Oxidative stress and lipid peroxidation products: effect of pinealectomy or exogenous melatonin injections on biomarkers of tissue damage during acute pancreatitis
2010-06-29CavitKahramanDinleruzHasdemirOktaykaikandlerBudayci
Cavit Çöl, Kahraman Dinler, Oğuz Hasdemir, Oktay Büyükaşik and Güler Buğdayci
Bolu, Turkey
Oxidative stress and lipid peroxidation products: effect of pinealectomy or exogenous melatonin injections on biomarkers of tissue damage during acute pancreatitis
Cavit Çöl, Kahraman Dinler, Oğuz Hasdemir, Oktay Büyükaşik and Güler Buğdayci
Bolu, Turkey
BACKGROUND:Melatonin (N-acetyl-5-methoxytripta-mine) is a free radical scavenger and a strong antioxidant, secreted by the pineal gland. In this study, we evaluated the effects of decreasing and increasing serum melatonin levels on malonyldialdehyde (MDA), superoxide dismutase (SOD), and reduced glutathione (GSH) levels in pancreatic tissue from rats with experimental acute pancreatitis.
METHODS:Experimental acute pancreatitis was induced in three groups of Wistar albino rats (10 animals per group) by pancreatic ductal ligation. The first group had only acute pancreatitis and served as the control. Surgical pinealectomy was added to acute pancreatitis in the second group, removing the source of endogenous melatonin (low melatonin levels group). The third group was given 0.1 ml daily intraperitoneal injections of 20 mg/ml melatonin solution for one week (high melatonin levels group). The effects of melatonin levels were evaluated by comparison of the levels of MDA, SOD, and GS in pancreatic tissue.
RESULT:We found that intraperitoneal melatonin injections decreased the levels of MDA and increased the levels of SOD and GSH in pancreatic tissue.
CONCLUSION:Exogenous melatonin has a preventive effect on lipid peroxidation and oxidative damage in acute pancreatitis. (Hepatobiliary Pancreat Dis Int 2010; 9: 78-82)
pineal gland; acute pancreatitis; melatonin; oxidative damage
Introduction
Oxygen-free radicals (OFRs) and products of oxidative cell damage are known as biomarkers that escalate in the physiopathology of many diseases. In the early stages of acute pancreatitis, hypovolemia develops due to liquid secretion into the peripancreatic area and abdominal cavity; in later stages, septic complications occur because of bacterial translocation. The extravasation of pancreatic secretions into the interstitial area activates enzymes and starts the process of autolysis.
As a result of prevalent edema, degeneration in the microcirculation, and cellular ischemia/reperfusion damage, lipid peroxidation products and OFRs begin to accrue within the tissue. Measuring the antioxidants produced against oxidative stress and the OFRs that play an important role in the physiopathology of the disease can give information about how serious the oxidative damage in the tissue is.
The role of OFRs in acute pancreatitis was first shown experimentally in 1984. In the following years, many studies were carried out on lipid peroxidation products and changes in glutathione metabolism.[1,2]However, the number of studies on the effects of melatonin and OFRs has been quite limited.
Melatonin (N-acetyl-5-methoxytriptamine) is a free radical scavenger and a strong antioxidant, secreted by the pineal gland. Melatonin impacts on toxic radicals independent of receptors and breaks down many free radicals, such as the highly toxic hydroxyl and peroxyl radicals, OFRs, and peroxynitrite anions. Moreover,melatonin affects receptors in the genome and has a stimulatory effect on antioxidant enzyme systems such as superoxide dismutase (SOD) and reduced glutathione (GSH). The oxidative damage that occurs as a result of lipid peroxidation disrupts the functional integrity of the cell membrane in acute pancreatitis.[3]
Malonyldialdehyde (MDA) is a peroxidative decomposition product of polyenic fatty acids, and an increase in tissue levels indicates an expansive lipid peroxidation. The most significant method used to assess oxidative stress and an increase in OFRs is to check the rise in plasma and tissue levels of SOD, one of the endogenous antioxidant enzymes, reduced glutathione, and MDA, one of lipid peroxidation products.[4]In the hepatic mitochondria of rats with ligated bile ducts, there were a decrease in antioxidative capacity and an increase of MDA among the lipid peroxidation products in tissue homogenates.[5,6]
There are studies on the idea that exogenous melatonin decreases cellular levels of oxidative damage. However, there are no studies on the effect of elimination of endogenous melatonin on oxidative damage in acute pancreatitis. In this study, the effect of removal of the endogenous melatonin source in rats with experimental acute pancreatitis through pinealectomy on oxidative damage products was compared with that of hypermelatoninemia created by exogenous melatonin in the same conditions.
Methods
Thirty male Wistar albino rats weighing 200-250 g were kept at normal room temperature (20-22 ℃) in a 12-hour light and 12-hour dark cycle and fed with standard rat pellets and tap water. This study was conducted in accordance with the principles and procedures outlined in theEuropean Communities Council Directivesand carried out with ethical approval obtained from the Animal Care Ethics Committee of Abant Izzet Baysal University Medical School.
The experimental acute pancreatitis model alone was developed in the first group of rats, and they were not given any other surgical or medical treatment; this group served as the control (group Ⅰ). Surgical pinealectomy was added to pancreatic ductal ligation in the second group (group Ⅱ), and this reduced the level of melatonin. In the third group (group Ⅲ), 20 mg/kg melatonin (Sigma, St. Louis, MO, USA) was injected intraperitoneally daily for one week in order to eliminate the damage caused by acute pancreatitis.
Surgical procedure
The technique of surgical ligation of the main pancreatic duct was used to develop the experimental acute pancreatitis model. All of the surgical procedures were performed under general anesthesia and standard sterile conditions on the same day. In all rats, 2.5 mg/kg Enrofloxacin (Baytril 10%, Bayer-Istanbul) intramuscular injection was administered as a prophylactic antibiotic. Intramuscular ketamine HCl (20 mg/kg; Ketalar, Eczacibasi, Istanbul) and xylazine HCl (5 mg/ kg; Alphazin amp) were used for general anesthesia.
Biochemical parameters
The pancreatic tissue was cleaned with cold saline after removal and was kept at -70 ℃ until further investigation. After this, 200 mg wet tissue was homogenized in 1.8 ml phosphate-buffered saline (PBS, pH 7.4) (1/9; weight/volume) with a Teflon pestle homogenizer (IKA Ultra Turrax T 8, IKA Labortechnic, Staufen, Germany) at 16 000 rpm and 4 ℃ for 3 minutes. This mixture was centrifuged at 4000 × g and 4 ℃ for 15 minutes; samples of the supernatant were used for MDA, SOD, and GSH measurements and tissue protein was measured by the Lowry method.[7]
MDA
The MDA thiobarbituric acid (TBA) heating method was used. TBA reacts with MDA and substrates similar to MDA and forms a stable pink color at 532 nm. Samples were precipitated with trichloroacetic acid (TCA) then TBA and N-butanol were added and spectrophotometric measurements were made at 532 nm (Digilab Hitachi U-2800, Hitachi High-Technologies, Tokyo, Japan); results were recorded as nmol/mg wet tissue weight.[8]
SOD
Measurement of SOD activity is based on the principle of formation of tetrazolium salts in the presence of free radicals formed by xanthine oxidase and hypoxanthine. SOD activity was measured using a commercial colorimetric kit (Cayman Chemical Company, Catalog No: 7006002, Ann Arbor, USA). The colorimetric plate where the reactions occurred was measured in a microplate reader (BioRAD Benchmark Plus ELISA) at 450 nm. The activity of SOD was defined by using the regression determined in the calibration curve and results were given in Unit/mg wet tissue weight.[9]
Reduced glutathione (GSH)
GSH was determined using Elman reactive 5, 5'-dithiobis (2-nitrobenzoic acid)-DTNB; D8130 (Sigma-Aldrich-Sigma Chemical, St. Louis, Mo, USA). First,the supernatant was added to Na2HPO4·2H2O solution and then Elman was added to the mixture; the mixture was immediately read at 412 nm by spectrophotometer (Digilab Hitachi U-2800, Hitachi High-Technologies, Tokyo, Japan). The results were recorded as nmol/mg wet tissue weight.[10]
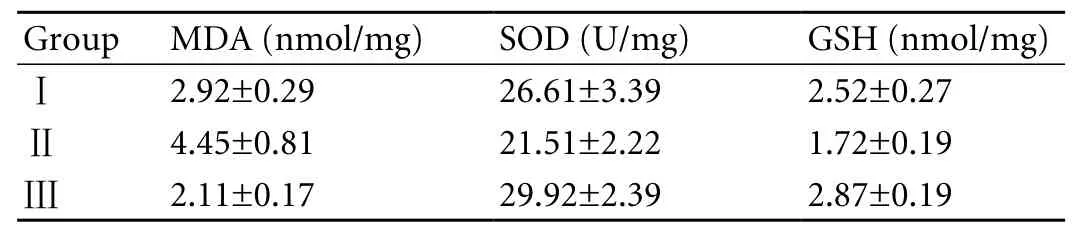
Table. The levels of MDA, SOD and GSH in rats with experimental acute pancreatitis
Statistical analysis
Statistical analysis of data was made using the SPSS statistical software package. The levels of MDA, SOD, and GSH were correlated with the results of Student'sttest. The level of statistical significance was set atP<0.05.
Results
The levels of MDA, SOD, and GSH tissue, which are indicators of oxidative stress in tissue samples, were measured in rats with experimental acute pancreatitis (Table).
The MDA level in pancreatic tissue was 2.92 nmol/mg in rats from the control group with acute pancreatitis (group Ⅰ), whereas the level in the group with pinealectomy (group Ⅱ) increased to 4.45 nmol/mg. The increased MDA level in group Ⅱ rats was much higher than in group Ⅰ (t=5.3,P<0.001). After injecting exogenous melatonin intraperitoneally into group Ⅲrats with acute pancreatitis for 7 days, the MDA level decreased to 2.11 nmol/mg (t=7.08,P<0.001).
The SOD level was 26.61 U/mg in group Ⅰ, 21.51 U/ mg in group Ⅱ, and 29.92 U/mg in group Ⅲ. Compared to the SOD level in group Ⅰ, the differences in levels between groups Ⅱ and Ⅲ were significant (t=3.6,P<0.01 for groups Ⅰ and Ⅱ,t=2.3,P<0.05 for groups Ⅰ and Ⅲ).
The GSH level was 2.52 nmol/mg in group Ⅰ, and 1.72 nmol/mg in group Ⅱ (t=6.70,P<0.001). Exogenous melatonin injection led to an excessive increase in GSH levels up to 2.87 nmol/mg in group Ⅲ. The difference in GSH levels between groups Ⅰ and Ⅲ was significant (t=3,P<0.01).
Discussion
Melatonin is a hormone that directly and indirectly affects cellular immunity and the general immune system. Melatonin can penetrate all the morphophysiological barriers in the human body due to its lipophilic and hydrophilic characteristics and can have antioxidant effects over quite a wide area.[11-13]Since there is no barrier to melatonin, it can cross the bloodbrain barrier and the placenta easily, and can reach all intracellular components without difficulty. Thus, melatonin can effectively protect cell walls, organelles, and nuclei from damage by free radicals.[11,12]
Being able to reach even to nuclei provides melatonin with an advantage in the protection of DNA from oxidative damage. By affecting receptors in the genome, melatonin also has a stimulant effect on the increase of the gene expression or activity of antioxidant enzyme systems such as catalase, SOD, glutathione peroxidase, glutathione reductase, and γ-glutamylcysteine synthetase. Through these effects, melatonin protects the cell from oxidative damage, decreases inflammation, and impedes the progress of tissue edema.[11,12]
The inflammatory cytokines and OFRs that appear because of tissue damage in acute pancreatitis disrupt the structure of phospholipids in the nucleus and cause peroxidation in membrane lipids. It is wellknown that there is a parallelism between the severity of acute pancreatitis, increase of lipid peroxidation products, and oxidative stress.[14]Tissue MDA level increases when lipid peroxidation products react with thiobarbituric acid.[4,15,16]Another increase occurs in lipid peroxidation products because of inflammatory cells in damaged tissue.[17]
In our study, the MDA level increased to some extent in the control group, and to a much higher level in the group that underwent pinealectomy. Increased level of MDA in tissue homogenates indicates increased lipid peroxidation in rats with the main bile ducts ligated.[6]
We found that MDA level decreased to below the control levels after injection of exogenous melatonin. We also evaluated groups Ⅰ and Ⅱ and found that hypomelatoninemia eliminated antioxidant reactions and caused lipid peroxidation products to increase, whereas hypermelatoninemia resulted in an evident antioxidant effect and a significant decrease in lipid peroxidation products. Hence, melatonin, as a free radical scavenger and a strong antioxidant does not undergo reactions producing hydroxyl radicals.[18]Melatonin has its antioxidant effect in its first contact with the cell membrane, by attaching to the external surface of the phospholipid layer, reacting with radicals, and detoxifying them before the membrane does; thus, it can protect the cell membrane.
Evaluating antioxidant enzymes SOD and GSH, showedthat an increase of their levels in acute pancreatitis, a slight decrease after pinealectomy, and a significant increase after exogenous melatonin injection. Hence as a result of reactions due to defense mechanisms in acute pancreatitis the level of SOD increased to a higher level with the effect of melatonin while decreasing the defense mechanism after pinealectomy.
In order to protect the cells from oxidative stress, a number of enzymatic and non-enzymatic mechanisms step in. Among the OFRs, SOD is responsible for enzymatic antioxidant reactions, and GSH for nonenzymatic ones.[19]These radicals are effective in the pathogenesis of acute pancreatitis, as in many inflammatory events. GSH is a tripeptide that can be synthesized in the liver. SOD is an antioxidant enzyme that catalyzes the transformation of superoxide (O2-) free radical into hydrogen peroxide (H2O2) and molecular oxygen (O2). GSH demonstrates its protective effect when free radicals react with peroxides, whereas SOD shows this effect by protecting cells from the harmful effects of O2
-such as lipid peroxidation.[17,20]In conclusion, MDA, a lipid peroxidation product, increases in the tissue, and this is provoked when endogenous melatonin secretion is blocked by removal of the pineal gland, whereas injection of exogenous melatonin prevents the increase and causes a decrease in MDA concentration. Similarly, SOD acts against oxidative damage. The levels of SOD and GSH in tissue are increased after pancreatitis, decreased after pinealectomy, and increased after injection of exogenous melatonin. Oxidative damage caused by acute pancreatitis is aggravated in the absence of melatonin or if its level is reduced, and injection of exogenous melatonin decreases the damage significantly.
Acknowledgment
The authors wish to thank Dr. Bulent Gunduz, Dr. Aysu Kiyan, Dr. Aysel Kukner and Dr. Tulin Firat for their collaboration.
Funding:None.
Ethical approval:This experimental animal study was conducted in accordance with the principles and procedures outlined in theEuropean Communities Council Directivesand carried out with the ethical approval obtained from the Animal Care Ethics Committee of Abant Izzet Baysal University Medical School.
Contributors:CÇ proposed the study and wrote the manuscript. DK and BO participated in the design of the study. HO and BG have participated adequately in the study for substantial contributions to conception and design, acquisition of data. CÇ is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Sanfey H, Bulkley GB, Cameron JL. The role of oxygenderived free radicals in the pathogenesis of acute pancreatitis. Ann Surg 1984;200:405-413.
2 Schoenberg MH, Büchler M, Gaspar M, Stinner A, Younes M, Melzner I, et al. Oxygen free radicals in acute pancreatitis of the rat. Gut 1990;31:1138-1143.
3 Leja-Szpak A, Jaworek J, Tomaszewska R, Nawrot K, Bonior J, Kot M, et al. Melatonin precursor; L-tryptophan protects the pancreas from development of acute pancreatitis through the central site of action. J Physiol Pharmacol 2004;55:239-254.
4 Gutteridge JM. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem. 1995;41:1819- 1828.
5 Sokol RJ, McKim JM Jr, Goff MC, Ruyle SZ, Devereaux MW, Han D, et al. Vitamin E reduces oxidant injury to mitochondria and the hepatotoxicity of taurochenodeoxycholic acid in the rat. Gastroenterology 1998;114:164-174.
6 Ljubuncic P, Tanne Z, Bomzon A. Evidence of a systemic phenomenon for oxidative stress in cholestatic liver disease. Gut 2000;47:710-716.
7 Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265-275.
8 Estebauer H, Cheeseen KH. Oxygen radicals in biological systems, methods in enzymology. In Packer C, Glazer AN, Ed. California: Academic Press; 1990:407-421.
9 Sun E, Xu H, Liu Q, Zhou J, Zuo P, Wang J. The mechanism for the effect of selenium supplementation on immunity. Biol Trace Elem Res 1995;48:231-238.
10 Beutler E. Red Blood cell metabolism: A manual of biochemical methods. In Beutler E, 2nd Ed. New York: Grune & Stratton Inc; 1984:74-76.
11 Bülbüller N, Dogru O, Umac H, Gürsu F, Akpolat N. The effects of melatonin and pentoxiphylline on L-arginine induced acute pancreatitis. Ulus Travma Acil Cerrahi Derg 2005;11:108-114.
12 Konturek SJ, Konturek PC, Brzozowska I, Pawlik M, Sliwowski Z, Cześnikiewicz-Guzik M, et al. Localization and biological activities of melatonin in intact and diseased gastrointestinal tract (GIT). J Physiol Pharmacol 2007;58: 381-405.
13 Reiter RJ. Melatonin: clinical relevance. Best Pract Res Clin Endocrinol Metab 2003;17:273-285.
14 Park BK, Chung JB, Lee JH, Suh JH, Park SW, Song SY, et al. Role of oxygen free radicals in patients with acute pancreatitis. World J Gastroenterol 2003;9:2266-2269.
15 Chen HM, Chen JC, Ng CJ, Chiu DF, Chen MF. Melatonin reduces pancreatic prostaglandins production and protects against caerulein-induced pancreatitis in rats. J Pineal Res 2006;40:34-39.
16 Weber H, Merkord J, Jonas L, Wagner A, Schröder H, Käding U, et al. Oxygen radical generation and acute pancreatitis: effects of dibutyltin dichloride/ethanol and ethanol on rat pancreas. Pancreas 1995;11:382-388.
17 Rau B, Poch B, Gansauge F, Bauer A, Nüssler AK, Nevalainen T, et al. Pathophysiologic role of oxygen free radicals in acute pancreatitis: initiating event or mediator of tissue damage? Ann Surg 2000;231:352-360.
18 Palaoglu OS, Beskonakli E. Pineal gland and aging. Turkish J Geriatrics 1998;1:13-18.
19 Bulger EM, Maier RV. Antioxidants in critical illness. Arch Surg 2001;136:1201-1207.
20 Dabrowski A, Konturek SJ, Konturek JW, Gabryelewicz A. Role of oxidative stress in the pathogenesis of caeruleininduced acute pancreatitis. Eur J Pharmacol 1999;377:1-11.
Accepted after revision November 4, 2009
I have but one lamp wait which my feet are guided; and that is the lamp of experience.iknow of no way of judging of the future but by the past.
— Patrick Henry
July 31, 2009
Author Affiliations: Department of General Surgery (Çöl C, Dinler K, Hasdemir O and Büyükasık O) and Department of Biochemistry (Buğdaycı G), Abant Izzet Baysal University Medical School, Bolu, Turkey
Cavit Çöl, MD, Abant İzzet Baysal Üniversitesi Tıp Fakultesi Genel Cerrahi Anabilimdalı, 14280 Bolu, Turkey (Tel: 90374-2541000/3515; Fax: +90374-2534559; Email: cavitcol@yahoo.com)
© 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Clinical management of hepatitis B virus infection correlated with liver transplantation
- Is bactibilia a predictor of poor outcome of pancreaticoduodenectomy?
- Acinar cell carcinoma of the pancreas in a young patient with chronic pancreatitis
- Letters to the Editor
- Simplifying living donor liver transplantation
- Meetings and Courses
