Genetic Polymorphism of PSCA and Risk of Advanced Precancerous Gastric Lesions in a Chinese Population
2010-07-18HongmeiMuChenWuLianZhangKaifengPanJunlingMaYangZhangWenqingLiHuakangTuHongmeiZengWeidongLiuTongZhouDongxinLinWeichengYou
Hong-mei Mu, Chen Wu, Lian Zhang*, Kai-feng Pan, Jun-ling Ma, Yang ZhangWen-qing Li, Hua-kang Tu, Hong-mei Zeng, Wei-dong Liu, Tong ZhouDong-xin Lin, Wei-cheng You
1Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Department of Epidemiology, Peking University School of Oncology, Beijing Cancer Hospital & Institute, Beijing 100142, China;2Department of Etiology and Carcinogenesis, Cancer Institute and Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China;3Linqu Public Health Bureau, Linqu, Shandong, China
Genetic Polymorphism of PSCA and Risk of Advanced Precancerous Gastric Lesions in a Chinese Population
Hong-mei Mu1, Chen Wu2, Lian Zhang1*, Kai-feng Pan1, Jun-ling Ma1, Yang Zhang1Wen-qing Li1, Hua-kang Tu1, Hong-mei Zeng1, Wei-dong Liu3, Tong Zhou1Dong-xin Lin2, Wei-cheng You1
1Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Department of Epidemiology, Peking University School of Oncology, Beijing Cancer Hospital & Institute, Beijing 100142, China;2Department of Etiology and Carcinogenesis, Cancer Institute and Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China;3Linqu Public Health Bureau, Linqu, Shandong, China
Objective:To evaluate the relationship between the genetic polymorphism of prostate stem cell antigen (PSCA) and the risk of advanced precancerous gastric lesions including intestinal metaplasia(IM) and dysplasia(Dys), a population-based study was conducted in Linqu County, a high-risk area of gastric cancer (GC) in China.
Methods:The prevalence of gastric lesions including superficial gastritis(SG), chronic atrophic gastritis(CAG), IM and Dys was determined by histopathologic examination. The genotypes were determined by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) technique. The effects ofPSCAgenetic variant on the risks of IM and Dys were calculated by unconditional logistic regression.
Results:Multivariate analysis revealed subjects carryingPSCArs2294008 CT/TT genotype were associated with an increased risk of IM (OR=1.38, 95% CI=1.11-1.71) and Dys (OR=1.75, 95% CI=1.36-2.26), especially for subjects withH.pyloriinfection (IM∶ OR=1.34, 95% CI=1.05-1.71; Dys∶ OR=1.82, 95% CI=1.37-2.42). Furthermore,H. pyloriinfection andPSCArs2294008 CT/TT genotype were observed to jointly elevate the risk of IM (OR=3.32, 95% CI=2.33-4.71) and Dys (OR=4.58, 95% CI=2.99-7.04).
Conclusion:This study suggested that PSCA rs2294008 might have an impact on the risk of IM or Dys among the high risk population of GC.
Polymorphism; Prostate stem cell antigen; Advanced precancerous gastric lesions;Helicobacter pylori
INTRODUCTION
Gastric cancer (GC) is one of the most common cancers in the world[1]. Linqu County, Shandong Province, China, has one of the highest mortality ratesof GC in the world (70/105for males)[2]. In this region, chronic atrophic gastritis (CAG) was nearly universal, while intestinal metaplasia (IM) and dysplasia (Dys) affected 33% and 20% of the adult residents, respectively[2]. Studies have shown that the process of intestinal gastric carcinogenesis involves a continuous stepwise evolution from glandular atrophy to IM, followed by Dys, and finally, to carcinoma[3,4]. Previous studies in Linqu identified several environmental risk factors associated with IM, Dys or GC, includingHelicobacter pylori(H. pylori)infection, smoking, consumption of sour pancakes or salty foods[5-7]. In addition, the genetic factors play a crucial role in GC and precancerous gastric lesions pathogenesis as well[8,9].
The prostate stem cell antigen (PSCA) protein is a cell surface antigen originally identified in the Los Angeles prostate cancer-4 (LAPC-4) xenograft mouse[10].PSCAis located on chromosome 8q24.2, encoding a 123-amino-acid glycoprotein[10]. In humans,PSCAexpression is largely restricted to the prostate, bladder, stomach and oesophagus[10,11]. In the stomach,PSCAis predominantly expressed in the isthmus, the middle portion of the gastric epithelium where contains stem cells and precursors of three cell lineages (pit-cell lineage, parietal-cell lineage, and zymogenic-cell lineage)[12]. Also,PSCAexpression associated with proliferation activities[12]. Studies showed thatPSCAexpression was suppressed in gastric cancer cells[11-13].
PSCArs2294008 is located in the first exon. This genetic polymorphism changes the amino acid, which may decrease transcriptional activity ofPSCAand lead to a change in the function ofPSCAprotein[12].
In the present study, we investigated genetic polymorphism ofPSCAwith the risk of IM and Dys in a Chinese population, and its joint effect with environmental factors in the risks of advanced precancerous gastric lesions.
MATERIALS AND METHODS
Study Population
A large population-based cross-sectional study of precancerous gastric lesions in Linqu in 1989 was described previously[3,14]. Briefly, a total of 3,433 subjects participated in an endoscopic examination; representing 83% of the eligible residents aged 34 to 65 in 14 villages selected at random in Linqu County. Blood samples were collected from all subjects. During the examination, each subject was interviewed with a structured questionnaire to obtain the information on cigarette and alcohol consumptions, diet, socioeconomic status, and other variables[15].
The study was approved by the Institutional Review Board of the National Cancer Institute and the Beijing Institute for Cancer Research, and all subjects provided written informed consent.
For the current study, a total of 2350 subjects were randomly selected. A total of 1169 subjects with SG and CAG in the control were assayed compared with subjects who had IM (n = 739) or Dys (n = 442).
Gastric Histopathology
Details of the pathologic procedures and classification criteria of SG, CAG, IM, Dys, and quality control procedures were provided elsewhere[3,14]. Briefly, three experienced gastroenterologists carried out the endoscopic examinations using fiber-optic gastroscopes (Olympus, Tokyo, Japan). Seven biopsies were obtained from standard locations of the stomach, two in the body, one in the angulus, and four in the antrum. Then, three senior pathologists from Beijing Institute for Cancer Research made histopathologic diagnoses. The presence or absence of SG, CAG, IM or Dys was recorded for each biopsy, and each subject was assigned a global diagnosis based on the most severe lesion among the seven biopsies.
H. pyloriAntibody Assays
Details of serologic assay were previously described[6]. In brief,H. pyloristrains cultured from gastric biopsies of two patients in Linqu were used to provide a local antigen preparation for serology. SerumH. pyloriIgG and IgA antibody concentrations were measured by ELISA at baseline at Beijing Institute for Cancer Research. An individual was considered the positive ofH. pyloriinfection if the mean ELISA absorbance reading for either the IgG or IgA was above 1.0, a cutoff value based on negative control. Quality-control samples were assayed at Vanderbilt University.
DNA Preparation
The blood clot was washed extensively with TE buffer (50 mmol/L Tris-HCl, pH 8.5 and 1 mmol/L EDTA). After centrifugation, the pellet was incubated with rotation in the lysis buffer (TE buffer containing 0.2% SDS and 200 µg/ml proteinase K) for 12 h at 55°C. The lysate was then extracted with phenolchloroform and precipitated with isopropyl alcohol. The precipitate was washed with 70% ethanol and dissolved in TE buffer. The purity and concentration of DNA were determined by spectrophotometry at A260nmand A280nm.
PCR Amplification and Determination of Genotypes
PSCArs2294008 genotypes were determined by polymerase chain reaction –restriction fragment length polymorphism (PCR-RFLP) technique, using the PCR primer pairs∶ F5’-GAAACCCGCTGGT GTTGACTGT-3’/R5’-GGGCAAGCAGCACAGCCC-3’. PCR products were digested with Nco I (New England BioLabs, Inc., Beverly, MA),and digestedproducts were separated on 3.5% agarose gel containing ethidium bromide and visualized under UV light. Genotypes revealed by PCR-RFLP were further confirmed by DNA sequencing of the PCR products, and genotyping was conducted without information of subjects’ histopathologic diagnoses. The reaction was started at 95°C for 2 min followed by 35 PCR cycles. The temperatures for denaturing, annealing, and elongation in each cycle were 94°C (30s), 66°C (30s) and 72°C (30s), respectively. At the end, the reaction was extended for 10 min at 72°C. PCR was accomplished with a 25-µl reaction mixture containing 100ng of genomic DNA, 0.1mmol/L of each primer, 0.2 mmol/L of dNTP , 1.0 U Taq DNA polymerase in 2×GC bufferⅠ (5mol/L Mg2+Plus).
Quality Control Procedures
Rigorous quality control procedures were applied throughout the genotyping process. The coding and analysis of DNA samples were double-blinded. Two investigators checked sample codes and data entry into the electronic database. To avoid PCR contamination, reagents for PCR reaction were carefully aliquoted and each aliquot was used no more than three times. For each assay, a negative control (with no DNA template) was added to monitor PCR contamination. Pilot experiments were conducted to optimize the restriction digestion conditions. After genotyping of all samples, approximately 10% to 15% of the samples in each genotype group were randomly selected for repeated assays to validate the results. The average concordance rate was 99.9% (range 97-100%).
Statistical Methods
The Hardy-Weinberg equilibrium equation was used to determine whether the proportion of each genotype obtained was in agreement with the expected values as calculated from allele frequencies. The difference in age between the SG/CAG and IM or Dys was evaluated with the Mann-Whitney U test. The χ2test was used to examine the differences between gender,H. pyloriinfection, smoking and drinking. Odds ratios (ORs) and 95% confidence intervals (CIs) for the association of polymorphism with IM or Dys were computed by unconditional logistic regression, adjusting for age, gender,H. pyloriinfection, smoking and drinking. All statistical tests were two-sided and the level of significance was set at 0.05. Rothman’s synergy index (S) was calculated to determine the joint effect ofPSCAgenotypes andH. pyloriinfection on the risk of IM or Dys[16,17]. The index (S) was calculated as follows[18]∶
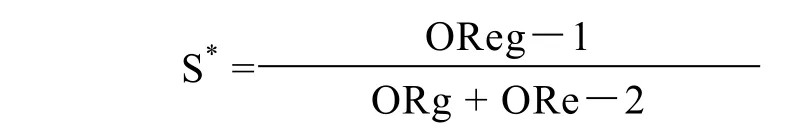
*S>1.0, positive interaction; S=1.0, no interaction; S<1.0 negative interaction
The OR for subjects exposed to both environmental and genetic factors in comparison to those not exposed to the two factors was defined as OReg. The ORs for subjects exposed to genetic factors only or environmental factors only were defined as ORg and ORe, respectively. These analyses were carried out with Statistical Analysis System Software (version8.0; SAS Institute, Cary, NC).
RESULTS
The proportion of gastric lesions in the study population (SG/CAG∶ 49.7%; IM∶ 31.5%; Dys∶18.8%) was similar to that in the population of this region[2]. The baseline characteristics of subjects are shown in Table 1. The information on smoking status, drinking status andH. pyloriseropositivity was available for 2178, 2177 and 2066 subjects, respectively. The distributions of cigarette smoking and alcohol drinking between SG/CAG and IM or Dys were similar. The distributions of gender between SG/CAG and IM were similar. However, the distributions of age between SG/CAG and IM was different (P<0.001). The distributions of age and gender in Dys were different (P<0.001) from that in the SG/CAG. The percentages ofH. pyloriinfection were higher in IM or Dys than in SG/CAG (P<0.001, Table 1).
The frequencies ofPSCArs2294008 TT, CT, and CC genotypes were 7.6%, 32.9%, and 59.5% respectively among the 1169 subjects with SG/CAG. The allele frequencies for the T and C were 24.1% and 75.9% in SG/CAG, respectively. The genotype distributions of the polymorphism of all subjects fitted the Hardy-Weinberg equilibrium. The frequencies ofPSCArs2294008 TT, CT, and CC genotypes were 7.7%, 41.7%, and 50.6% among 739 subjects with IM and 7.2%, 46.2%, and 46.6% among 442 subjects with Dys respectively (Table 2). Elevated risks of IM and Dys were found for CT carriers. The ORs were 1.47 (95% CI∶ 1.17-1.85) and 1.92 (95% CI∶ 1.47-2.50), respectively. Dominant genetic model revealed that subjects with CT/TT genotype were associated with increased risks of IM (OR∶ 1.38, 95% CI∶ 1.11-1.71) and DYS (OR∶ 1.75, 95% CI∶1.36-2.26) (Table 2).
The risks of IM and Dys associated withPSCArs2294008 were further examined with stratificationbyH. pyloriinfection (Table 3). The association ofPSCArs2294008 CT/TT genotype with risk of IM was more pronounced for subjects withH. pyloriinfection (OR 1.34, 95% CI 1.05-1.71). Similar results were observed for Dys. Subjects withPSCArs2294008 CT/TT genotype andH. pyloriinfection have an elevated risk of Dys (OR 1.82, 95% CI 1.37-2.42). (Table 3).
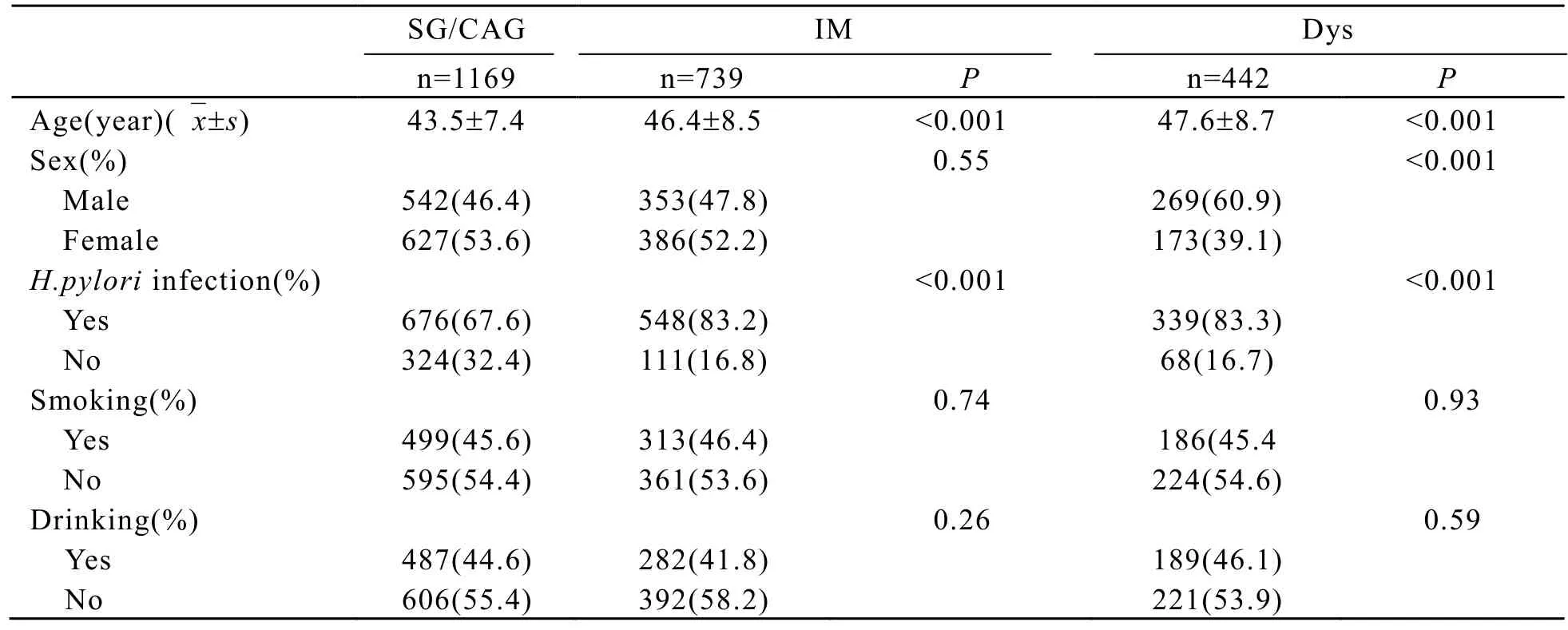
Table 1. The baseline characteristics of subjects with gastric precancerous lesions
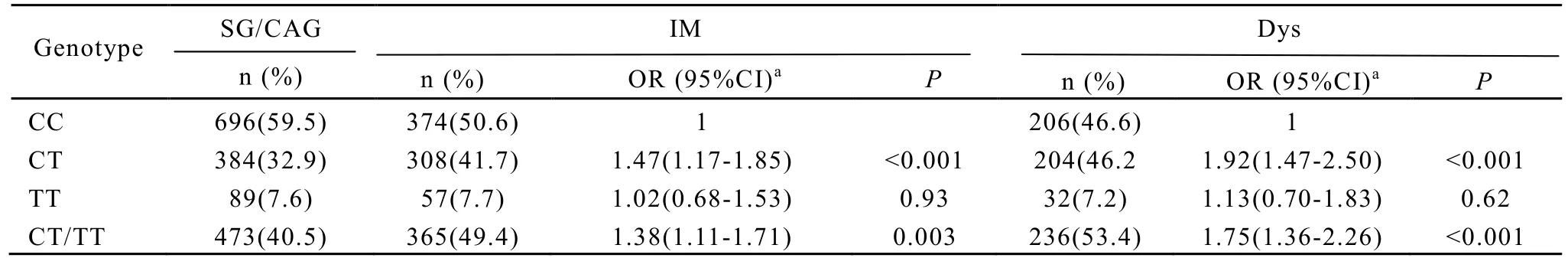
Table 2. The frequencies of PSCA rs2294008 in SG/CAG, IM and Dys
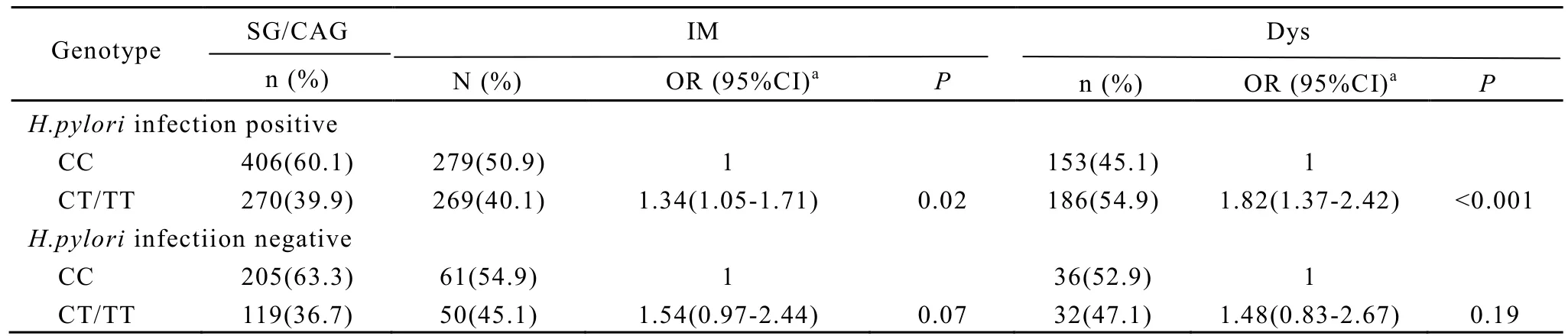
Table 3. Risks of IM and Dys Associated PSCA-ThrlMet by H.pylori Infection
Furthermore, we examined the joint effects ofPSCArs2294008 andH. pyloriinfection. Compared with carriers of CC genotype withoutH. pyloriinfection, the subjects of TT or CT genotype withoutH. pyloriinfection did not increase the risks of IM and Dys. However, among the subjects who carried the TT/CT genotype and were infected withH. pyloriinfection, there was a more than 2-fold elevated risk for IM (OR 3.32, 95% CI, 2.33-4.71) and a more than 3-fold increased risk for Dys (OR 4.58, 95% CI, 2.99-7.04) in this study (Table 4). The Rothman’s synergy indices of the interaction betweenPSCArs2294008 CT/TT genotype andH. pyloriinfection were 1.21 in subjects with IM and 1.85 in subjects with Dys, respectively.
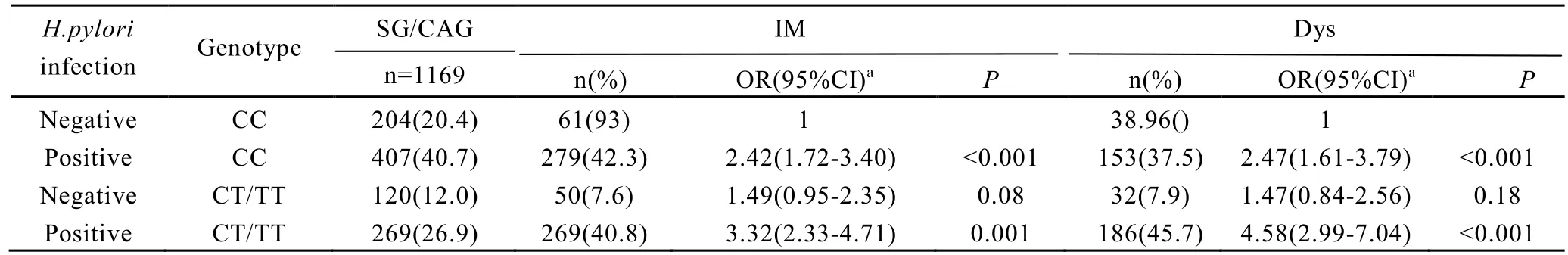
Table 4. The joint effect of H.pylori infection and PSCA genotypes
DISCUSSION
Previous study showed thatPSCArs2294008 was associated with risk of GC[19]. In our present study, a relatively large sample was selected from a Chinese population with a high mortality of GC to evaluate the associations between genetic polymorphism ofPSCAand risk of advanced precancerous gastric lesions. Our study indicated elevated risk of IM or Dys correlated withPSCArs2294008 CT/TT genotype, especially for subjects withH. pyloriinfection. To our best knowledge, this is the first study that explores the impact ofPSCArs2294008 on the risk of advanced precancerous gastric lesions.
Sakamoto et al[19]reported that subjects carrying at least onePSCArs2294008 T allele were associated with an increased risk of GC(diffuse GC∶ OR=1.67, 95%CI=1.47-1.90; intestinal GC∶ OR=1.29, 95%CI=1.11-1.49,P<0.001). In the present study, we found that subjects who carriedPSCArs2294008 CT/TT genotype had an increased risk for advanced precancerous gastric lesions (IM and Dys). Present study provides a population-base evidence of the relationship betweenPSCArs2294008 polymorphism and gastric cancer.
ThePSCAgene was originally identified in the prostate cancer cells in 1998[20]. Studies reportedPSCAwas highly expressed in a large proportion of human prostate tumors, which had an importance association with the occurrence of prostate cancer[20-22]. But the subsequent series of studies indicated thatPSCAwas suppressed in the cancers of the bladder, esophagus, skin and stomach[19,23,24]. Sakamoto et al[19]found that among gastric cancer tissues, quantitative RT-PCR and immunohistochemical analyses showed frequent suppression ofPSCAexpression in both the intestinal and diffuse types. In vitro, the growth of GC cells with stablePSCAexpression was slower than the GC cells withoutPSCAexpression[19], which suggested thatPSCAmay have a tumor suppressor-like character in gastric cancers.
The regulation mechanism ofPSCAexpression is largely unknown. Sakamoto et al[19]sequencedPSCAand its 5’ upstream region and identified 17 SNPs, including a missense SNP located at the presumed translation-initiating codon, rs2294008. Substitution of C with T atPSCArs2294008 can alter the threonine to methionine. This genetic variation changes the length of the N-terminal signal peptide, which may alter the protein folding, intracellular processing, and subcellular localization. Meanwhile, research showed that rs2294008 T-allele can decrease transcriptional activity of the upstream region ofPSCA[19].
Similar with the evidences of previous functional study[19]or association studies[19,23], our study provided evidence thatPSCArs2294008 polymorphism may have an important role in the development of IM and Dys. Previous studies in Linqu indicated thatH. pyloriinfection, cigarette smoking, and low levels of dietary vitamin C were the important environmental factors for GC[7,14]. In the present study, stratified analysis found that the presence of bothPSCArs2294008 CT/TT genotype andH. pyloriinfection significantly elevated the risks of IM and Dys.
H. pyloriis a bacterium that colonizes in the human gastric epithelial cells. The infection wasdemonstrated as an important etiological factor of GC and precancerous gastric lesions[6,7,25]. We have conducted a randomized intervention trial in Linqu Country and found that eradication ofH.pyloricaused a significant decrease in the combined prevalence of severe CAG, IM and Dys[26]. However, the outcome of subjects withH. pyloriinfection is highly variable, and only a small fraction of infected individuals (probably<3%) developed GC[27], which may be influenced by both microbial and host factors[28]. Accumulating evidences have shown thatH. pylorican modulate cell proliferation, which plays an important role in the development of GC and precancerous gastric lesions[29]. Several studies have suggested thatPSCAinvolved in the cell growth regulation[19,30-33].PSCArs2294008 CT/TT genotype andH. pyloriinfection may co-contribute to the development of IM and Dys by modulating cell proliferation. Further studies are needed to test this hypothesis.
In conclusion, this study suggested thatPSCArs2294008 CT/TT genotype increased the genetic susceptibility of IM and Dys, especially for subjects withH. pyloriinfection, suggesting that this polymorphism may play a role in the occurrence of advanced precancerous gastric lesions.
REFERENCES
[1] Parkin D M, Muir C S. Cancer Incidence in Five Continents. Comparability and quality of data[J]. IARC Sci Publ 1992; 120∶ 45-173.
[2] You WC, Blot WJ, Li JY, et al. Precancerous gastric lesions in a population at high risk of stomach cancer[J]. Cancer Res 1993; 53∶1317-21.
[3] You WC, Li JY, Blot WJ, et al. Evolution of precancerous lesions in a rural Chinese population at high risk of gastric cancer[J]. Int J Cancer 1999; 83∶615-9.
[4] Correa P. A human model of gastric carcinogenesis [J]. Cancer Res 1988; 48∶3554-60.
[5] You WC, Blot WJ, Chang YS, et al. Diet and high risk of stomach cancer in Shandong, China[J]. Cancer Res 1988; 48∶3518-23.
[6] Zhang L, Blot WJ, You WC, et al. Helicobacter pylori antibodies in relation to precancerous gastric lesions in a high-risk Chinese population[J]. Cancer Epidemiol Biomarkers Prev 1996; 5∶ 627-30.
[7] You WC, Zhang L, Gail MH, et al. Gastric dysplasia and gastric cancer∶ Helicobacter pylori, serum vitamin C, and other risk factors[J]. J Natl Cancer Inst 2000; 92∶1607-12.
[8] Kato S, Onda M, Matsukura N, et al. Genetic polymorphisms of the cancer related gene and Helicobacter pylori infection in Japanese gastric cancer patients. An age and gender matched case-control study[J]. Cancer 1996; 77(8 Suppl)∶1654-61.
[9] Gonzalez C A, Sala N, Capella G. Genetic susceptibility and gastric cancer risk[J]. Int J Cancer 2002; 100∶249-60.
[10] Reiter RE, Gu Z, Watabe T, et al. Prostate stem cell antigen∶ a cell surface marker overexpressed in prostate cancer[J]. Proc Natl Acad Sci USA 1998; 95∶1735-40.
[11] Bahrenberg G, Brauers A, Joost HG, et al. Reduced expression of PSCA, a member of the LY-6 family of cell surface antigens, in bladder, esophagus, and stomach tumors[J]. Biochem Biophys Res Commun 2000; 275∶783-8.
[12] Sakamoto H, Yoshimura K, Saeki N, et al. Genetic variation in PSCA is associated with susceptibility to diffuse-type gastric cancer[J]. Nat Genet 2008; 40∶730-40.
[13] Lee J Y, Eom E M, Kim D S, et al. Analysis of gene expression profiles of gastric normal and cancer tissues by SAGE[J]. Genomics 2003; 82∶ 78-85.
[14] Kneller RW, You WC, Chang YS, et al. Cigarette smoking and other risk factors for progression of precancerous stomach lesions[J]. J Natl Cancer Inst 1992; 84∶1261-6.
[15] You WC, Blot WJ, Chang YS, et al. Allium vegetables and reduced risk of stomach cancer[J]. J Natl Cancer Inst 1989; 81∶162-4.
[16] Rothman K J. Synergy and antagonism in cause-effect relationships[J]. Am J Epidemiol 1974; 99∶385-8.
[17] Rothman K J. The estimation of synergy or antagonism[J]. Am J Epidemiol 1976; 103∶ 506-11.
[18] Semenza J C, Ziogas A, Largent J, et al. Geneenvironment interactions in renal cell carcinoma[J]. Am J Epidemiol 2001; 153∶851-9.
[19] Sakamoto H, Yoshimura K, Saeki N, et al. Genetic variation in PSCA is associated with susceptibility to diffuse-type gastric cancer[J]. Nat Genet 2008; 40∶730-40.
[20] Reiter RE, Gu Z, Watabe T, et al. Prostate stem cell antigen∶ a cell surface marker overexpressed in prostate cancer[J]. Proc Natl Acad Sci USA 1998; 95∶1735-40.
[21] Ross S, Spencer S D, Holcomb I, et al. Prostate stem cell antigen as therapy target∶ tissue expression and in vivo efficacy of an immunoconjugate[J]. Cancer Res 2002; 62∶ 2546-53.
[22] Gu Z, Thomas G, Yamashiro J, et al. Prostate stem cell antigen (PSCA) expression increases with high gleason score, advanced stage and bone metastasis in prostate cancer[J]. Oncogene 2000; 19∶1288-96.
[23] Lee JY, Eom EM, Kim DS, et al. Analysis of gene expression profiles of gastric normal and cancer tissues by SAGE[J]. Genomics 2003; 82∶ 78-85.
[24] Bahrenberg G, Brauers A, Joost H G, et al. Reduced expression of PSCA, a member of the LY-6 family of cell surface antigens, in bladder, esophagus, and stomach tumors[J]. Biochem Biophys Res Commun 2000; 275∶783-8.
[25] Schistosomes, liver flukes and Helicobacter pylori.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994[J]. IARC Monogr Eval Carcinog Risks Hum 1994; 61∶1-241.
[26] You WC, Brown LM, Zhang L, et al. Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions[J]. J Natl Cancer Inst 2006; 98∶ 974-83.
[27] Peek RM, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas[J]. Nat Rev Cancer 2002; 2∶ 28-37.
[28] Blaser MJ. Polymorphic bacteria persisting in polymorphic hosts∶ assessing Helicobacter pylorirelated risks for gastric cancer[J]. J Natl Cancer Inst 2002; 94∶ 1662-3.
[29] Ding SZ, Smith MF, Goldberg JB. Helicobacter pylori and mitogen-activated protein kinases regulate the cell cycle, proliferation and apoptosis in gastric epithelial cells[J]. J Gastroenterol Hepatol 2008; 23(7 Pt 2)∶ 67-78.
[30] Gu Z, Yamashiro J, Kono E, et al. Anti-prostate stem cell antigen monoclonal antibody 1G8 induces cell death in vitro and inhibits tumor growth in vivo via a Fc-independent mechanism[J]. Cancer Res 2005; 65∶9495-500.
[31] Saffran DC, Raitano AB, Hubert RS, et al. Anti-PSCA mAbs inhibit tumor growth and metastasis formation and prolong the survival of mice bearing human prostate cancer xenografts[J]. Proc Natl Acad Sci USA 2001; 98∶ 2658-63.
[32] Tran C P, Lin C, Yamashiro J, et al. Prostate stem cell antigen is a marker of late intermediate prostate epithelial cells[J]. Mol Cancer Res 2002; 1∶113-21.
[33] Sharom FJ, Radeva G. GPI-anchored protein cleavage in the regulation of transmembrane signals[J]. Subcell Biochem 2004; 37∶ 285-315.
R735.2 Document code: A Article ID: 1000-9604(2010)02-0099-07
10.1007/s11670-010-0099-3
2009−09−08; Accepted 2010−01−12
This work was supported by a grant from the Program of National Natural Science Foundation of China(No.30772515); the National“863” High-Tech Res & Dev Program of China (No. 2006A A02A402)
*Corresponding author.
E-mail∶ zhanglmail@yahoo.com.cn
© Chinese Anti-Cancer Association and Springer-Verlag Berlin Heidelberg 2010
杂志排行
Chinese Journal of Cancer Research的其它文章
- Cortactin Overexpression Correlates with Poor Prognosis in Hepatocellular Carcinoma
- Glioma-conditioned Medium Blocks Endothelial Cells’ Apoptosis Induced by Hypoxia and Promotes Its Angiogenesis via Up-regulation of u-PA/u-PAR
- Synergistic Action of fMLP-boanmycin Combination on the Growth of Mouse Colon Carcinoma and Its Action Mechanisms
- Differential Diagnosis of Warthin's Tumor Complicated with Lung Adenocarcinoma by 18F- FDG PET/CT Imaging and Radioisotope Scanning with Tc-99m Pertechnetate: A Case Report and Literature Review
- Expression of Embryonic Stem Cell Marker Oct-4 and Its Prognostic Significance in Rectal Adenocarcinoma
- CGI-100 Specific shRNA Inhibits Proliferation and Induces Differentiation in Leukemia K562 Cells
