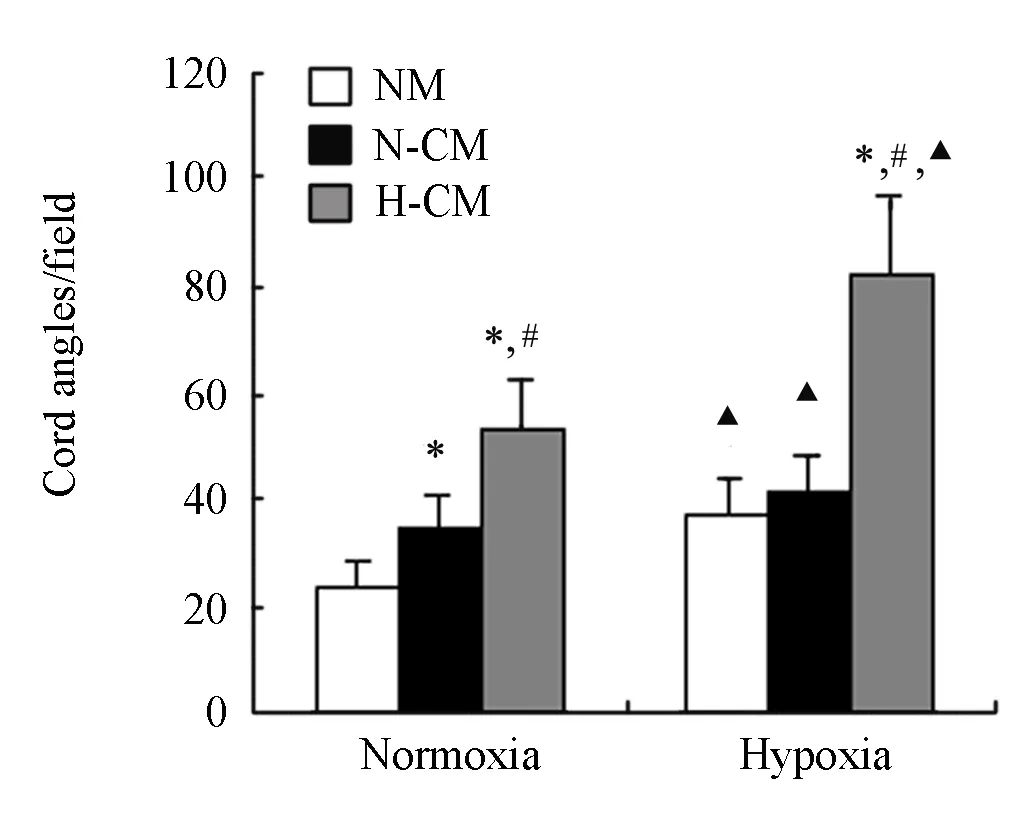
Figure 3. Effect of U251 H-CM on cord formation of ECV304 cells plated on matrigel-coated wells and incubated 24 h under normoxic or hypoxic condition. Quantitative analysis revealed the different capacity of cord formation of ECV304 cells under different conditioned medium.*P<0.05vs.NM cultured cells under normoxic or hypoxic condition;#P<0.05 H-CMvs. N-CM;▲P<0.05 hypoxic group vs. normoxic group under different culturing medium. Studentt-test was used for the comparison between 2 sample groups and one-way ANOVA test for the multiple comparisons.
Role of u-PA/u-PAR in the Survival and Cord Formation of ECV304 Cells Stimulated by U251 H-CM
As u-PA/u-PAR system has been implicated in a number of processes involved in tumor progression, including extracellular matrix degradation, tumor cell invasion, and angiogenesis, we investigate the possible involvement of u-PA/u-PAR in survival and cord formation of ECV304 cells promoted by U251 H-CM. The expressions of u-PA/u-PAR in ECV304 cells were examined. Quantitative real-time RT–PCR demonstrated that mRNA levels of uPA and uPAR in ECV304 cells cultured with U251 H-CM increased respectively, compared with cells cultured with NM or U251 N-CM under hypoxia (Figure 4A, 4B). Then, the specific u-PA and u-PAR monoclonal antibodies were employed to antagonize its activation. Matrigel assay showed that specific u-PA and u-PAR antibodies attenuated the cord formation ability of ECV304 cells promoted by U251 H-CM. Significant reduction was observed in cord formation by either antibody (Figure 5).

Figure 4. Expression of u-PA and u-PAR mRNA in ECV304 cells cultured with different culturing medium uner normoxia and hypoxia. Under hypoxia while not normoxia, quantitative real-time RT-PCR demonstrated that u-PA(A) and u-PAR (B) mRNA levels in ECV304 cells with U251 H-CM were significantly increased.#P<0.01vs.NM;▲P<0.01vs.N-CM. Studentt-test was used for the comparison between 2 sample groups and one-way ANOVA test for the multiple comparisons.

Figure 5. u-PA/u-PAR antibodies affected cord formation of ECV304 cells incubated with U251 H-CM. After 90% confluence of ECV304 cells, medium was replaced by U251 H-CM and specific u-PA and u-PAR antibodies were added respectively at the same time point under hypoxia. Matrigel cord formation assay was performed after 48h incubation. Both u-PA and u-PAR specific antibodies attenuated the cord formation ability of ECV304 cells.#P<0.05vs. untreated control group by one-way ANOVA test.
DISCUSSION
Angiogenesis is a complex process in which ECs respond to various proangiogenic factors, including growth factors and chemokines produced by tumor and stromal cells. In the process of angiogenesis of tumor tissue, new blood vessels are initiated by endothelial cells from various sources, including the sprouts of preexisting vessels adjacent to the tumor. These endothelial cells form new microvessels through proliferation, tubulogenesis and remodeling[13,14]. The proliferation of endothelial cell, as a crucial step of angiogenesis is triggered and modulated by a variety of stimuli including hypoxic microenviroment which is believed to be a potent and crucial stimulus in tumor angiogenesis[15]. Some researchers have reported that hypoxia induced endothelial cells proliferation[16,17], while others have shown a reduction of endothelial cell number in hypoxia and an arrest in the cell cycle[2,8,19]. In this study we first investigated the responses of human endothelial ECV304 cells to hypoxia mimicked inin vitroexperiment. Reduction of ECV304 cell numbers and increase of apoptotic cells were observed in hypoxic conditions, compared with ECV304 cells cultured in normoxic conditions. Our findings are similar to that of Ezhilarasan R et al[2]who reported that hypoxia caused a reduction in clonogenic cell survival response and an increase of the sub-G1 phase of the cell cycle in endothelial cells, and suggested thatcaspase-dependent mechanism involved in the hypoxia-induced endothelial cell apoptosis.
Although damage of endothelial cells was observed under hypoxic condition inin vitroexperiment, endothelial cells survive and sprout new vessels in solid tumors existing intratumoral hypoxia. This process occurs most prominently in glioblastoma in which necrotic lesions are detectable at early stages of tumor development. The relative mechanisms are not completely clarified[20]. Because of the crucial role of survival and proliferation of endothelial cells in the angiogenic process, we tried to investigate whether glioma cells under hypoxic condition can protect hypoxia-induced endothelial cell damage. Here we demonstrated that the culture medium of U251 glioma cells treated with hypoxia rescued endothelial cells from death induced by hypoxia. Both the secretion of VEGF from glioma cells under hypoxic culturing condition and activation of nuclear factor-κB (NF-κB) in endothelial cells induced by TNF-α are claimed to be necessary for endothelial cell survival as they increase the expression of antiapoptotic genes including Bcl-2, Bcl-XL, survivin and X-chromosome-linked inhibitor of apoptosis protein in endothelial cells[3].
In addition to block the ECV304 cells death induced by hypoxia, the enhancement of angiogenic ability of ECV304 cells by exposure to hypoxic conditioned medium of U251 glioma cells were also shownin vitroby matrigel cord formation assay. Compared with U251 N-CM, more cord like structures were observed in cells treated with H-CM. Our findings also provided evidence supporting the notion that glioma cells under hypoxic conditions may secrete soluble factors triggering a variety of biological responses that regulate endothelial cells proliferation and angiogenesis. It has been verified that the most important factor orchestrating hypoxiainduced angiogenesis is HIF-1, which is a heterodimer that consists of a HIF-1α and a HIF-1β subunit. Under normoxic condition HIF-1α is ubiquitinated and degradated through its interaction with Von Hippel-Lindau tumor suppressor protein. During hypoxia, HIF-1α is activated, interacts with its co-activators p300 and CBP and binds to hypoxia- responsive elements (HRE), which leads to up- regulation of several genes involving in cell cycle control, stress response, and neovasularization, such as cyclin G2, insulin-like growth factor binding protein 3, VEGF, NOS, and other adaptive response molecules or chemokines[21-23].
In these angiogenic regulators, u-PA and u-PAR are also required for activation of many signal transduction pathways. A large body of evidence suggests that u-PA/u-PAR system plays a key role in tumor tissue morphogenesis, cell differentiation, migration and invasion by virtue of its ability to activate plasminogen, which degrades many ECM components and activates latent collagenases[24,25]. In gliomas, u-PA and u-PAR are up-regulated and their levels are increased with tumor grade. U-PA and u-PAR were also detected in glioma endothelial cells of the tumor vasculature, which were predominantly located at sites of vascular proliferation and at the leading edges of the tumor. Taking into consideration the correlation of uPA expression with microvessel quantity, it was suggested that both u-PA and u-PAR play a significant role, not only in tumor invasion but also in glioma angiogenesis[8,9]. In this study, we showed that U251 H-CM increased u-PA and u-PAR expression in ECV304 cells, and that u-PA/u-PAR antibodies inhibited the promotion of cord formation in ECV304 cells stimulated by U251 H-CM. Our data suggested that modulation of u-PA/u-PAR expression altered the angiogenic potential of the ECV304 cells under hypoxic condition. By using RNA interference directed toward u-PAR and MMP-9, Lakka et al[26]also achieved specific inhibition of uPAR and MMP-9, which inhibited glioma cell proliferation, and significantly reduced the levels of phosphorylated forms of MAPK, ERK, and AKT signaling pathway molecules, and inhibited the formation of capillarylike structures in bothin vitroandin vivomodels of angiogenesis.
In summary, we have shown that hypoxia induced apoptosis of endothelial cells and H-CM of glioma cells rescued endothelial cells from apoptosis and promoted capillary tube formation. We further demonstrated that u-PA/u-PAR system would play important roles in these biological processes. These results provide insight into the mechanisms by which hypoxia stimulates glioma angiogenesis. It may be useful in the design of new antiangiogenic therapy.
Acknowledgement
This work was supported by the Foundation from the Health Department of Hubei province (No. JX1B019).
REFERENCES
[1] Jouanneau E. Angiogenesis and gliomas∶ current issues and development of surrogate markers[J]. Neurosurgery 2008; 62∶31-50.
[2] Ezhilarasan R, Mohanam I, Govindarajan K, et al. Glioma cells suppress hypoxia- induced endothelial cell apoptosis and promote the angiogenic process[J]. Int J Oncol 2007; 30∶ 701-7.
[3] Ueda Y, Nakagawa T, Kubota T,et al. Glioma cells under hypoxic conditions block the brain micro-vascular endothelial cell death induced by serum starvation[J]. J Neurochem 2005; 95∶ 99-110.
[4] Gardsvoll H,Ploug M. Mapping of the vitronectin-binding site on the urokinase receptor∶involvement of a coherent receptor interface consisting of residues from both domain I and the flanking interdomain linker region[J]. J Biol Chem 2007; 282∶ 13561-72.
[5] Barinka C,Parry G,Callahan J, et al. Structural basis of interaction between urokinase-type plasminogen activator and its receptor[J]. J Mol Biol 2006; 363∶482-95.
[6] Dass K,Ahmad A,Azmi AS, et al. Evolving role of u-PA/u-PAR system in human cancers[J]. Cancer Treat Rev 2008; 34∶122-36.
[7] Dass CR,Nadesapillai AP,Robin D, et al. Downregulation of uPAR confirms link in growth and metastasis of osteosarcoma[J].Clin Exp Metastasis 2005; 22∶ 643-52.
[8] Yamamoto M, Sawaya R, Mohanam S, et al. Expression and localization of urokinase-type plasminogen activator receptor in human gliomas [J].Cancer Res 1994; 54∶ 5016-20.
[9] Yamamoto M, Sawaya R, Mohanam S, et al. Expression and localization of urokinase-type plasminogen activator in human astrocytomasin vivo[J]. Cancer Res 1994; 54∶ 3656-61.
[10] Chu SH, Zhu ZA, Yuan XH, et al.In vitroandin vivopotentiating the cytotoxic effect of radiation on human U251 gliomas by the c-Met antisense oligodeoxynucleotides[J]. J Neurooncol 2006; 80∶ 143-9.
[11] Williams CK, Li JL, Murga M, et al. Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function[J]. Blood 2006; 107∶931-9.
[12] Gondi CS, Lakka SS, Dinh DH, et al. Intraperitoneal injection of a hairpin RNA-Expression plasmid targeting urokinase- type plasminogen activaor(uPA) receptor and uPA retards angiogenesis and inhibits intracranial tumor growth in nude mice[J]. Clin Cancer Res 2007; 13∶ 4051-60.
[13] Bian XW, Jiang XF, Chen JH, et al. Increased angiogenic capabilities of endothelial cells from microvessels of malignant human gliomas[J]. Int Immunopharmacol 2006; 6∶ 90-9.
[14] Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors[J]. Nat Med 2003; 9∶ 669-76.
[15] Kaur B, Tan C, Brat DJ, et al. Genetic and hypoxic regulation of angiogenesis in gliomas[J]. J Neuro Oncol 2004; 70∶ 229-43.
[16] Nomura M, Yamagishi S, Harada S, et al. Possible participation of autocrine and paracrine vascular endothelial growth factors in hypoxia-induced proliferation of endothelial cells and pericytes[J]. J Biol Chem 1995; 270∶ 28316-24.
[17] Li W, Petrimpol M, Molle KD, et al. Hypoxiainduced endothelial proliferation requires both mTORC1 and mTORC2[J]. Circ Res 2007; 100∶79-87.
[18] Zhang J, Tan Z, Tran ND. Chemical hypoxiaischemia induces apoptosis in cerebromicrovascular endothelial cells[J]. Brain Res 2000; 877∶ 134-40.
[19] Iida T, Mine S, Fujimoto H, et al. Hypoxia- inducible factor-1α induces cell cycle arrest of endothelial cells [J]. Genes Cells 2002; 7∶ 143-9.
[20] Folkman J. Is angiogenesis an organizing principle in biology and medicine[J]? J Pediatric Surg 2007; 42∶1-11.
[21] Zhao S, Lin Y, Xu W, et al.Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha[J].Science 2009; 324∶ 261-5.
[22] Ragel BT, Couldwell WT, Gillespie Dl, et al. Identification of hypoxia-induced genes in a malignant glioma cell line (U-251) by cDNA microarray analysis[J]. Neurosurg Rev 2007; 30∶ 181-7.
[23] Li ZQ,Yuan XH, Jiang PC, et al. Inhibition effect of vascular endothelial growth factor antisense oligodeoxynucleotides on C6 glioma[J]. Chin J Cancer Res 2003; 15∶ 210-2.
[24] Chandrasekar N, Mohanam S, Gujrati M, et al. Downregulation of uPA inhibits migration and PI3k/Akt signaling in glioblastoma cells[J]. Oncogene 2003; 22∶ 392-400.
[25] Danø K, Rømer J, Nielsen BS, et al. Cancer invasion and tissue remodeling--cooperation of protease systems and cell types[J]. APMIS 1999; 107∶120-7.
[26] Lakka SS, Gondi CS, Dinh DH, et al. Specific interference of urokinase-type plasminogen activator receptor and matrix metalloproteinase-9 gene expression induced by double-stranded RNA results in decreased invasion, tumor growth and angiogenesis in gliomas[J]. J Biol Chem 2005; 280∶ 21882-92.
R739.41 Document code: A Article ID: 1000-9604(2010)02-0119-07
10.1007/s11670-010-0119-3
2009−09−10; Accepted 2010−02−08
This work was supported by the Foundation from the Health Department of Hubei Province (No. JX1B019)
*Corresponding author.
E-mail∶ lifenzhi@yahoo.com.cn
© Chinese Anti-Cancer Association and Springer-Verlag Berlin Heidelberg 2010
杂志排行
Chinese Journal of Cancer Research的其它文章
- Genetic Polymorphism of PSCA and Risk of Advanced Precancerous Gastric Lesions in a Chinese Population
- Cortactin Overexpression Correlates with Poor Prognosis in Hepatocellular Carcinoma
- Synergistic Action of fMLP-boanmycin Combination on the Growth of Mouse Colon Carcinoma and Its Action Mechanisms
- Differential Diagnosis of Warthin's Tumor Complicated with Lung Adenocarcinoma by 18F- FDG PET/CT Imaging and Radioisotope Scanning with Tc-99m Pertechnetate: A Case Report and Literature Review
- Expression of Embryonic Stem Cell Marker Oct-4 and Its Prognostic Significance in Rectal Adenocarcinoma
- CGI-100 Specific shRNA Inhibits Proliferation and Induces Differentiation in Leukemia K562 Cells
