Norcantharidin inhibits growth of human gallbladder carcinoma xenografted tumors in nude mice by inducing apoptosis and blocking the cell cycle in vivo
2010-06-29YueZuFanZeMingZhaoJinYeFuChunQiuChenandWeiSun
Yue-Zu Fan, Ze-Ming Zhao, Jin-Ye Fu, Chun-Qiu Chen and Wei Sun
Shanghai, China
Norcantharidin inhibits growth of human gallbladder carcinoma xenografted tumors in nude mice by inducing apoptosis and blocking the cell cycle in vivo
Yue-Zu Fan, Ze-Ming Zhao, Jin-Ye Fu, Chun-Qiu Chen and Wei Sun
Shanghai, China
BACKGROUND:Gallbladder carcinoma, a lethal malignant neoplasm with poor prognosis, has dismal results of surgical resection and chemoradiotherapy. We previously reported that norcantharidin (NCTD) is useful against growth, proliferation, and invasion of human gallbladder carcinoma GBC-SD cellsin vitro. In this study, we further studied the inhibitory effect of NCTD on the growth of xenografted tumors of human gallbladder carcinoma in nude micein vivoand the underlying mechanisms.
METHODS:The tumor xenograft model of human gallbladder carcinoma in nude micein vivowas established with subcutaneous GBC-SD cells. The experimental mice were randomly divided into control, 5-FU, NCTD, and NCTD+5-FU groups which were given different treatments. Tumor growth in terms of size, growth curve, and inhibitory rate was evaluated. Cell cycle, apoptosis, and morphological changes of the xenografted tumors were assessed by flow cytometry and light/electron microscopy. The expression of the cell cyclerelated proteins cyclin-D1 and p27 as well as the apoptosisrelated proteins Bcl-2, Bax, and survivin were determined by the streptavidin-biotin complex (SABC) method and RT-PCR.
RESULTS:NCTD inhibited the growth of the xenografted tumors in a dose- and time-dependent manner. Tumor volume decreased (5.61±0.39 vs. 9.78±0.61 cm3,P=0.000) with an increased tumor inhibitory rate (42.63% vs. 0%,P=0.012) in the NTCD group compared with the control group. The apoptosis rate increased (15.08±1.49% vs. 5.49±0.59%,P= 0.0001) along with a decreased percentage of cells in S phase(43.47±2.83% vs. 69.85±1.96%,P=0.0001) in the NTCD group compared with the control group. The morphological changes of apoptosis such as nuclear shrinkage, chromatin aggregation, chromosome condensation, and typical apoptosis bodies in the xenografted tumor cells induced by NCTD were observed by light and electron microscopy. The expression of cyclin-D1, Bcl-2 and survivin proteins/mRNAs decreased significantly, with increased expression of p27 and Bax proteins/mRNAs in the NCTD group compared with the control group.
CONCLUSION:NCTD inhibits the growth of xenografted tumors of human gallbladder carcinoma in nude mice by inducing apoptosis and blocking the cell cyclein vivo.
(Hepatobiliary Pancreat Dis Int 2010; 9: 414-422)
norcantharidin; gallbladder neoplasm; tumor growth; apoptosis; cell cycle
Introduction
Gallbladder carcinoma is the most common malignancy of the biliary tract, the fifth or sixth most common malignant neoplasm of the digestive tract, and the leading cause of cancerrelated deaths in Western countries and in China.[1-6]Late diagnosis, absence of effective treatment, and poor survival of many patients remain the characteristics of this disease.[5-8]Even with the recent improvements in treatment of gallbladder carcinomas, the only potentially curative therapy is radical surgery.[9-13]Unfortunately, most patients with gallbladder carcinoma present with advanced and unresectable disease and only 10%-30% of the patients are indicated for surgery,[5,6,9-13]or palliative treatment such as chemotherapy and radiotherapy.[5,6,14-20]However, these adjuvant therapies for the disease are disappointing.[5,6,14-20]Clearly, new therapeutic agents areneeded to treat gallbladder carcinomain vivo.
Evidence has shown that traditional Chinese medicines contain anticancer ingredients. Norcantharidin (NCTD) is a demethylated form of cantharidin with anti-tumor properties, and is an active ingredient of the traditional Chinese herbal medicineMylabris.[21-23]It is effective in inhibiting the proliferation of several tumor cell lines, including HeLa, CHO, CaEs-17, BEL-7402, SMMC-7721, human hepatoma HepG2, colon cancer HT29, human myeloid leukemia K562 and HL-60, and human epidermoid laryngocarcinoma.[24-30]Because of its anti-tumor activity, fewer side-effects and leukocytosis, NCTD is reported clinically as an anti-tumor drug against hepatoma, esophageal and gastric carcinoma, and leucopenia in China. However, few reports have investigated the effect of NCTD on human gallbladder carcinomasin vitroandin vivo. We previously reported the effects of NCTD on proliferation and invasion of human gallbladder carcinoma GBC-SD cellsin vitro.[31-33]In the present study, we further studied the inhibitory effect of NCTD on the growth of xenografted tumors of human gallbladder carcinomas in nude micein vivoand the underlying mechanisms.
Methods
Acute toxicity test
Sixty-six white rats obtained from Shanghai Animal Resources Center, Chinese Scientific Institute, China were randomly divided into 6 groups (11 rats in each group). Maximal (250 mg/kg) or minimal (102.4 mg/kg) lethal dose (LD50) was calculated in preliminary experiments. The dose of NCTD (Beijing Fourth Pharmaceutical Works, China) was diluted among the groups at a NCTD/normal saline ratio of 1/0.8. White rats in the 6 groups were injected intraperitoneally with the mentioned dose of NCTD, respectively. Acute toxic reaction and death in white rats were observed. The concentration for 50% of LD50was calculated from the formula LD50=lg-1[Xm-I (∑p-0.5)].
Tumor xenografted model in nude mice and inhibitory experimentin vivo
GBC-SD cell lines of human gallbladder carcinoma (Shanghai Cell Biology Research Institute, China) were cultured in RPMI-1640 medium (Gibco, USA) supplemented with 10% bovine calf serum (Hangzhou Sijiqing Biological Co., China) in an incubator with 5% CO2at 37 ℃. When the cells became confluent, they were digested with 0.25% trypsin (Gibco, USA), cultured at 37 ℃ in 5% CO2for 24 hours, washed twice with Hanks' balanced salt solution (HBSS, Gibco, USA), and then used in experiments.
All of the procedures were performed on nude mice according to the official recommendations of the Chinese Community Guidelines. Balb/c nu/nu mice from the Shanghai Laboratory Animal Center (Shanghai, China) were housed in specific pathogen-free conditions. Equal numbers of male and female mice, 5 weeks old and about 20 g, were used. The tumor xenograft model was established by subcutaneous implantation of cultured GBC-SD cells (1.8×107/ml) in 0.2 ml HBSS into the right axil back region. The ears of mice that received cell injections were tagged for identification. By 2 weeks a solid tumor was apparent in all mice that received GBC-SD cell injections.
The mice with the xenografted tumors used in experiments were randomly divided into a control group (n=9) receiving intraperitoneal (i.p.) injections of 0.1 ml normal saline alone twice each week, a NCTD group (n=8), a 5-FU (Shanghai Xudonghaipu Pharmaceutical Co., China) group (n=8), and a NCTD+5-FU group (n=8), in which each mouse received consecutive i.p. injections of 28 mg/kg NCTD at a dose of 1/5 LD50given in 0.1 ml of normal saline, 24 mg/kg 5-FU at a dose of 1/5 LD50, or 28 mg/kg NCTD+24 mg/kg 5-FU, twice each week for 6 weeks in all. The size of the implanted tumor and body weight of each mouse were determined weekly by a single observer. In addition, the maximum (a) and minimum (b) diameters of the xenografted tumor were measured with calipers twice each week. The tumor volume was calculated by the formula: V (cm3)=1/6πab2. Also, the tumor growth curve and tumor inhibitory rate in each group was evaluated. Tumor inhibitory rate=(volume in the control group-volume in the experimental group)/volume in the control group× 100%.
Flow cytometry
Ten samples of fresh xenografted tumors were collected from each group, washed with phosphatebuffered saline (PBS), cut into tissue blocks (1 mm3), griddled, sieved, and made up into a cell suspension. The cells were centrifuged at 1500 g for 5 minutes, treated with 150 μl RNaseA at 37 ℃ for 15-20 minutes, and suspended with PBS (1×106cells/ml). Tumor DNA was then stained for 10 minutes with 100 μl (50 μg/ml) propidium iodide (PI, Sigma, USA). Using a standard control of DNA assessment with healthy lung cells from nude mice, DNA values, cell cycle, and apoptotic rate of the xenografted tumor cells in each group were determined with an apoptosis detection kit (BioDev Co., China) and a fluorescence-activated cell sorter (420type FACS Flow Cytometer, Becton-Dickinson, CA). Ten separate experiments were performed.
Light and electron microscopy
The xenografted tumor specimens in each group were fixed in 10% formalin, embedded in paraffin, cut into 4 μm sections, and then stained with hematoxylin and eosin (HE). Cell morphology was examined under a light microscope (Olympus Co., Japan). Ten specimens of the fresh xenografted tumors were taken from each group, cut into tissue blocks (1 mm3), and fixed in 2.5% glutaraldehyde-1% osmium tetroxide buffered with PBS (pH 7.2). The specimens were dehydrated in a graded ethanol series and embedded in 618 epoxy resin. Then they were cut into 50-70 nm sections, doublestained with uranyl acetate-lead citrate, and analyzed by standard procedures in a transmission electron microscope (H-500, Japanese Hitachi Co., Japan).
Immunohistochemistry
Cyclin-D1, p27, Bcl-2, Bax, and survivin protein products from the xenografted tumor cells of each group were determined by the SABC method. The paraffin sections (4 μm) from each group were dehydrated in xylene and graded ethanol series, and added in order with primary antibody [cyclin-D1 (mouse monoclonal antibody, Neomarker, USA), p27 (rabbit polyclonal antibody, Wuhan Boster Co., China), Bcl-2 (1∶50; rabbit polyclonal antibody, Santa Cruz, USA), Bax (rabbit polyclonal antibody, Santa Cruz), survivin (1∶100; mouse monoclonal antibody, Neomarker)], biotinylated secondary antibody (horse serum 1∶200, Vector, USA; or goat serum 1∶100, Wuhan Boster Co.), SABC reagents and DAB solution (Wuhan Boster Co.). For negative control, the sections were treated with PBS instead of primary antibody. Ten sample sections from each group were chosen for analysis. More than 10 visual fields were observed or more than 500 cells were counted per section. The following equation was used: the positive percentage of each protein=protein-positive cells/all cells ×100%.
RT-PCR analysis
Expressions of cyclin-D1, p27, Bcl-2, Bax, and survivin mRNAs from the xenografted tumors in the control and NCTD groups (1/5 LD50dose) were determined by RTPCR assay. Total RNA was isolated using the guanidinium thiocyanate-phenol-chloroform extraction method. PCR was designed to amplify specific mRNAs, using published sequences, at Shanghai Saibaisheng Gene Technology Co., Ltd., China (Table 1). PCR was performed using the gene-specific annealing temperatures and cycle numbers shown in Table 1. PCR was performed with 29 cycles with control GAPDH, as follows: 94 ℃ for 2 minutes, 94 ℃ for 30 seconds, 61 ℃ for 45 seconds, 72 ℃ for 1 minute, and 72 ℃ for 6 minutes. In addition, agarose gel electrophoresis of the PCR products, followed by staining with ethidium bromide, was performed to confirm the specificity of the amplification.
Statistical analysis
Statistical analyses were performed using SPSS 11.5 and Microsoft Excel Office 2007 for Windows. All data were presented as mean±SD. Statistical differences were evaluated using Student'sttest or the Chi-square test.P<0.05 was considered statistically significant.
Results
Growth inhibition of xenografted tumors in nude mice by NCTD
Based on the acute toxicity test and the LD50formula, the LD50of NCTD for small white rats was calculated as139.96 mg/kg. The xenografted tumor volume of nude mice in the NCTD group was smaller than that of the control group, with an increased tumor inhibitory rate. There were significant differences in tumor volume and tumor inhibitory rate in the NCTD+5-FU group compared with the other groups (Table 2 and Fig. 1).
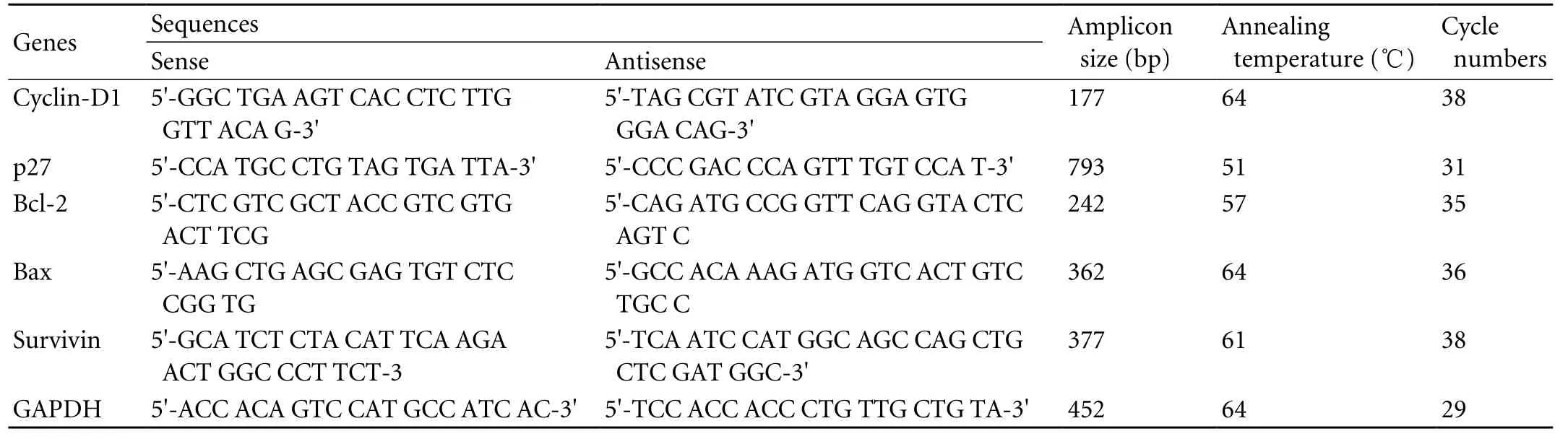
Table 1. RT-PCR conditions and reagents of cell cycle- and apoptosis-related genes from xenografted tumors of human gallbladder carcinoma in nude mice in vivo
Cell cycle and apoptosis of the xenografted tumor cells by NCTD
Flow cytometry in each group showed that NCTD decreased the cells in S phase from the tumors in the NCTD and NCTD+5-FU groups compared with the control and 5-FU groups, and the results were consistent with those of the healthy lung cells (standard control) (Table 3 and Fig. 2). But there was no difference in G2+M phase cells among all groups. Furthermore, after treatment with NCTD, the percentage apoptosis of xenografted tumor cells in nude mice was increased.
Apoptotic morphology of xenografted tumor cells with NCTD
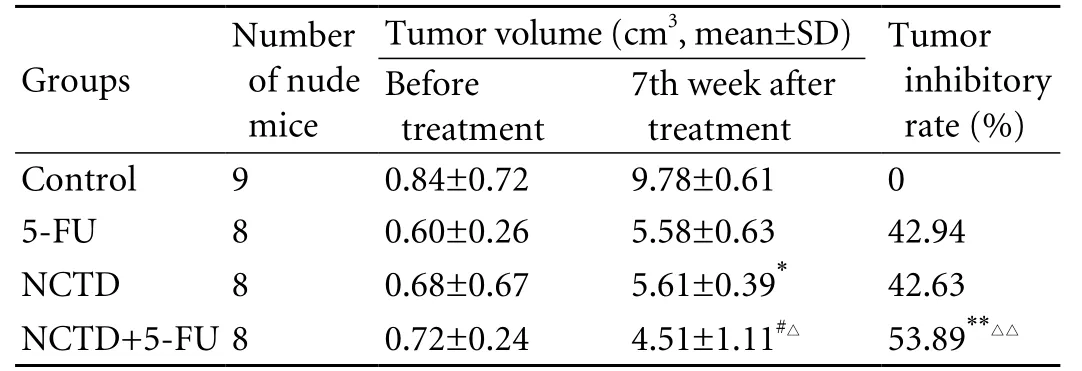
Table 2. Inhibitory effect of NCTD on growth of xenografted tumors in nude mice in vivo
The morphologic changes of the xenografted tumors in nude mice treated with or without NCTD, 5-FU, or NCTD+5-FU for 48 hours were examined by light (Fig. 3A-D) and electron microscopy (Fig. 3E-H). We found that xenografted tumor cells with irregular nuclei, more mitoses, invaded fat tissues and skeletal muscles around tumors, and cancer cell nests occurred in the control group (Fig. 3A). Different sizes of glands invaded by cancer cells and cancer cell nests were destroyed to different degrees in the 5-FU group (Fig. 3B). Fat tissues, skeletal muscles around tumors, and cancer cell nests were also destroyed and parts of apoptotic cancer cells were seen in the NCTD group (Fig. 3C). Many destroyed cancer cells and apoptotic cancer cells among blood vessels were observed in the NCTD and 5-FU+NCTD groups (Fig. 3D). Also found were irregular cells with abundant microvilli, clear cell organelles, large nucleus/cytoplasm ratio, irregular nuclei, and chromatin enrichment in the control group (Fig. 3E). Decreasing microvilli, golgiosome atrophy, mitochondrial swelling, cytoplasmic vacuoles, nuclear shrinkage, chromosome condensation, chromatinaggregation, and typical apoptotic bodies were found in the treatment groups (Fig. 3F-H).
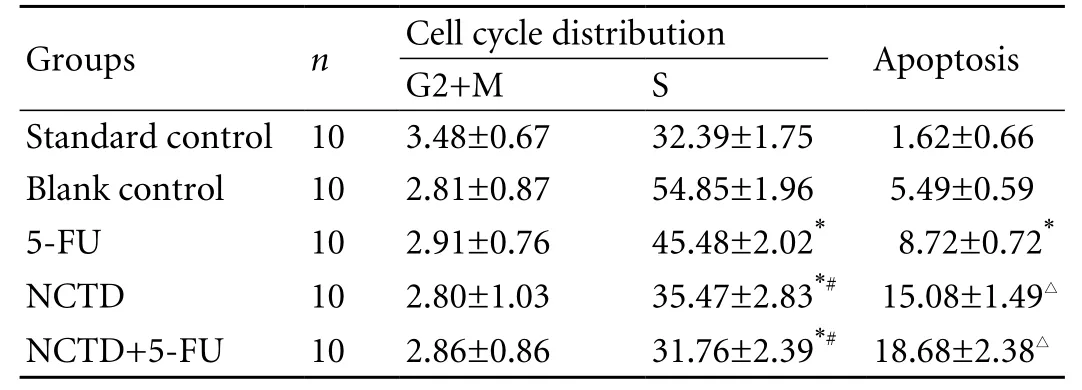
Table 3. Cell cycle and apoptosis of xenografted tumor cells from nude mice (%, mean±SD)
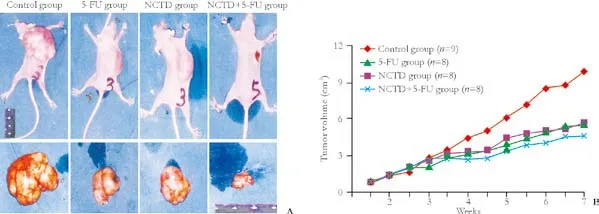
Fig. 1. Inhibitory effect of NCTD on growth of xenografted tumors of human gallbladder carcinoma from nude mice in vivo. A: Volume of implanted tumor in each group: maximal tumor volume (13 cm3) in the control group, less tumor volume in the 5-FU group and in the NCTD group, minimal tumor volume (2.18 cm3) in the NCTD+5-FU group. B: Growth curves of implanted tumors in each group.
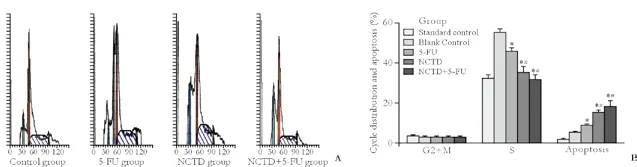
Fig. 2. Cell cycle distribution and apoptosis in xenografted tumor cells from nude mice by flow cytometry in each group. A: Flow cytometric profiles; B: histogram of cell cycle distribution and apoptosis: *: P<0.05 vs. control group; #: P<0.05 vs. 5-FU group; #: P<0.05 vs. control or 5-FU group.
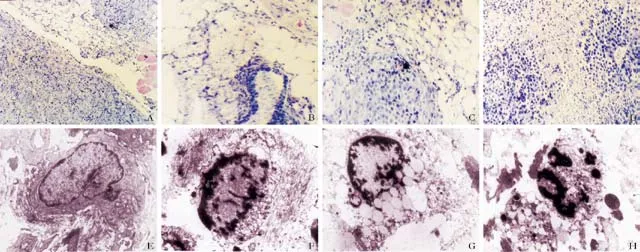
Fig. 3. Apoptotic morphology of xenografted tumors of human gallbladder carcinoma from nude mice. A-D: HE staining, light microscopy. Tumor cells invaded fat tissues and skeletal muscles, cancer nests present in the control group (A, original magnification × 100). Different-sized glands were invaded by tumor cells and cancer nests were destroyed to different degrees in the 5-FU group (B, original magnification ×400). Fat tissues and skeletal muscles around tumor and cancer nests were destroyed; parts of apoptotic cancer cells were seen in the NCTD group (C, original magnification ×400). Many destroyed tumor cells and apoptotic tumor cells among blood vessels were observed in the NCTD group and in the NCTD+5-FU group (D, original magnification ×200). E-H: Microstructural morphology under electron microscopy. Irregular tumor cells with abundant microvilli, clear cell organelles, and chromatin enrichment in the control group (E, original magnification ×4000); disappearing microvilli, mitochondrial swelling, golgiosome atrophy, cell organelle vacuoles, nuclear shrinkage, chromatin aggregation, chromosome condensation, and typical apoptosis bodies in the 5-FU group (F, original magnification ×5000), NCTD group (G, original magnification ×6000) and NCTD+5-FU group (H, original magnification ×6000).
Effect of NCTD on cell cycle- and apoptosis-related genes in xenografted tumors
Expression of cyclin-D1, p27, Bcl-2, Bax, and survivin proteins
The positive expression sites of cyclin-D1, p27, Bcl-2, Bax, and survivin presented as yellow-brown reactant in the nucleoli or cytoplasm; after treatment with NCTD, the expression of cyclin-D1, Bcl-2, and survivin proteins in tumor cells was significantly decreased, along with increased expression of the p27 and Bax proteins (P<0.05) compared with the control group. The most marked changes of these proteins were found in the NCTD+5-FU group (P<0.05) (Table 4 and Fig. 4A, B).
Expression of cyclin-D1, p27, Bcl-2, Bax, and survivin mRNAs
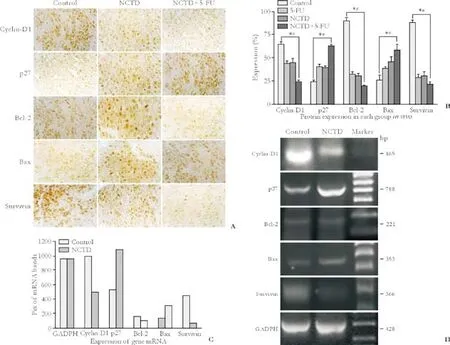
Fig. 4. Expression of cell cycle- and apoptosis-related proteins (A, B, immunohistochemistry SABC method, original magnification ×400) or mRNAs (C, D, RT-PCR) of xenografted tumor cells from nude mice. A, B: expression of cyclin-D1, Bcl-2 and survivin proteins was significantly decreased, with increased expression of p27 and Bax proteins in the NCTD group than in the control group; most significant changes of these proteins occurred in the NCTD+5-FU group. *: P<0.05 vs. control group; #: P<0.05 vs. 5-FU and NCTD groups. C, D: After treatment with NCTD, the expression of cyclin-D1 (989 vs. 498), Bcl-2 (155 vs. 96) and survivin (444 vs. 67) mRNAs was decreased significantly, with increased expression of p27 (533 vs. 1088) and Bax (133 vs. 302) mRNAs. No difference was observed on the Pix or relative level of GADPH mRNA (960 vs. 958).
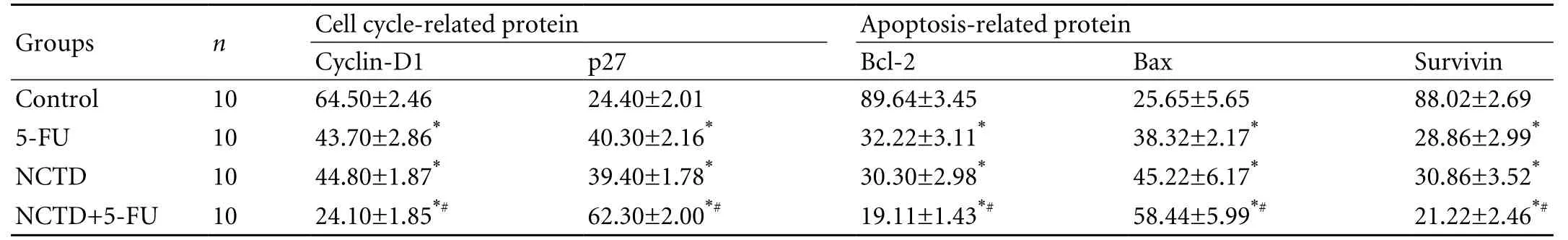
Table 4. Effect of NCTD on expression of cell cycle- and apoptosis-related proteins of xenografted tumors in nude mice in vivo (percent positive, mean±SD)
The lengths of the bands for GADPH, cyclin-D1, p27, Bcl-2, Bax, and survivin were 428, 165, 788, 221, 353 and 366bp, respectively. No difference was found in the Pix or relative level of GADPH mRNA (960 vs. 958) of the xenografted tumor cells without or with NCTD. After treatment with NCTD, the Pix or relative levels of cyclin-D1 mRNA (989 vs. 498), Bcl-2 mRNA (155 vs. 96) and survivin mRNA (444 vs. 67) were decreased significantly, whereas the Pix or relative levels of p27 mRNA (533 vs. 1088) and Bax mRNA (133 vs. 302) were increased significantly (Fig. 4C, D).
Discussion
Modern oncology research has shown that the genesis and development of tumors are clearly related to proliferation and apoptosis of the cells. Proliferation and apoptosis of normal cells maintain a balance. If apoptosis is arrested or proliferation exceeds apoptosis, the tissue grows. One of the most important mechanisms by which many anti-cancer agents inhibit the growth of tumors is the inducement of apoptosis in tumor cells.[24-30]Many reports demonstrated that NCTD inhibits the growth of the human tumor cell lines HepG2, HT29, CT26, K562, and HL-60 by inducing apoptosis.[24-30,34-36]The present study showed that NCTD inhibited the growth of xenografted tumors of human gallbladder carcinomas by inducing apoptosis of the tumors in nude micein vivo. This finding is consistent with the report about the inducement of apoptosis in tumor cells by NCTD.[24-30,34-36]Apoptosis, the death process of cells, has unique morphologic and biologic characteristics. The most important morphological features of apoptosis are nuclear shrinkage, chromosome condensation, chromatin aggregation, and typical apoptosis bodies. We found in the study that, after treatment with NCTD, destroyed fat tissues or skeletal muscles around tumors and destructed cancer-cell nests were observed by light microscopy, whereas decreasing microvilli, golgiosome atrophy, mitochondrial swelling, nuclear shrinkage, chromosome condensation, chromatin aggregation, and typical apoptosis bodies were seen under an electron microscope. Thus, NCTD induced the apoptosis of xenografted tumor cells of human gallbladder carcinoma in nude mice.
Studies reports demonstrated that NCTD is an inhibitor of protein phosphatase types 1, 2A and 2B, arrests the cell cycle at G2/M phase in K562 human myeloid leukemia cells, and inhibits DNA synthesis in HL-60 cells.[25,37-40]Our previous results showed that NCTD markedly increases the GBC-SD cells in G2/M phase, and decreases those in S phasein vitro.[31-33]We also found by flow cytometry that NCTD decreased the cells in S phase in xenografted tumors from nude micein vivo. This is also consistent with the report about the inducement of apoptosis in tumor cells by NCTD.[25,37-40]Possibly inducement of apoptosis in gallbladder carcinoma cells might correlate with arresting cells in G2/M phase and decreasing cells in S phase, inhibition of DNA replication, and an influence on cell metabolism. It has been reported that the cell cycle and cell proliferation are regulated by cell cycle-related genes and proteins. The regulatory system includes cyclins, cyclindependent kinases (CDKs), and CDK inhibitors (CDKIs) which play important roles in cell proliferation.[41-43]Cyclin-D1 and p27 are markers and proteins reflecting cell cycle regulation and proliferation. Cyclin-D1 is the most important, positive regulatory gene in G1 phase. Its excess expression and disordered regulation induces abnormal regulation of the cell cycle, and results in the genesis of tumors.[41-43]p27kip1 protein is one of the Cip/Kip family of CDKIs, a functional CDKI and new tumor suppressor gene, which restrains the activity of corresponding CDKs, regulates cell-cycle progression, and induces blockade of G1 phase and apoptosis.[44,45]In our study, after treatment with NCTD, the expression of cyclin-D1 protein and mRNA in xenografted tumor cells was significantly decreased, with increased expression of p27 protein and mRNA (P<0.05) as determined by immunohistochemical staining and RT-PCR. Of course, it was essential to further verify the expression changes of cyclin-D1 and p27 at the protein level by Western blotting in our study. We demonstrated that the cell cycle-related genes cyclin-D1 and p27 participated in the process of inhibiting growth of the xenografted tumor cells caused by NCTDin vivo. This has further demonstrated that inducement of apoptosis in gallbladder carcinoma cells correlates with arresting cells in G2/M phase and decreasing cells in S phase, inhibition of DNA replication, and an influence on cell metabolism.
Great progress has been made in research into the genetic regulation of apoptosis in recent years. Apoptosis-related genes and their regulatory molecules regulate the apoptosis of cells. Thus, changing the genetic regulation of apoptosis may be an important mechanism by which many anti-cancer agents inhibit tumor growth. Bcl-2, Bax, and survivin are apoptosisrelated proteins. Bcl-2 participates in the apoptotic process under specific circumstances, suppresses apoptosis, and results in the genesis of some tumors; so it is an important marker of apoptosis.[46,47]Bax, a proapoptotic gene, is one of the proteins that accelerates cell apoptosis. The anti-apoptotic gene Bcl-2 and the pro-apoptotic gene Bax might form homogenous or heterogenous dimers, so that the ratio of anti-apoptotic dimers to pro-apoptotic dimers in cells, namely Bcl-2/ Bax is the important defining factor for apoptosis,[46-49]and both influence the prognosis of patients with some tumors.[50]In addition, caspases and especially caspase-3, a family of cysteine proteases that are activated during the apoptotic processes, are crucial mediators in the apoptotic pathway.[47]Survivin, one of the inhibitors of apoptosis proteins and anti-apoptotic genes is selectively expressed in the G2/M phase. It was shown that checking the combination of survivin with canaliculus proteins accelerates the activity of caspase-3, andaccordingly induces the apoptosis of G2/M phase cells; and that survivin is an independent prognostic marker for risk stratification in some cancer patients.[51,52]In this study, after treatment with NCTD, the expression of Bcl-2 and survivin proteins/mRNAs of the xenografted tumor cells were decreased significantly, with increased expression of Bax when compared with the control group, as measured by immunocytochemistry and RTPCR. Of course, it was also essential to further verify the expression change of Bcl-2, Bax and survivin in protein level by Western blotting. We also showed that the anti-apoptotic genes Bcl-2 and survivin, and the pro-apoptotic gene Bax participated in the process of inducing the apoptosis in gallbladder carcinoma cells caused by NCTD; NCTD induced apoptosis of the xenografted tumor cells by down-regulation of the antiapoptotic genes Bcl-2 and survivin, and up-regulation of the pro-apoptotic gene Bax. This may be one of the mechanisms by which NCTD induces apoptosis of the xenografted tumor cells of human gallbladder carcinomain vivo.
Acknowledgements
We are grateful to Professor Yao-Qing Yang (Tumor Cell Biology Research Institute, Tongji University Medicine of School, China) for his advice, technical assistance, and experimental help.
Funding:None.
Ethical approval:Not needed.
Contributors:FYZ and ZZM designed the research, analyzed the data, and wrote the paper. FYZ, ZZM and FJY performed the research. CCQ and SW contributed new reagents/analytic tools. FYZ is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Gourgiotis S, Kocher HM, Solaini L, Yarollahi A, Tsiambas E, Salemis NS. Gallbladder cancer. Am J Surg 2008;196:252-264.
2 Reddy SK, Clary BM. Surgical management of gallbladder cancer. Surg Oncol Clin N Am 2009;18:307-324, ix.
3 Shukla PJ, Barreto SG. Gallbladder cancer: we need to do better! Ann Surg Oncol 2009;16:2084-2085.
4 Lazcano-Ponce EC, Miquel JF, Muñoz N, Herrero R, Ferrecio C, Wistuba II, et al. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin 2001;51:349-364.
5 Li LD, Zhang SW, Lu FZ, Mu R, Sun XD, Huangpu XM. Research on characteristics of mortality spectrum and type composition of malignant tumors in China. Zhonghua Zhong Liu Za Zhi 1997;19:323-328.
6 Wang BS, Qin J, Deng J, Zhang BH, Han TQ, Shen MC, et al. A survey on the diagnosis and treatment of biliary tract cancers in Shanghai. Zhonghua Wai Ke Za Zhi 2005;43:455-459.
7 Chakravarty KD, Yeh CN, Jan YY, Chen MF. Factors influencing long-term survival in patients with T3 gallbladder adenocarcinoma. Digestion 2009;79:151-157.
8 Konstantinidis IT, Deshpande V, Genevay M, Berger D, Fernandez-del Castillo C, Tanabe KK, et al. Trends in presentation and survival for gallbladder cancer during a period of more than 4 decades: a single-institution experience. Arch Surg 2009;144:441-447.
9 Pilgrim C, Usatoff V, Evans PM. A review of the surgical strategies for the management of gallbladder carcinoma based on T stage and growth type of the tumour. Eur J Surg Oncol 2009;35:903-907.
10 Taner CB, Nagorney DM, Donohue JH. Surgical treatment of gallbladder cancer. J Gastrointest Surg 2004;8:83-89.
11 Singh S, Agarwal AK. Gallbladder cancer: the role of laparoscopy and radical resection. Ann Surg 2009;250:494-495.
12 Wang JD, Liu YB, Quan ZW, Li SG, Wang XF, Shen J. Role of regional lymphadenectomy in different stage of gallbladder carcinoma. Hepatogastroenterology 2009;56:593-596.
13 Hueman MT, Vollmer CM Jr, Pawlik TM. Evolving treatment strategies for gallbladder cancer. Ann Surg Oncol 2009;16:2101-2015.
14 Ishii H, Furuse J, Yonemoto N, Nagase M, Yoshino M, Sato T. Chemotherapy in the treatment of advanced gallbladder cancer. Oncology 2004;66:138-142.
15 Morine Y, Shimada M, Ikegami T, Imura S, Kanemura H, Arakawa Y, et al. Usefulness of gemcitabine combined with 5-fluorouracil and cisplatin (GFP) in patients for unresectable biliary carcinoma. Hepatogastroenterology 2009;56:307-312.
16 Morise Z, Sugioka A, Tanahashi Y, Okabe Y, Ikeda M, Kagawa T, et al. Treatment of patients with unresectable advanced carcinoma of biliary tract-chemotherapy and surgical resection. Anticancer Res 2009;29:1783-1786.
17 Gold DG, Miller RC, Haddock MG, Gunderson LL, Quevedo F, Donohue JH, et al. Adjuvant therapy for gallbladder carcinoma: the Mayo Clinic Experience. Int J Radiat Oncol Biol Phys 2009;75:150-155.
18 Mahantshetty UM, Palled SR, Engineer R, Homkar G, Shrivastava SK, Shukla PJ. Adjuvant radiation therapy in gall bladder cancers: 10 years experience at Tata Memorial Hospital. J Cancer Res Ther 2006;2:52-56.
19 Kresl JJ, Schild SE, Henning GT, Gunderson LL, Donohue J, Pitot H, et al. Adjuvant external beam radiation therapy with concurrent chemotherapy in the management of gallbladder carcinoma. Int J Radiat Oncol Biol Phys 2002;52:167-175.
20 Mojica P, Smith D, Ellenhorn J. Adjuvant radiation therapy is associated with improved survival for gallbladder carcinoma with regional metastatic disease. J Surg Oncol 2007;96:8-13.
21 Wang GS. Medical uses of mylabris in ancient China and recent studies. J Ethnopharmacol 1989;26:147-162.
22 Liu J, Gao J, Liu X. Advances in the study of Cantharidin and its derivatives. Zhong Yao Cai 2003;26:453-455.
23 Ho YP, To KK, Au-Yeung SC, Wang X, Lin G, Han X. Potential new antitumor agents from an innovative combination of demethylcantharidin, a modified traditional Chinese medicine, with a platinum moiety. J Med Chem 2001;44:2065-2068.
24 Yang EB, Tang WY, Zhang K, Cheng LY, Mack PO.Norcantharidin inhibits growth of human HepG2 celltransplanted tumor in nude mice and prolongs host survival. Cancer Lett 1997;117:93-98.
25 Yi SN, Wass J, Vincent P, Iland H. Inhibitory effect of norcantharidin on K562 human myeloid leukemia cells in vitro. Leuk Res 1991;15:883-886.
26 Kok SH, Hong CY, Kuo MY, Lee CH, Lee JJ, Lou IU, et al. Comparisons of norcantharidin cytotoxic effects on oral cancer cells and normal buccal keratinocytes. Oral Oncol 2003;39:19-26.
27 Hong CY, Huang SC, Lin SK, Lee JJ, Chueh LL, Lee CH, et al. Norcantharidin-induced post-G(2)/M apoptosis is dependent on wild-type p53 gene. Biochem Biophys Res Commun 2000; 276:278-285.
28 Chen YN, Chen JC, Yin SC, Wang GS, Tsauer W, Hsu SF, et al. Effector mechanisms of norcantharidin-induced mitotic arrest and apoptosis in human hepatoma cells. Int J Cancer 2002;100:158-165.
29 Chen YN, Cheng CC, Chen JC, Tsauer W, Hsu SL. Norcantharidin-induced apoptosis is via the extracellular signal-regulated kinase and c-Jun-NH2-terminal kinase signaling pathways in human hepatoma HepG2 cells. Br J Pharmacol 2003;140:461-470.
30 Peng F, Wei YQ, Tian L, Yang L, Zhao X, Lu Y, et al. Induction of apoptosis by norcantharidin in human colorectal carcinoma cell lines: involvement of the CD95 receptor/ligand. J Cancer Res Clin Oncol 2002;128:223-230.
31 Fan YZ, Fu JY, Zhao ZM, Chen CQ. The in vitro effect of norcantharidin on proliferation and invasion of human gallbladder carcinoma GBC-SD cells and its mechanism. Zhonghua Zhong Liu Za Zhi 2004;26:271-274.
32 Fan YZ, Fu JY, Zhao ZM, Chen CQ. Effect of norcantharidin on proliferation and invasion of human gallbladder carcinoma GBC-SD cells. World J Gastroenterol 2005;11:2431-2437.
33 Fan YZ, Fu JY, Zhao ZM, Chen CQ. Inhibitory effect of norcantharidin on the growth of human gallbladder carcinoma GBC-SD cells in vitro. Hepatobiliary Pancreat Dis Int 2007;6:72-80.
34 Liao HF, Su SL, Chen YJ, Chou CH, Kuo CD. Norcantharidin preferentially induces apoptosis in human leukemic Jurkat cells without affecting viability of normal blood mononuclear cells. Food Chem Toxicol 2007;45:1678-1687.
35 Peng C, Liu X, Liu E, Xu K, Niu W, Chen R,et al. Norcantharidin induces HT-29 colon cancer cell apoptosis through the alphavbeta6-extracellular signal-related kinase signaling pathway. Cancer Sci 2009;100:2302-2308.
36 Chen YJ, Kuo CD, Tsai YM, Yu CC, Wang GS, Liao HF. Norcantharidin induces anoikis through Jun-N-terminal kinase activation in CT26 colorectal cancer cells. Anticancer Drugs 2008;19:55-64.
37 Liu XH, Blazsek I, Comisso M, Legras S, Marion S, Quittet P, et al. Effects of norcantharidin, a protein phosphatase type-2A inhibitor, on the growth of normal and malignant haemopoietic cells. Eur J Cancer 1995;31A:953-963.
38 McCluskey A, Ackland SP, Gardiner E, Walkom CC, Sakoff JA. The inhibition of protein phosphatases 1 and 2A: a new target for rational anti-cancer drug design? Anticancer Drug Des 2001;16:291-303.
39 Hart ME, Chamberlin AR, Walkom C, Sakoff JA, McCluskey A. Modified norcantharidins; synthesis, protein phosphatases 1 and 2A inhibition, and anticancer activity. Bioorg Med Chem Lett 2004;14:1969-1973.
40 Li JL, Cai YC, Liu XH, Xian LJ. Norcantharidin inhibits DNA replication and induces apoptosis with the cleavage of initiation protein Cdc6 in HL-60 cells. Anticancer Drugs 2006;17:307-314.
41 Marx J. How cells cycle toward cancer. Science 1994;263: 319-321.
42 Chen YC, Chang SC, Wu MH, Chuang KA, Wu JY, Tsai WJ, et al. Norcantharidin reduced cyclins and cytokines production in human peripheral blood mononuclear cells. Life Sci 2009;84:218-226.
43 Hui AM, Li X, Shi YZ, Takayama T, Torzilli G, Makuuchi M. Cyclin D1 overexpression is a critical event in gallbladder carcinogenesis and independently predicts decreased survival for patients with gallbladder carcinoma. Clin Cancer Res 2000;6:4272-4277.
44 Hui AM, Li X, Shi YZ, Torzilli G, Takayama T, Makuuchi M. p27(Kip1) expression in normal epithelia, precancerous lesions, and carcinomas of the gallbladder: association with cancer progression and prognosis. Hepatology 2000;31:1068-1072.
45 Noguchi T, Kikuchi R, Ono K, Takeno S, Moriyama H, Uchida Y. Prognostic significance of p27/kip1 and apoptosis in patients with colorectal carcinoma. Oncol Rep 2003;10: 827-831.
46 Qiu Z, Zhu J, Harms JS, Friedrichsen J, Splitter GA. Bovine herpesvirus VP22 induces apoptosis in neuroblastoma cells by upregulating the expression ratio of Bax to Bcl-2. Hum Gene Ther 2005;16:101-108.
47 Zheng XL, Sun HX, Liu XL, Chen YX, Qian BC. Astilbic acid induced COLO 205 cell apoptosis by regulating Bcl-2 and Bax expression and activating caspase-3. Acta Pharmacol Sin 2004;25:1090-1095.
48 Bowen JM, Gibson RJ, Keefe DM, Cummins AG. Cytotoxic chemotherapy upregulates pro-apoptotic Bax and Bak in the small intestine of rats and humans. Pathology 2005;37:56-62.
49 Kok SH, Cheng SJ, Hong CY, Lee JJ, Lin SK, Kuo YS, et al. Norcantharidin-induced apoptosis in oral cancer cells is associated with an increase of proapoptotic to antiapoptotic protein ratio. Cancer Lett 2005;217:43-52.
50 Santini D, Tonini G, Vecchio FM, Borzomati D, Vincenzi B, Valeri S, et al. Prognostic value of Bax, Bcl-2, p53, and TUNEL staining in patients with radically resected ampullary carcinoma. J Clin Pathol 2005;58:159-165.
51 Morinaga S, Nakamura Y, Ishiwa N, Yoshikawa T, Noguchi Y, Yamamoto Y, et al. Expression of survivin mRNA associates with apoptosis, proliferation and histologically aggressive features in hepatocellular carcinoma. Oncol Rep 2004;12: 1189-1194.
52 Dai DJ, Lu CD, Lai RY, Guo JM, Meng H, Chen WS, et al. Survivin antisense compound inhibits proliferation and promotes apoptosis in liver cancer cells. World J Gastroenterol 2005;11:193-199.
January 23, 2010
Accepted after revision May 29, 2010
Author Affiliations: Department of Surgery, Tongji Hospital, Tongji University School of Medicine, Shanghai 200065, China (Fan YZ, Zhao ZM, Chen CQ and Sun W); and Department of Surgery, Ninth People's Hospital, Shanghai Jiaotong University, Shanghai 200065, China (Fu JY)
Yue-Zu Fan, Professor, Department of Surgery, Tongji Hospital, Tongji University School of Medicine, Shanghai 200065, China (Tel: 86-21-66111109; Fax: 86-21-56050502; Email: fanyuezu_shtj@ yahoo.com.cn)
© 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Efficacy of Shanvac-B recombinant DNA hepatitis B vaccine in heaIth care workers of Northern India
- Inflammatory boweI diseases and hepatitis C virus infection
- Radiofrequency ablation, heat shock protein 70 and potential anti-tumor immunity in hepatic and pancreatic cancers: a minireview
- Outcome of hepatocellular carcinoma treated by liver transplantation: comparison of living donor and deceased donor transplantation
- Meetings and Courses
