Clinical management of hepatitis B virus infection correlated with liver transplantation
2010-06-29JianZhangLinZhouandShuSenZheng
Jian Zhang, Lin Zhou and Shu-Sen Zheng
Hangzhou, China
Clinical management of hepatitis B virus infection correlated with liver transplantation
Jian Zhang, Lin Zhou and Shu-Sen Zheng
Hangzhou, China
BACKGROUND:As a radical cure for post-hepatitis B virus (HBV)-related liver cirrhosis and hepatocellular carcinoma, liver transplantation has been applied in many medical centers. Before the use of effective measures, hepatitis B recurrence and the existence of HBsAg(+) donors, patients with hepatitis B-related diseases are contraindicated for liver transplantation. Application of interferon, hepatitis B immunoglobulin (HBIG), and nucleotide analogues (e.g., lamivudine) has made great progress in the clinical care of HBV. However, there are still many shortcomings such as low viral suppression rate, rising expense, and the induction of HBV tyrosine-methionineaspartate-aspartate (YMDD) mutation. This article systematically reviews the current evidence that immunotherapy, conventional drug combinations, and some special fields of HBV infection correlate with liver transplantation.
DATA SOURCES:Studies were identified by searching MEDLINE and PubMed for articles using the keywords "hepatitis B virus", "hepatitis B vaccination", "lamivudine", "adefovir", "entecavir", "tenofovir", "HBV genotype", and "liver transplantation" up to October 2009. Additional papers were identified by a manual search of the references from the key articles.
RESULTS:Hepatitis B vaccine and human monoclonal antibody have very good clinical prospects. Compared with traditional therapies, the new medical regimens have many benefits such as boosting viral suppression rate and decreasing medical expenses. The triple therapy for YMDD mutation also has an excellent therapeutic effect and a low barrier to resistance. New nucleos(t)ide analogues (entecavir and tenofovir) eliminate virus more effectively with few adverse reactions, and may replace lamivudine or HBIG in future.CONCLUSIONS:Hepatitis B vaccine needs further largescale and rigorous randomized controlled trials to confirm its effective dose and injection frequency. Monoclonal antibody is still experimental, and the next step is to carry out the relevant animal and human studies. A consensus standard regimen for the treatment of hepatitis B should be developed.
(Hepatobiliary Pancreat Dis Int 2010; 9: 15-21)
hepatitis B vaccination;hepatitis B immunoglobulin; lamivudine; liver transplantation; adefovir; hepatitis B virus; genotype
Introduction
Hepatitis B is a viral infection that attacks the liver and can cause both acute and chronic diseases. About 2 billion people worldwide have been infected with the virus and about 350 million live with chronic infection. An estimated number of 600 000 persons die each year from the acute or chronic consequences of hepatitis B.
Liver transplantation is a radical cure for posthepatitis B virus (HBV)-related cirrhosis and hepatocellular carcinoma. In Japan, the morbidity of post-HBV-related liver cirrhosis is about 13.9%.[1]In China, HBV related diseases is the most common primary reason for liver transplantation (78.6%).[2]Although liver transplantation can alleviate clinical symptoms such as ascites, hepatic encephalopathy, hypoalbuminemia, and infection, the adverse effects of immunosuppressive drugs raise the possibility of HBV recurrence or virus breakthrough.[3]Choosing the right prophylactic drug to control the HBV DNA load at an acceptable level is very important.
Immunotherapy is a promising treatment that can enhance the body's ability to resist viruses. Traditional non-specific immune therapy is a passive one such as HBIG. Then studies have been concentrated onthe specific immune therapy for stimulating active immunity-vaccines. The development of new vaccines that stimulate an effective immune response when patients receive an organ transplant is a hot topic. Recently, Pan et al[4]synthesized engineered mAbs that showed inhibition of HBVin vitro.
Various monotherapies and combinations have been developed for the treatment of hepatitis B.[5,6]These drugs have played important therapeutic roles, and they also have inherent disadvantages such as adverse reactions, hepatitis B recurrence, and long-term drugresistant strains. Viral resistance mechanisms and the pharmacoeconomics of combination therapy have been reported.[7]
Immunotherapy
Hepatitis B vaccination: dose and phase?
Inducing an active immune response against the hepatitis B surface antigen (HBsAg) leads to continuous secretion of specific antibodies. The vaccine is given in either three or four separate doses as part of the existing routine immunization schedule. The safe anti-HBs titer for continuous prevention of HBV reinfection varies in some studies. Relatively low seroconversion rates and serum anti-HBs concentrations are reported among chronic HBV-infected liver transplantation recipients; only a minority of vaccines may have stable antibody levels >100 IU/L.[8]In the past, anti-HBs-positive individuals (seropositivity: anti-HBs titer of >10 IU/L) have always been considered safe to receive orthotopic liver transplantation for non-HBV-related diseases. Five of ninede novoHBV-infected patients were anti-HBs positive before orthotopic liver transplantation.[9]So, for prevention ofde novoHBV infection after transplantation, the protective anti-HBs titer should be >200 IU/L.[10]
It has been reported that the response rate to HBV vaccine is significantly lower in patients with liver cirrhosis, immunocompromised patients, or organ transplant recipients.[8]When discussing immune status including T-cell and B-cell activity with response rate, Tahara et al[11]found that T-cell interactions which lead to an effective immune response to hepatitis B vaccination can cause activation of anti-HBsAg-specific T cells and suppression of anti-donor-specific T cells. Patients showing a donor-specific hyporesponse with a well-maintained response to a third-party (exogenous) stimulus always have a sustained immune response to the vaccine. Another study on T-cell immunity found that in 12 orthotopic liver transplantation recipients with HBV recurrence, the HBV-specific T-cell immunity was enhanced to a level comparable to that of patients with chronic hepatitis B, and the level was dependent on the serum viral load. Thus this raises an interesting question whether the higher response in theresearch of Tahara et al is really due to the donor-specific hyporesponse or just to the activity of anti-HBsAgspecific T cells or both. Schumann et al[12]demonstrated that HBV-specific humoral and cellular immunity can be transferred by liver transplantation after vaccination of the donor. The transfer of B-cell and T-cell immunity correlates with the magnitude of the immune responses in the donor.
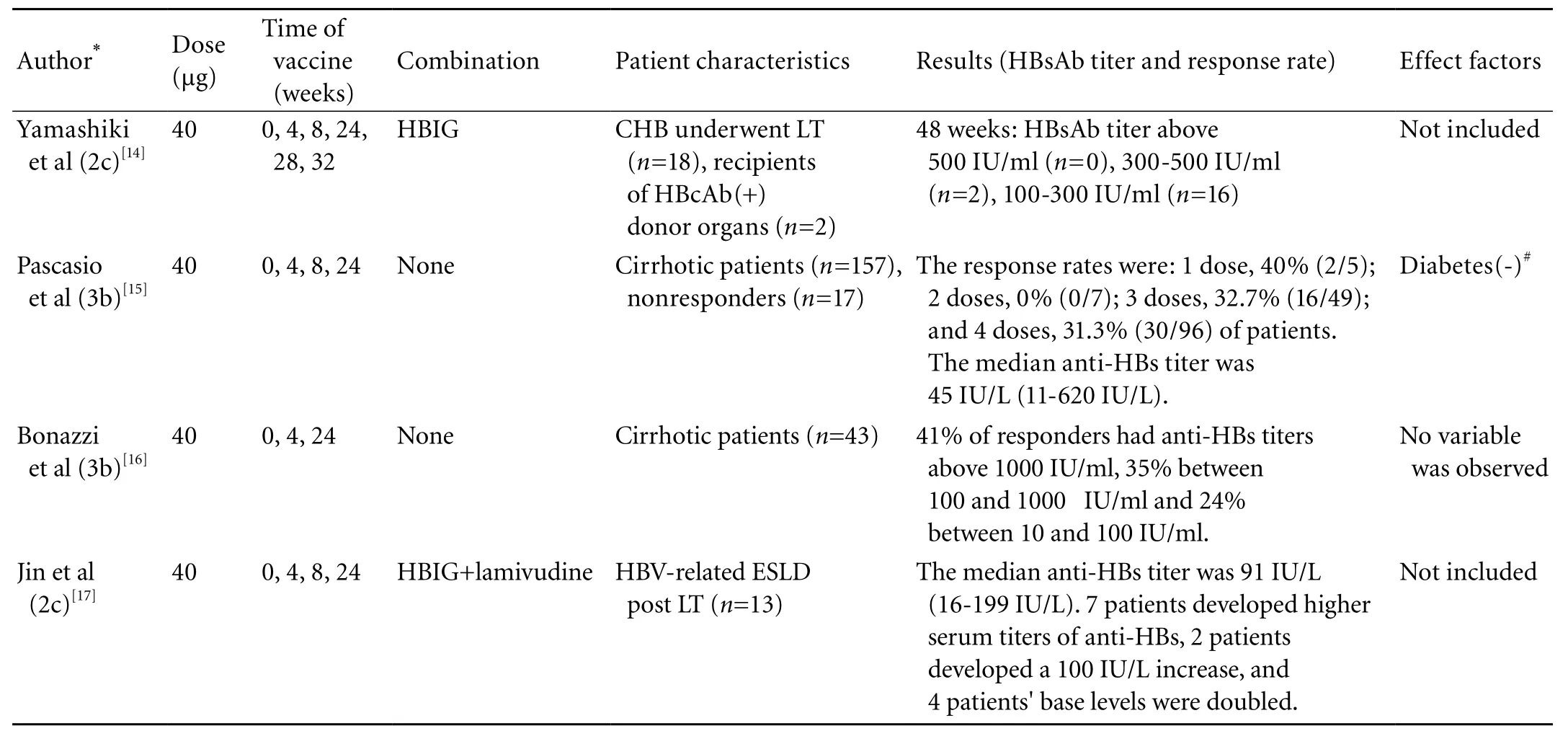
Table. Comparison of four studies of hepatitis B vaccination
The response rate of liver transplantation candidates (with HBV-unrelated liver failure) to recombinant hepatitis B vaccination varies from 16% to 62%.[13]Many variables are associated with the response rate, the most important being the injection frequency and dose per injection. The Table shows four different studies of hepatitis B vaccination. Although these studies may be incompletely randomized or lack proper controls they show that 3-4 doses (40 μg), which can stimulate the necessarily response rate (30%-41%), are optimal for vaccination. But the HBsAb titer of responders varies a lot. Larger randomized controlled trials with good quality controls and design will provide more distinct answers about the dose, phase and response rate of hepatitis B vaccination.
In special patients, the existing routine immunization schedules may not be equally effective as in normal people. Yamashiki et al[14]investigated 18 patients who underwent liver transplantation for chronic hepatitis B and 2 non-HBV-infected patients who received hepatitis B core antibody (HBcAb)-positive donor organs; doubledose double-phase use of second-generation recombinant vaccine was not effective in preventing hepatitis B recurrence in the study population. These populations should be targeted for a conventional vaccine regimen, and different approaches such as strong adjuvant or pre-S containing protein should be further tested.
Vaccination also shows differences in sex, both in adult and pediatric liver transplantation groups. Girls respond with higher anti-HBs titres than boys, as do women.[18,19]It has become an interesting controversy whether the treatment is individualized to different ethnicities or groups of people. Ni et al[19]found that more than half of the children who were primarily vaccinated with HBV vaccines maintained adequate anti-HBs titers 1 year later after liver transplantation. All consecutive patients maintain a certain degree of cellular immune response irrespective of their anti-HBs status after liver transplantation. We expect that one or more doses of booster may help children who receive transplantation to develop protective response to anti-HBs.
Human mAb: a novel approach?
High specificity monoclonal antibodies to pathogens such as human papilloma virus have greatly expanded therapeutic methodology. Genetically engineered mAbs specific to the surface antigens of HBV would be a good alternative for the immunoprophylaxis of HBV infection.[20]Thus Pan et al[4]cloned the gene of a high Fab-affinity antibody from human peripheral blood and constructed Fab antibody rAAV-HB. After evaluating the activity of antibody hepatitis B in tree shrews treated with intramuscular injection of rAAV-HB andin vitroexperiments, they concluded that human antibody will be useful for the immunoprophylaxis of HBV infection, but this needs further study in human experiments. The safety and efficiency of transgenic drugs is controversial, which may restrict the use of engineered monoclonal antibodies.
Drug regimens
HBIG and lamivudine combination: low-dose intramuscular or high-dose intravenous HBIG?
The HBIG (10 000 IU per month) and lamivudine regimen used to be the gold standard for prophylaxis against HBV recurrence after liver transplant. This therapy reduces the risk of recurrence to less than 5% at 5 years.[21]However, the cost of HBIG has driven people to investigate a lowdose of intramuscular HBIG (400-800 IU per month) and lamivudine in combination. This combination can achieve the same prophylaxis effect at a lower cost.[22]In Tashiro's research,[23]although two patients were preoperatively HBV DNA-positive shown by a transcription-mediated amplification assay method, all 14 patients receiving a high dose (10 000 IU/day) of HBIG and lamivudine in combination postoperatively became HBV-DNA-negative and HBsAg-negative.
In Chinese liver transplant patients, the efficacy of HBIG was found to be associated with human leukocyte Fcγ receptor 3A gene polymorphisms (at nucleotide site 559). Among the 559G carrier group (n=42, 54.5%), the risk of HBV recurrence was 9.5%, and the 1- and 2-year recurrence-free survival rates were 95.2% and 88.7%, respectively. In the 559G noncarrier group (n=35, 45.5%), the risk of HBV recurrence was 28.6%, and the 1-and 2-year recurrence-free survival rates were 74.3% and 69.3%. Detecting FCGR3A genotypes can be a clinical reference for treatment before HBIG administration.[24]
Hooman et al[25]pointed out that administration of intravenous HBIG (2000 IU per six weeks) and intramuscular administration of HBIG (2000 IU per six weeks) are equally effective with antibody against hepatitis B surface antigen (anti-HBs) in pharmacokinetics. Intramuscular HBIG may not be dose-saving; however, it may be cost-effective if the price per unit of intramuscular HBIG is lower. Individualized dosing intervals should be further evaluated as a cost-effective alternative to fixed dose schemes.
Saab et al[26]compared prophylaxis with lamivudine and adefovir (strategy 1), with intramuscular HBIG and lamivudine (strategy 2) with the addition of adefovir in patients who subsequently developed hepatitis B recurrence. The medical costs for strategies 1 and 2 after 10 years of therapy were $151819 and $166246, respectively, and the cost saving was $14427. They developed a Markov model to provide pharmacoeconomic support for the use of strategy 1 as first-line therapy in hepatitis B prophylaxis in liver transplant recipients 1 year after transplantation. Strategy 1 not only decreased the total fee for preventing hepatitis B after liver transplantation, but also improved the quality of life. Administration of HBIG requires frequent blood tests to assess antibody titers. On the other hand, adefovir is an oral medication with rare adverse effects and less demand for frequent monitoring.
High-dose HBIG during the ahepatic period and in the early stage of post transplantation can fulfill the treatment target as a long-term lamivudine therapy before liver transplantation. Long-term preoperative lamivudine treatment may result in an earlier HBV mutation in YMDD and increase the HBV recurrence rate and risk in the first year after transplantation.[27]Yilmaz et al[28]found that recurrence can be prevented in E-antigen-negative patients with HBIG alone or in E-antigen-positive patients with a combination of HBIG and an anti-viral agent. Eight out of fifteen E-antigen-positive patients who received HBIG alone developed recurrence after a mean of 17 months. As long as the anti-HBV surface remained detectable, no absolute minimum serum level appeared to lead to recurrent HBV. But the question is raised whether this combination or use of HBIG alone should be continued within 1 year after operation.
Polyvalent immunoglobulin can cause nephrotoxicity. Pathologic examination of the kidneys generally reveals changes typical of osmotic nephrosis. The inappropriate use of HBIG can increase the risk of renal dysfunction, particularly in combination with nephrotoxic drugs.[29]
Lamivudine and adefovir dipivoxil regimen: effective and easy to get
Lamivudine and adefovir represent two different classes of anti-HBV agents (L-nucleoside and acyclic phosphonate) with different mechanisms of action, and there is no cross-resistance between them. Their combination greatly reduces the risk of subsequent adefovir resistance in nontransplantation patients.[30]Prophylaxis combined with adefovir/lamivudine therapy without the use of long-term HBIG is effective and well tolerated.[31]Meanwhile we cannot exclude the possibility that with a prolonged follow-up, some patients may experience viral breakthrough due to the appearance of lamivudine/adefovir resistance.[5]
Before liver transplantation, lamivudine was proposed to be down-graded from the first to second-line therapy. In contrast, adefovir dipivoxil has been approved not only as the first-line therapy but also as rescue therapy for patients with lamivudine resistance.[32]A retrospective cohort study evaluated the safety and efficacy of adefovir in 68 elderly and cirrhotic patients with lamivudine-resistant chronic hepatitis B, among whom 75.4% received a combination treatment with lamivudine and adefovir for a median duration of 12.6 months; the remaining patients received adefovir overlapping with lamivudine treatment for a median duration of 7.9 months. At the end of the follow-up, 41.2% of the patients had undetectable HBV DNA (≤2000 copies/ml) with a median reduction of lg3.4 copies/ ml. No resistance mutations and no significant side effects were observed.[33]Even though no combination treatment can cover all the possible mutation strains, we focus on reducing the resistance as much as possible to get a good prognosis.
A seven-year follow-up study of 24 patients showed that HBV recurrence developed in 7 patients with cumulative probabilities of 8%, 13%, 28%, 35%, 35%, and 49% in 1, 2, 3, 4, 5, and 6 years. Four patients had rtM204I mutation, and in 3 of them HBV DNA levels were too low for sequencing. After use of lamivudine followed by adefovir salvage for 150 weeks (91-193), 6 (86%) patients had normal ALT levels. HBV DNA was undetected in 2 (29%) patients, 100-1000 copies/ml in 2 (29%) and 10 000-100 000 copies/ml in 3 (43%) on the last visit. No genotypic resistance to adefovir was detected,[34]showing that lamivudine followed by adefovir salvage is effective for prophylaxis of recurrence of HBV for up to 7 years after liver transplantation.
Management of YMDD mutant: lamivudine, adefovir and HBIG triple therapy
YMDD mutation is common in the application of lamivudine in treating HBV. In 183 adult liver transplantation patients who lived more than 6 months and were followed up for 14.6 months, the rate of YMDD mutation in the lamivudine group was 8.49% (9/106), markedly higher than that in the lamivudine+HBIG group (1.30%, 1/77;P=0.035). The patients with YMDDmutation improved after treatment with adefovir.[35]
4.烤箱预热至160度,取烤盘、上置一烤架,鸡置于烤架上(鸡背向上),烤盘置于烤箱最下层,烘烤时间为每1000克鸡烤35-40分钟。每过45分钟将烤盘中的汤汁和鸡油,收集起来倒在另一盆内。同时将鸡翻一次身。
In patients with YMDD mutation at the time of transplantation, combined use of HBIG and lamivudine is not consistently successful in preventing reinfection. In this situation, the addition of another antiviral agent combating the drug-resistant virus (i.e., adefovir, tenofovir, or entecavir) would be expected to further reduce the risk of prophylaxis failure.[36]
As described in a ten-year study from Ikegami et al,[37]patients with YMDD mutation (9 of 29 patients), except an HBsAg-positive donor, were successfully protected by the triple therapy of lamivudine, adefovir, and HBIG. No graft loss was due to the recurrence of HBV. YMDD mutation should be closely monitored during lamivudine treatment.[38]
Other oral nucleos(t)ide analogues: entecavir and tenofovir
Entecavir is a guanosine nucleotide analogue. It is a new, highly potent antiviral agent; phase Ⅱ and Ⅲstudies have demonstrated this drug to be superior to placebo and lamivudine in patients with chronic HBV.[39]Because of the low resistance barrier, it used to be a substitute for lamivudine-resistant patients. Kurashige et al[40]recommended that lamivudine-to-entecavir switching treatment may be suitable in chronic hepatitis B patients without evidence of lamivudine resistance during the preceding lamivudine treatment with great attention to the emergence of entecavir-resistance.
Kamar et al[41]reported that 10 male transplant patients with chronic HBV infection who were adefovir-(n=9) or lamivudine-resistant (n=1) were given entecavir at 0.5 to 1 mg/d. All patients were HBsAg-positive. After a median follow-up of 16.5 months, entecavir therapy reduced the HBV DNA viral load from lg3.86 (lg2.71-lg6.46) copies/ml at baseline to lg2.94 (lg2.15-lg4) copies/ml at the last follow-up (P=0.004). The host biological response was better in kidney transplant patients than in liver transplant patients because HBV has a liver tropism which may be more resistant and more aggressive in a transplanted liver than in a native one.[41]This study showed good results of entecavir in treating kidney or liver transplant patients, which may be used in treatment of liver-kidney transplantation patients.
Tenofovir is an acyclic nucleotide analog, reverse transcriptase inhibitor.[42]Zhu et al[43]compared different regimens of tenofovir with lamivudine, entecavir, and adefovir. They found that of combinations lamivudine+tenofovir, entecavir+tenofovir, and adefovir+ tenofovir showed additive anti-HBV effectsin vitro. This supports the use of tenofovir as a component in combination regimens with currently available anti-HBV nucleoside analogues.
Benefits and risks of combination therapy
Many reports describe the benefits and risks of hepatitis B drug treatment. This section just mentions some special points of liver transplantation. Combination therapy for hepatitis B is recommended in specific patients: those who have undergone liver transplantation, those with decompensated cirrhosis, those coinfected with human immunodeficiency virus and HBV who are on antiretroviral therapy, and those with drug-resistant HBV infection.[44]In successful antiviral combination therapy, different drug regimens may cover major virus mutation pathways to minimize possible drug resistance. However, investigators still find many different mutations in response to the first line drugs such as lamivudine with rtM204V, rtL180M, and rtS202G mutations.[40]These mutations may cause virus recurrence and lead to transplant failure. Thus it is important to monitor the status of patients during therapy. Monitoring of treatment response includes tests for serum aminotransferase level, HBV DNA level, hepatitis B e antigen (HBeAg) and antibody (anti-HBe), hepatitis B surface antigen (HBsAg) or antibody (anti-HBs), and liver histology.[45]Early discovery of possible drug resistance may contribute to a good prognosis of liver transplantation.
Genetic factors and drug therapy: individual treatment
Eight distinct genotypes of HBV A to H have been identified, each having a characteristic geographic distribution.[46,47]Each genotype can be divided into many subtypes like B1-B4, and C1-C4. In China, B2 and C2 predominate.[48]Genotype can effect graft survival rate and the cumulative rate of viral breakthrough. Lo et al[49]found that genotype B patients have more pre-transplant acute flare, poorer liver functions, and higher end-stage liver disease scores. Fewer genotype B patients have HBeAg (13% vs. 32%;P=0.017). The 3-year graft survival rate is 83% for genotype B and 89% for genotype C (P=0.2). The graft survival due to lamivudine-resistant mutation at 3 years is 4% for genotype B and 21% for genotype C (P=0.017). Liver biopsy after viral breakthrough showed recurrent hepatitis B in 7 of 10 genotype C patients.
Gene polymorphism also contributes to host immune behavior to HBV and drugs, such as mutations of the gene coding for the major hydrophilic region of HBsAg may be associated with anti-HBs immunoglobulins or vaccine escape.[50]In Chinese liver transplantation, the organ recipients with CTLA-4 +49 GG genotype have a reduced risk (6.67%,n=11) of HBV recurrence comparedwith non-CTLA-4 +49 GG-carrying individuals (20.7%) (relative risk 3.098;P=0.032).[51]
After ending of the human genome project, the concept of molecular medicine has emerged. The evidence mentioned above can lead to early diagnosis and genetic prediction of HBV infection or recurrence. Before prescribing a drug in future we may review the genetic characteristics of a patient and decide the best choice of therapy.
In conclusion, further research is needed to provide evidence-based recommendations about optimal antiviral therapy in adults with chronic hepatitis B infection. Monoclonal antibodies are still in the experimental stage, and the next step is taken to do relevant animal and human studies. A consensus on a standard regimen for the treatment of hepatitis B should be achieved.
Funding:This study was supported by grants from the Key Program of National Natural Science Foundation of China (30730085) and the National High Technology Research and Development Program of China (863 Program; 2006AA 02A412).
Ethical approval:Not needed.
Contributors:ZJ wrote the main body of the article under the supervision of ZL and ZSS. ZL and ZSS provided advice on medical aspects. ZSS is the guarantor.
Competing interest:No bene fits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Michitaka K, Nishiguchi S, Aoyagi Y, Hiasa Y, Tokumoto Y, Onji M, et al. Etiology of liver cirrhosis in Japan: a nationwide survey. J Gastroenterol 2009.
2 Zhao J, Yan L, Li B, Zeng Y, Wen T, Zhao J, et al. Diabetes mellitus after living donor liver transplantation: data from mainland China. Transplant Proc 2009;41:1756-1760.
3 Lok AS. How to diagnose and treat hepatitis B virus antiviral drug resistance in the liver transplant setting. Liver Transpl 2008;14:S8-S14.
4 Pan T, Cai M, Tang L, Zhou LQ, Li BJ, Zhu T, et al. A novel approach of prophylaxis to HBV recurrence after liver transplantation. Virology 2008;382:1-9.
5 Angus PW, Patterson SJ, Strasser SI, McCaughan GW, Gane E. A randomized study of adefovir dipivoxil in place of HBIG in combination with lamivudine as post-liver transplantation hepatitis B prophylaxis. Hepatology 2008;48:1460-1466.
6 Zhang FK, Zhang Y, Zhang JY, Jia JD, Wang BE. Favorable outcome of de novo hepatitis B infection after liver transplantation with lamivudine and adefovir therapy. Transpl Infect Dis 2009;11:549-552.
7 Avolio AW, Nure E, Pompili M, Barbarino R, Basso M, Caccamo L, et al. Liver transplantation for hepatitis B virus patients: long-term results of three therapeutic approaches. Transplant Proc 2008;40:1961-1964.
8 Castells L, Esteban R. Hepatitis B vaccination in liver transplant candidates. Eur J Gastroenterol Hepatol 2001;13:359-361.
9 Bárcena Marugán R, García-Hoz F, Vázquez Romero M, Nash R, Mateos M, González Alonso R, et al. Prevention of de novo hepatitis B infection in liver allograft recipients with previous hepatitis B infection or hepatitis B vaccination. Am J Gastroenterol 2002;97:2398-2401.
10 Su WJ, Ho MC, Ni YH, Chen HL, Hu RH, Wu YM, et al. High-titer antibody to hepatitis B surface antigen before liver transplantation can prevent de novo hepatitis B infection. J Pediatr Gastroenterol Nutr 2009;48:203-208.
11 Tahara H, Tanaka Y, Ishiyama K, Ide K, Shishida M, Irei T, et al. Successful hepatitis B vaccination in liver transplant recipients with donor-specific hyporesponsiveness. Transpl Int 2009;22: 805-813.
12 Schumann A, Lindemann M, Valentin-Gamazo C, Lu M, Elmaagacli A, Dahmen U, et al. Adoptive immune transfer of hepatitis B virus specific immunity from immunized living liver donors to liver recipients. Transplantation 2009;87:103-111.
13 Takemura N, Sugawara Y, Tamura S, Makuuchi M. Liver transplantation using hepatitis B core antibody-positive grafts: review and university of Tokyo experience. Dig Dis Sci 2007; 52:2472-2477.
14 Yamashiki N, Sugawara Y, Tamura S, Kaneko J, Matsui Y, Togashi J, et al. Double-dose double-phase use of second generation hepatitis B virus vaccine in patients after living donor liver transplantation: Not an effective measure in transplant recipients. Hepatol Res 2009;39:7-13.
15 Pascasio JM, AoufiS, Gash A, Sousa JM, Perea R, Sayago M, et al. Response to a vaccination schedule with 4 doses of 40 microg against hepatitis B virus in cirrhotic patients evaluated for liver transplantation. Transplant Proc 2008;40:2943-2945.
16 Bonazzi PR, Bacchella T, Freitas AC, Osaki KT, Lopes MH, Freire MP, et al. Double-dose hepatitis B vaccination in cirrhotic patients on a liver transplant waiting list. Braz J Infect Dis 2008;12:306-309.
17 Jin SJ, Lu SC, Lai W, Dai J, Zhao J, Li YP, et al. Enhancing active immunity against hepatitis B virus by HBV vaccine immunization in patients with HBV-related end-stage liver diseases treated with liver transplantation. Zhonghua Gan Zang Bing Za Zhi 2008;16:261-264.
18 Fang JW, Lai CL, Chung HT, Wu PC, Lau JY. Female children respond to recombinant hepatitis B vaccine with a higher titre than male. J Trop Pediatr 1994;40:104-107.
19 Ni YH, Ho MC, Wu JF, Chen HL, Wu YM, Hu RH, et al. Response to booster hepatitis B vaccines in liver-transplanted children primarily vaccinated in infancy. Transplantation 2008;86:1531-1535.
20 Hong HJ, Ryu CJ, Hur H, Kim S, Oh HK, Oh MS, et al. In vivo neutralization of hepatitis B virus infection by an anti-preS1 humanized antibody in chimpanzees. Virology 2004;318:134-141.
21 Papatheodoridis GV, Cholongitas E, Archimandritis AJ, Burroughs AK. Current management of hepatitis B virus infection before and after liver transplantation. Liver Int 2009; 29:1294-1305.
22 Angus PW, McCaughan GW, Gane EJ, Crawford DH, Harley H. Combination low-dose hepatitis B immune globulin and lamivudine therapy provides effective prophylaxis against posttransplantation hepatitis B. Liver Transpl 2000;6:429-433.
23 Tashiro H, Itamoto T, Fudaba Y, Ohdan H, Fukuda S, KohashiT, et al. Prophylaxis against recurrence of HBV hepatitis after living-donor liver transplantation. Hepatogastroenterology 2008;55:1746-1749.
24 Wang WL, Zhang GL, Wu LH, Yao MY, Jin J, Jia CK, et al. Efficacy of hepatitis B immunoglobulin in relation to the gene polymorphisms of human leukocyte Fcgamma receptor III (CD16) in Chinese liver transplant patients. Chin Med J (Engl) 2007;120:1606-1610.
25 Hooman N, Rifai K, Hadem J, Vaske B, Philipp G, Priess A, et al. Antibody to hepatitis B surface antigen trough levels and half-lives do not differ after intravenous and intramuscular hepatitis B immunoglobulin administration after liver transplantation. Liver Transpl 2008;14:435-442.
26 Saab S, Ham MY, Stone MA, Holt C, Tong M. Decision analysis model for hepatitis B prophylaxis one year after liver transplantation. Liver Transpl 2009;15:413-420.
27 Lu AW, Zheng SS, Wu MP, Shen Y, Yang RW. Reevaluation of the effect of lamivudine therapy preoperative to prevent HBV recurrence after liver transplantation. Hepatobiliary Pancreat Dis Int 2008;7:357-361.
28 Yilmaz N, Shiffman ML, Todd Stravitz R, Sterling RK, Luketic VA, Sanyal AJ, et al. Prophylaxsis against recurrance of hepatitis B virus after liver transplantation: a retrospective analysis spanning 20 years. Liver Int 2008;28:72-78.
29 Angeli P, Scaglione F. Nephrotoxicity of intravenous immunoglobulin in the setting of liver transplantation or HBV-related cirrhosis: an undervalued topic. Minerva Gastroenterol Dietol 2008;54:259-275.
30 Lampertico P, Viganò M, Manenti E, Iavarone M, Sablon E, Colombo M. Low resistance to adefovir combined with lamivudine: a 3-year study of 145 lamivudine-resistant hepatitis B patients. Gastroenterology 2007;133:1445-1451.
31 Patterson SJ, Angus PW. Post-liver transplant hepatitis B prophylaxis: the role of oral nucleos(t)ide analogues. Curr Opin Organ Transplant 2009;14:225-230.
32 Jiang L, Jiang LS, Cheng NS, Yan LN. Current prophylactic strategies against hepatitis B virus recurrence after liver transplantation. World J Gastroenterol 2009;15:2489-2499.
33 Zoulim F, Parvaz P, Marcellin P, Zarski JP, Beaugrand M, Benhamou Y, et al. Adefovir dipivoxil is effective for the treatment of cirrhotic patients with lamivudine failure. Liver Int 2009;29:420-426.
34 Limquiaco JL, Wong J, Wong VW, Wong GL, Tse CH, Chan HY, et al. Lamivudine monoprophylaxis and adefovir salvage for liver transplantation in chronic hepatitis B: a seven-year follow-up study. J Med Virol 2009;81:224-229.
35 Zhang XL, Zhu XF, Shi HJ, Cui SZ, Tang YQ, Ba MC, et al. Prevention and treatment of hepatitis B recurrence after liver transplantation. Zhonghua Yi Xue Za Zhi 2008;88:606-609.
36 Samuel D. The option of liver transplantation for hepatitis B: where are we? Dig Liver Dis 2009;41:S185-189.
37 Ikegami T, Soejima Y, Ohta R, Taketomi A, Yoshizumi T, Harada N, et al. Living donor liver transplantation for hepatitis B associated liver diseases: a 10-year experience in a single center. Hepatogastroenterology 2008;55:1445-1449.
38 Gwak GY, Huh W, Lee DH, Choi MS, Lee JH, Koh KC, et al. The incidence and clinical outcome of YMDD mutants in hepatitis B surface antigen-positive renal allograft recipients after prolonged lamivudine therapy. Transplant Proc 2007;39: 3121-3126.
39 Calleja JL, Penas B. Enferm Infecc Microbiol Clin 2008;26:S39-48.
40 Kurashige N, Ohkawa K, Hiramatsu N, Yakushijin T, Mochizuki K, Oze T, et al. Lamivudine-to-entecavir switching treatment in type B chronic hepatitis patients without evidence of lamivudine resistance. J Gastroenterol 2009;44:864-870.
41 Kamar N, Milioto O, Alric L, El Kahwaji L, Cointault O, Lavayssière L, et al. Entecavir therapy for adefovir-resistant hepatitis B virus infection in kidney and liver allograft recipients. Transplantation 2008;86:611-614.
42 Reynaud L, Carleo MA, Talamo M, Borgia G. Tenofovir and its potential in the treatment of hepatitis B virus. Ther Clin Risk Manag 2009;5:177-185.
43 Zhu Y, Curtis M, Qi X, Miller MD, Borroto-Esoda K. Antihepatitis B virus activity in vitro of combinations of tenofovir with nucleoside/nucleotide analogues. Antivir Chem Chemother 2009;19:165-176.
44 Terrault NA. Benefits and risks of combination therapy for hepatitis B. Hepatology 2009;49:S122-128.
45 Andersson KL, Chung RT. Monitoring during and after antiviral therapy for hepatitis B. Hepatology 2009;49:S166-173.
46 Fung SK, Lok AS. Hepatitis B virus genotypes: do they play a role in the outcome of HBV infection? Hepatology 2004;40: 790-792.
47 Echevarría JM, Avellón A. Hepatitis B virus genetic diversity. J Med Virol 2006;78:S36-42.
48 Norder H, Couroucé AM, Coursaget P, Echevarria JM, Lee SD, Mushahwar IK, et al. Genetic diversity of hepatitis B virus strains derived worldwide: genotypes, subgenotypes, and HBsAg subtypes. Intervirology 2004;47:289-309.
49 Lo CM, Cheung CK, Lau GK, Yuen MF, Liu CL, Chan SC, et al. Significance of hepatitis B virus genotype in liver transplantation for chronic hepatitis B. Am J Transplant 2005; 5:1893-1900.
50 Roque-Afonso AM, Ferey MP, Belkhiri D, Dussaix E. HBs antigen mutants: prevalence, clinical and diagnostic implications. Pathol Biol (Paris) 2005;53:563-568.
51 Jiang Z, Feng X, Zhang W, Gao F, Ling Q, Zhou L, et al. Recipient cytotoxic T lymphocyte antigen-4 +49 G/G genotype is associated with reduced incidence of hepatitis B virus recurrence after liver transplantation among Chinese patients. Liver Int 2007;27:1202-1208.
October 22, 2009
Accepted after revision December 23, 2009
Author Affiliations: Key Laboratory of Combined Multi-Organ Transplantation, Ministry of Public Health; Key Laboratory of Organ Transplantation, Zhejiang Province; First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Zhang J, Zhouland Zheng SS)
Shu-Sen Zheng, MD, PhD, FACS, Key Laboratory of Combined Multi-Organ Transplantation, Ministry of Public Health, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Tel: 86-571-87236601; Email: shusenzheng@zju.edu.cn)
© 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
猜你喜欢
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Is bactibilia a predictor of poor outcome of pancreaticoduodenectomy?
- Oxidative stress and lipid peroxidation products: effect of pinealectomy or exogenous melatonin injections on biomarkers of tissue damage during acute pancreatitis
- Acinar cell carcinoma of the pancreas in a young patient with chronic pancreatitis
- Letters to the Editor
- Simplifying living donor liver transplantation
- Meetings and Courses
