Expression of Embryonic Stem Cell Marker Oct-4 and Its Prognostic Significance in Rectal Adenocarcinoma
2010-07-18ChungenXingXueguanLuYongshengZhangFangZhouXiaopingXu
Chun-gen Xing, Xue-guan Lu, Yong-sheng Zhang, Fang Zhou, Xiao-ping Xu
1Department of General Surgery,2Department of Radiation Oncology,3Department of Pathology, Second Affiliated Hospital of Soochow University, Suzhou 215004, China
Expression of Embryonic Stem Cell Marker Oct-4 and Its Prognostic Significance in Rectal Adenocarcinoma
Chun-gen Xing1, Xue-guan Lu2*, Yong-sheng Zhang3, Fang Zhou2, Xiao-ping Xu2
1Department of General Surgery,2Department of Radiation Oncology,3Department of Pathology, Second Affiliated Hospital of Soochow University, Suzhou 215004, China
Objective:Recent evidence suggests that Oct-4 is highly expressed in several cancers, and its expression contributes to tumor growth. In this study, we investigated the level of Oct-4 expression in rectal adenocarcinoma, and evaluated the prognostic significance of Oct-4 expression in these cases.
Methods:The immunohistochemical expression of Oct-4 was evaluated in 52 formalin-fixed paraffin-embedded postoperative rectal adenocarcinoma tissue samples. The impact of the immunoreactivity of Oct-4 in regard to clinical outcome was determined by Kaplan-Meier and log-rank.
Results:The expression level of Oct-4 ranged from 0 to 18.5%. There was no significant association between Oct-4 expression and gender (P=0.772), age (P=0.123), clinical stage (P=0.391), and histological grade (P=0.056). The 3-year local recurrence-free rates with negative and positive expression of Oct-4 were 83.5% and 75.0%, respectively (P=0.583). The 3-year metastasis-free rates with negative and positive expression of Oct-4 were 88.6% and 61.9%, respectively (P=0.035). The 3-year overall survival rates with negative and positive expression of Oct-4 were 77.9% and 49.0%, respectively (P=0.037).
Conclusion:The results suggest that embryonic stem cell marker Oct-4 expression may have prognostic significance in patients with rectal adenocarcinoma. However, to confirm this more and larger studies are required.
Rectum; Adenocarcinoma; Oct-4; Stem cell; Prognosis
INTRODUCTION
Colorectal cancer is a leading cause of morbidity and mortality in developed countries[1]. Although most Asian countries show a much lower incidence, these countries have an increasing incidence of colorectal cancer. More information on factors that influence the increase, progression, and metastasis of colorectal cancer will lead to the development of novel diagnostic methods and treatment. One of these factors is cancer stem cells (CSC)[2,3]. In an increasing number of cancers, CSC is only made of a restricted subset of tumor cells which share characteristics withnormal stem cells, including the capacities for self renewal, multilineage differentiation and maintained proliferation[4]. Several studies have linked CSC to mechanisms of resistance to current therapies[5,6], suggesting that the existence of CSC may directly impact the prognosis of cancer. So it is important for us to develop biologic marker to identify CSC and separate these cells from bulk tumors. For example, CD133 (prominin-1), a 5-transmembrane glycoprotein, has been considered an important marker to represent the subset population of CSC in many cancers[3,6-8]. Recently, it has been proposed that Oct-4 acts as a multifunctional factor in CSC.
Embryonic stem cell marker Oct-4 (also known as Oct-3, Oct-3/4, and POU5F1), a member of the family of POU-domain transcription factors, is expressed in pluripotent embryonic stem cells and plays a key role in self-renewal and differentiation of these cells[9-10].Recent evidences suggest that Oct-4 is not only expressed in adult stem cells (including multipotent adult progenitor cells and hematopoietic stem cells)[11], but also in several cancers[12,13]. Moreover, its expression contributes to tumor growth[11,14]. But until now, little is known about Oct-4 expression in rectal cancer, especially there is no study to evaluate the prognostic significance of Oct-4 expression in patients with rectal adenocarcinoma. So in this study, we investigated the level of Oct-4 expression in rectal adenocarcinoma, and explored the prognostic significance of Oct-4 expression in these cases.
MATERIALS AND METHODS
Patients and Samples
Approval for current project was obtained from the local ethics committee. The study included 52 patients with rectal adenocarcinoma, who had been treated between 2005 and 2006. There were 28 men and 24 women with a median age of 63 years (range 17-88). All patients were proved to be adenocarcinoma by postoperative pathology. The staging was based on the findings at the surgery and the postoperative histopathologic report. According to UICC/AJCC stage system, 18 patients had stage I, 16 stage II, and 18 stage III. Patients’ characteristics are given in Table 1.
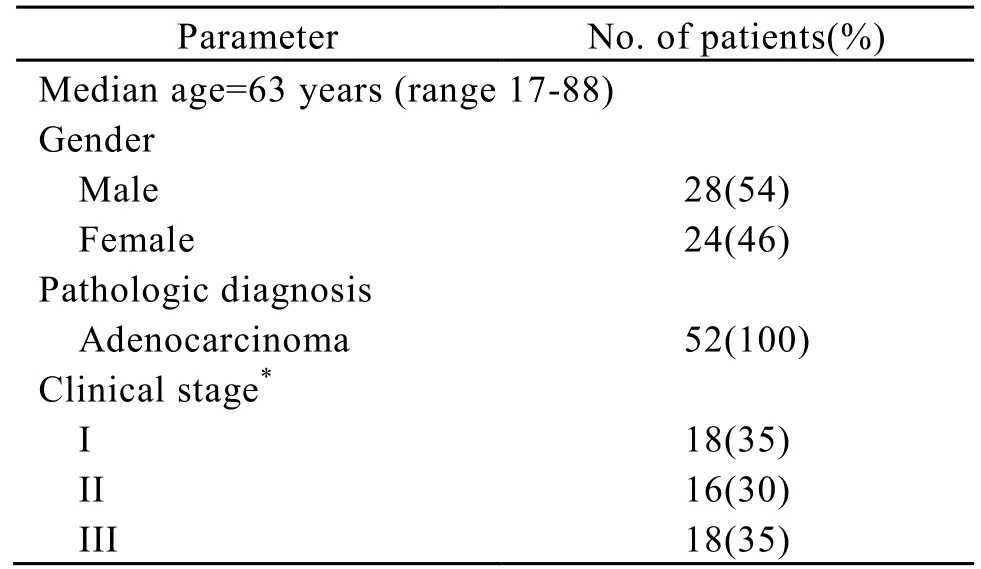
Table 1. Characteristics of 52 patients with rectal adenocarcinoma
All patients underwent surgical resection. There were 27 patients who received Miles’s surgery, 23 patients receiving Dixon’s surgery, 1 patient receiving Hartmann’s surgery, and 1 patient receiving localized resection. At the same time, thirty-six patients received total mesorectal excision. Forty-three of the 52 patients received adjuvant radiotherapy. There were 7 patients receiving preoperative irradiation, 30 patients receiving postoperative irradiation, and 6 patients receiving “sandwich” radiotherapy (including pre- and postoperative irradiation). The adjuvant radiation treatment consisted of high-energy photon (6 Mv x-ray) using a linear accelerator. Patients were treated with a three-field (posteroanterior and two lateral wedge fields) or two-field (posteroanterior and anteriopost fields). The top of the field was placed at the L4-5 junction, the lateral borders 1-2 cm outside the bony pelvis, and the inferior margin below the anal. The total dose for postoperative patients was 5000-5400cGy delivered by 200cGy/day for 5 days per week. The total dose for preoperative patients was 2000cGy delivered by 400cGy/day for 5 days per week. For the patients receiving “sandwich”radiotherapy, the total dose of preoperative irradiation was 2000cGy delivered by 400cGy/day for 5 days per week, and the total dose of postoperative irradiation was 4500cGy delivered by 180cGy/day for 5 days per week. Adjuvant chemotherapy was administered in 32 patients. The chemotherapeutic regimens used were 5-flurouracil and leucovorin and oxaliplatin. The patients’ treatment modality is given in Table 2.
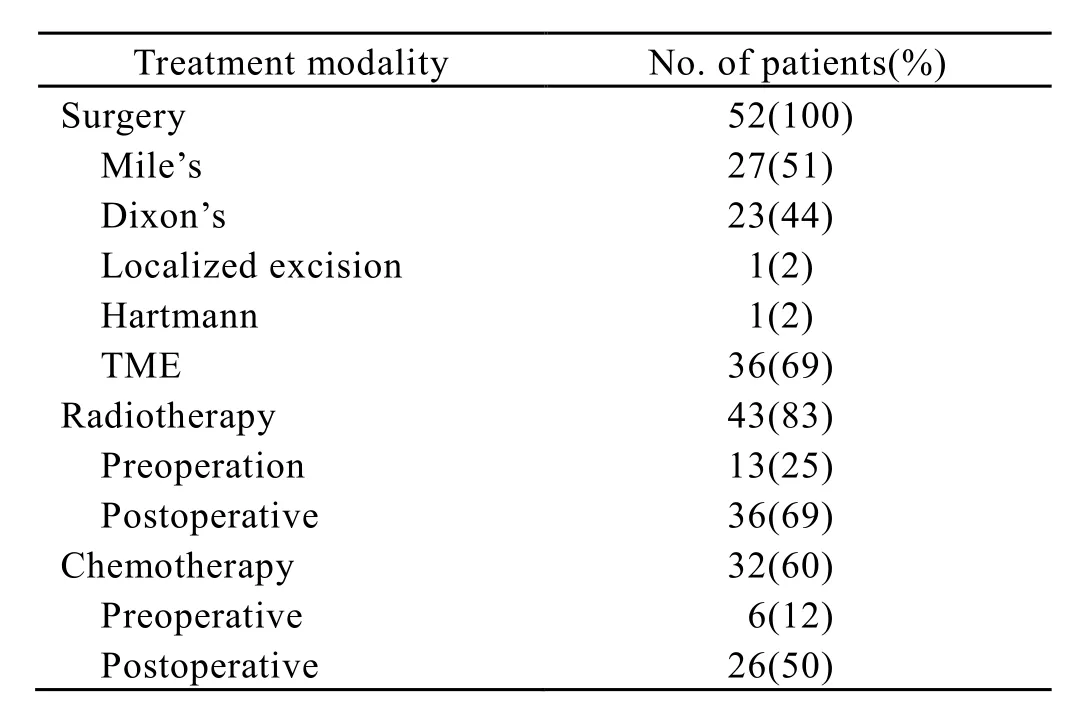
Table 2. Treatment modality
Immunohistochemistry
Formalin-fixed paraffin-embedded tissue sections were deparaffinized with xylene, and rehydrated in descending concentrations of ethanol and cooked for 20 min in citrate buffer (pH=6.0). Endogenous peroxidase activity was suppressed with 3% H2O2for 20 min. Sections were serum-blocked (with normal goat serum) and incubated with anti-Oct-4 polyclonal antibody (3576; 1∶50 dilution; BioVision) for 1 h at room temperature, and then stained with goat ABC staining system kit (Santa Cruz). Negative controls were done using an IgG2b isotype antibody (Dako),ensuring the same concentration of immunoglobins as for the anti-Oct-4. The staining intensity of Oct-4 was rated as follows∶ negative expression (<5% positive cells) and positive expression (≥5% positive cells).
Statistical Analysis
The relationship between Oct-4 expression and clinical variables was examined with chi-square tests. Local recurrence-free, metastasis-free, and overall survival rates were performed by the Kaplan-Meier method and log-rank test. For all tests, a two-sidedP<0.05 was considered significant. The duration of local recurrence-free was defined from the day of treatment beginning to the day of local recurrence or last follow-up. The duration of metastasis-free was defined from the day of treatment beginning to the day of distant metastasis appearance or last follow-up. Overall survival time was defined from the day of treatment beginning to the day of death or last follow-up.
RESULTS
Association of Oct-4 Expression and Clinicopathologic Characteristics
Oct-4 protein was detected as a dark brown precipitate and the staining was confined to the cytoplasm of tumor cells. The level of Oct-4 expression ranged from 0 to 18.5%. Negative and positive expressions of Oct-4 were recognized in 38 (73.1%) and 14 (26.9%) patients, respectively (Figure 1A,1B). There were no significant association between Oct-4 expression and gender (P=0.772), age (P=0.123), clinical stage (P=0.391), and histological grades (P=0.056) (Table 3).

Figure 1. Expression of Oct-4 by immunohitochemistry in rectal adenocarcinoma (magnification ×400). A∶ negative expression; B∶ positive expression.

Table 3. Association between Oct-4 and clinicopathologic features
Clinical Outcomes and Prognosis Analysis
The median duration of follow-up for these patients was 34 months (ranging, 2-42 months). The 3-year local recurrence-free, metastasis-free, and overall survival rates of all patients were 81.4%, 81.2%, and 70.0%, respectively. The 3-year local recurrence-free rates with negative and positive expression of Oct-4 were 83.5% and 75.0%, respectively (χ2=0.3,P=0.583; Figure 2A). The 3-year metastasis-free rates with negative and positive expression of Oct-4 were 88.6% and 61.9%, respectively (χ2=4.47,P=0.035; Figure 2B). The 3-year overall survival rates with negative and positive expression of Oct-4 were 77.9% and 49.0%, respectively (χ2=4.35,P=0.037; Figure 2C). All clinical outcomes are summarized in Table 4.

Table 4. Prognostic value of Oct-4 expression by the Kaplan-Meier method
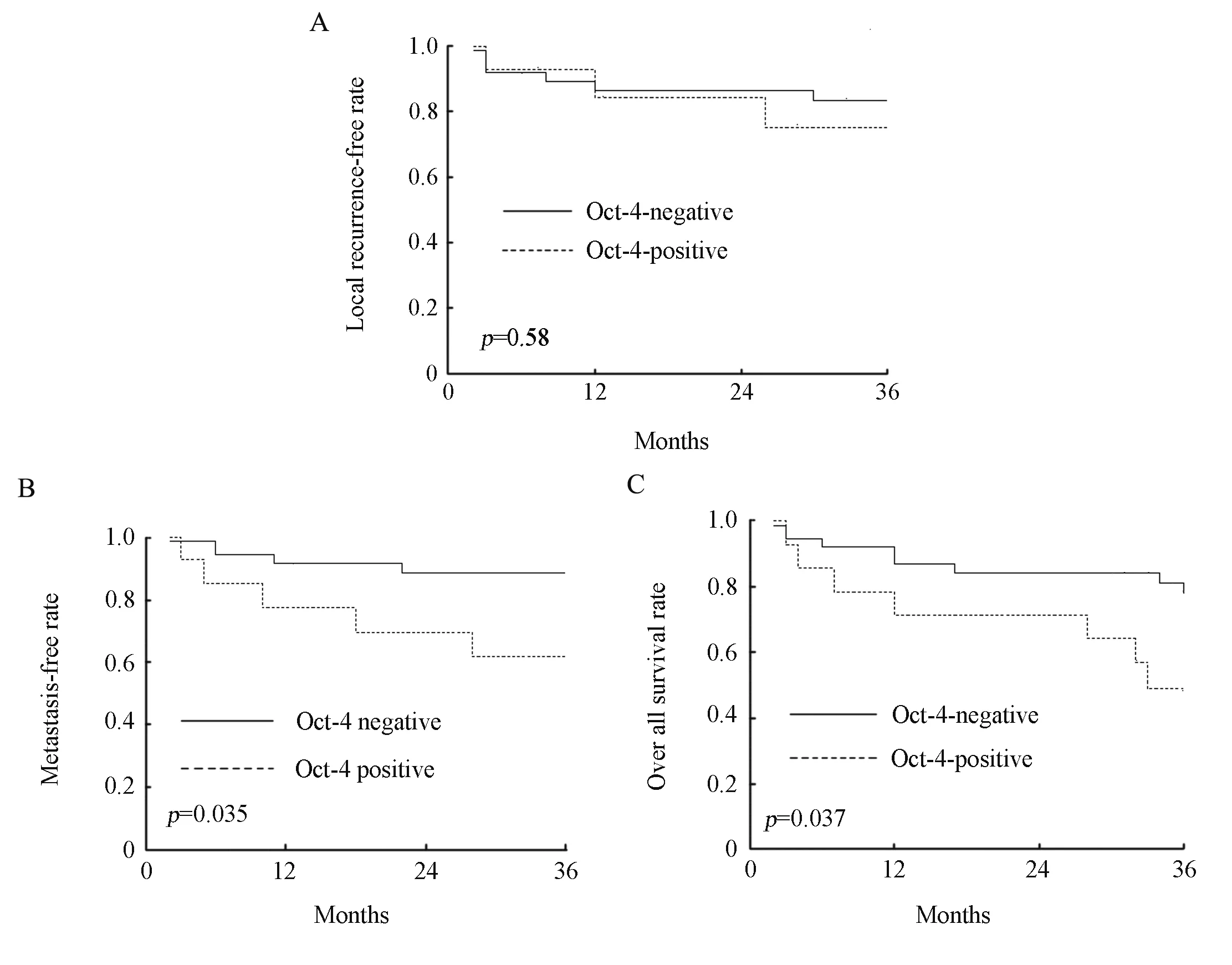
Figure 2. The local recurrence-free rate, metastasis-free rate and overall survival rate of the patients with rectal adenocarcinoma. A∶ Kaplan-Meier plot showed that the local recurrence-free rate of patients had no significance between negative and positive expression of Oct-4; B∶ Kaplan-Meier plot showed that the metastasis-free rate of patients with positive expression of Oct-4 was significantly lower than that in patients with negative expression of Oct-4; C∶ Kaplan-Meier plot showed that the overall survival rate of patients with positive expression of Oct-4 was significantly more unfavorable than that in patients with negative expression of Oct-4.
DISCUSSION
The hallmarks of CSC are self renewal, undifferentiated status, and capability to differentiate into heterogeneous mature cell types[4,15]. Oct-4 has been suggested as one of the important factors that render the reprogramming capability of adult cells into germ-line-competent induced pluripotent stemcells[16,17]. Recent evidences show that Oct-4 is highly expressed in several cancers[12-13,18]. Atlasi et al. have shown that Oct-4 expression by immunohistochemical staining is high in tumor biopsies of bladder cancer, with no or low immunoreactivity in nontumor cells[12]. Simlarly, Chiou et al. found that Oct-4 is also highly expressed in tumor biopsies of oral squamous cell carcinoma from 52 consecutive patients[18,19]. In the present study, we found that positive staining (≥5% positive cells) of Oct-4 was detected in 26.9% patients with rectal adenocarcinoma, with a variable proportion of positive tumor cells. To our knowledge, this study presented here is the first report of Oct-4 expression in tumor tissues of rectal carcinoma. We also identified that Oct-4 staining was confined to the cytoplasm of tumor cells in rectal carcinoma. Interestingly, the study of Atlasi et al. revealed that Oct-4 expression had a cytoplasmic distribution in some samples of bladder cancer, but Oct-4 was primarily localized in the nuclei of tumor cells[12]. These results showed that the intensity of expression and subcellular localization were variable among positive cells, suggesting that the cells within the tumors are heterogenous in term of Oct-4 expression.
In the current study, we also tried to explore the prognostic significance of Oct-4 expression in these patients. The Kaplan-Meier plots showed that positive expression of Oct-4 was associated with a higher distant metastasis (P=0.035), and a worse overall survival (P=0.037), but the level of Oct-4 expression had no impact on local control. Similarly, Chiou et al. investigated the expression of Oct-4 in patients with oral squamous cell carcinoma[18]. The results showed that the Oct-4 immunohistochemistry-positive cases were associated with a considerably worse overall survival rate compared with the negative ones. Moreover, patients with double-positive (Oct-4/ Nanog) or triple-positive (Oct-4/Nanog/CD133) expression predicted the worse survival rate compared with other oral cancer patients[18]. At same time, the results also showed that high expression of Oct-4 was positively correlated with the advanced stages and medium to poor differentiation of oral cancers. In contrast, our research revealed that there was no significant association between Oct-4 expression and clinical stages (P=0.391), and histological grades (P=0.056). It may be potentially limited by several factors. One is the relatively small number of patients in this retrospective study, and therefore the results may not be the most representative for the whole cases. Another factor is that the biological character of rectal adenocarcinoma may be different from that of oral squamous cancer. Taken together, these results indicate that abnormal expression of Oct-4 in tumor tissues might play a vital role in tumor transformation, tumorigenicity, and tumor metastasis. Guo et al found that Oct-4 is critical for survival/ antiapoptosis of murine embryonic stem cells through the STAT3/Survivin pathway[20], and the results partly revealed the mechanism through which Oct-4 gene regulates the tumor biology through the pathway of cancer stem cells.
In conclusion, the results suggest that embryonic stem cell marker Oct-4 expression may have prognostic significance in patients with rectal adenocarcinoma.
REFERENCES
[1] Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006[J]. CA Cancer J Clin 2006; 56∶ 106-30.
[2] Ieta K, Tanaka F, Haraguchi N, et al. Biological and genetic characteristics of tumor-initiating cells in colon cancer[J]. Ann Surg Oncol 2008; 15∶ 638-48.
[3] O’Brien CA, Pollett A, Gallinger S, et al. A human colon cancer cell capable of initiating tumor growth in immunodeficient mice[J]. Nature 2007; 445∶106-10.
[4] Jordan CT, Guzman ML, Noble M. Cancer stem cells[J]. N Engl J Med 2006; 355∶ 1253-61.
[5] Rich JN. The cancer stem cell∶ a new therapeutic paradigm[J]? Expert Rev Anticancer Ther 2006; 6∶1531-3.
[6] Bao S, Wu Q, Mclendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response[J]. Nature 2006; 444∶756-60.
[7] Collins AT, Berry PA, Hyde C, et al. Prospective identification of tumorigenic prostate cancer stem cells[J]. Cancer Res 2005; 65∶ 10946-51.
[8] Zeppernick F, Ahmadi R, Campos B, et al. Stem cell marker CD133 affects clinical outcome in glioma patients[J]. Clin Cancer Res 2008; 14∶ 123-9.
[9] Rosner MH, Vigano MA, Ozato K, et al. A POU-domain transcription factor in early stem cells and germ cells of the mammalian embryo[J]. Nature 1990; 345∶ 686-92.
[10] Schöler HR, Ruppert S, Suzuki N, et al. New type of POU domain in germ line-specific protein Oct-4[J]. Nature 1990; 344∶ 435-9.
[11] Covello KL, Kehler J, Yu H, et al. HIF-2α regulates Oct-4∶ effects of hypoxia on stem cell function, embryonic development, and tumor growth[J]. Genes & Development 2006; 20∶ 557-70.
[12] Atlasi Y, Mowla SJ, Ziaee SA, et al. Oct-4, an embryonic stem cell marker, is highly expressed in bladder cancer[J]. Int J Cancer 2007; 120∶ 1598-02.
[13] Gibbs CP, Kukekov VG, Reith JD, et al. Stem-like cells in bone sarcomas∶ implications for tumori-genesis[J]. Neoplasia 2005; 7∶ 967-76.
[14] Gidekel S, Pizov G, Bergman Y, et al. Oct-3/4 is a dose-dependent oncogenic fate determinant[J]. Cancer Cell 2003; 4∶ 361-70.
[15] Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells- perspectives on current status and future directions∶ AACR workshop on cancer stem cells[J]. Cancer Res 2006; 66∶ 9339-44.
[16] Okita K, Ichisaka T, Yamanaka S. Generation of germ-line-competent induced pluripotent stem cells[J]. Nature 2007; 448∶ 313-7.
[17] Park IH, Zhao R, West JA, et al. Reprogramming of human somatic cells to pluripotency with defined factors[J]. Nature 2008; 451∶ 141-6.
[18] Chiou SH, Yu CC, Huang CY, et al. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma[J]. Clin Cancer Res 2008; 14∶ 4085-95.
[19] Ezeh UI, Turek PJ, Reijo RA, et al. Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are expressed in both seminoma and breast carcinoma[J]. Cancer 2005; 104∶ 2255-65.
[20] Guo Y, Mantel C, Hromas RA, et al. Oct-4 is critical for survival/antiapoptosis of murine embryonic stem cell subjected to stress∶ effects associated with Stat3/Survivin[J]. Stem Cells 2008; 26∶ 30-4.
R735.3 Document code: A Article ID: 1000-9604(2010)02-0106-06
10.1007/s11670-010-0106-8
2009−11−26; Accepted 2010−02−23
This work was supported by the grant from Jiangsu Natural Science Program(No. BK2009126).
*Corresponding author.
E-mail∶ luxueguanok@yahoo.com.cn
© Chinese Anti-Cancer Association and Springer-Verlag Berlin Heidelberg 2010
杂志排行
Chinese Journal of Cancer Research的其它文章
- Triptolide Inhibits Cell Growth and Induces G0- G1 Arrest by Regulating P21wap1/cip1 and P27 kip1 in Human Multiple Myeloma RPMI-8226 Cells
- Different Outcome of Myeloid Sarcoma with Spinal Cord Compression Preceding Acute Myeloid Leukemia: Report of Two Cases and Review of Literatures
- Effects of Triptolide on Histone Acetylation and HDAC8 Expression in Multiple Myeloma in vitro
- CGI-100 Specific shRNA Inhibits Proliferation and Induces Differentiation in Leukemia K562 Cells
- Differential Diagnosis of Warthin's Tumor Complicated with Lung Adenocarcinoma by 18F- FDG PET/CT Imaging and Radioisotope Scanning with Tc-99m Pertechnetate: A Case Report and Literature Review
- Synergistic Action of fMLP-boanmycin Combination on the Growth of Mouse Colon Carcinoma and Its Action Mechanisms
