Chemical Mechanical Polishing of Glass Substrate with α-Alumina-g-Polystyrene Sulfonic Acid Composite Abrasive
2010-03-01LEIHongBUNaijingZHANGZefangandCHENRuling
LEI Hong, BU Naijing, ZHANG Zefang, and CHEN Ruling
Research Center of Nano-science and Nano-technology, Shanghai University, Shanghai 200444, China
1 Introduction
Chemical mechanical polishing(CMP) as the only technology to provide global planarization has been widely used in the manufacturing of semiconductor and digital compact disc(CD) glass substrate[1–3]. In CMP, abrasive is one of key influencing factors on the polished surface quality. The size and distribution, dispersibility, hardness and species of abrasive are crucial for a desired CMP performances[4–5]. Large particles resulted from particles agglomeration in CMP slurries, are believed to be main factor to cause polishing scratch and the increasing of surface roughness[6], and hence must be eliminated.
In CMP slurries, two types of abrasives are adopted: the traditional inorganic particles and the composite particles[6].Traditional inorganic abrasives, such as silica[2–3],alumina[8–9], ceria[10–11], have been widely studied and used in the commercial slurries, but just one kind of inorganic abrasives used in slurries often leads to undesired CMP performance. It has been proved that the nanoparticle impacts during CMP can lead to nano-deformation or damages in sub-surface layer of the polished surfaces[12–15].Recently, with the increasing demand of improving the polishing performances while minimizing roughness and defects of the polished surface, composite particles as abrasives in slurry have been paid much attentions[7,16–24].It is thought that the coating on the surface of particles with harder substance can improve the removal rate while keeping the density of defects constant, however the hard particles coated with a softer material can reduce CMP defects[17]. YANO, et al[18], developed a kind of slurry with inorganic/resin abrasive for the Al/low-k damascene wiring CMP. The slurry resulted in less scratching and better planarity. In SiO2CMP, it was found that composite abrasive slurry exhibited the reduction of the dishing and erosion depth, which was reduced to less than 80 nm after the first step CMP and less than 70 nm after the second step CMP[19]. KAWAHASHI, et al[20], presented composite particles consisting of polymer core covered with inorganic composition such as SiO2and Al2O3. The Cu-CMP slurry with these composite particles gave excellent dishing,erosion, and the over-polish-margin performances.Particularly these composite particles were useful to prevent increasing scratch on the tetraethyl orthosilicate and low-k dielectric materials surfaces in the second step CMP process[20]. Composite abrasives with polymer core and ceramic shell have been extensively published in Refs. [18, 21–22].
At present, α-Al2O3particles as a kind of abrasive has been widely used in CMP slurries[8–9], but its high hardness and poor dispersion stability often lead to more surface defects.
In the present paper, a novel α-alumina-g-polystyrene sulfonic acid (α-Al2O3-g-PSS) composite abrasive was prepared by surface graft polymerization in section 2.Subsequently, in section 3, its composition, structure and morphology were characterized and its CMP performances on glass substrate were investigated, followed by conclusions.
2 Experimental Methods
2.1 Preparation of α-Al2O3-g-PSS composite abrasive
2.1.1 Silylation
Prior to silylation, α-Al2O3powder was dried in an oven at 110 ℃ under vacuum for 12 h to get rid of the absorbed moisture. Subsequently, 50 g dried α-Al2O3powder (crystal particle diameter of about 50 nm) and 25 g KH570 silane couple agent (γ-methacryloxypropyl trimethoxy silane) in 500 mL anhydrous ethanol were charged into a 1 000 mL three-necked flask equipped with a reflux condenser. After dispersed with ultrasonic for 0.5 h, the mixture was refluxed at the boiling temperature of ethanol for 5 h under continuously stirring. Then the mixture was separated by centrifugation and the precipitate was washed by anhydrous ethanol for 6 times to remove excess KH570. In the end, the precipitate was dried at 80 ℃ in a vacuum oven for 24 h to remove the remaining solvent.
2.1.2 Graft polymerization
The graft polymerization reactions for styrene monomer were performed in a flask equipped with a condenser at 80 ℃ under vacuum. 25 g of the above prepared α-Al2O3powder, 8 mL 3% polyvinyl alcohol(PVA), and 492 mL deionized(DI) water were added into the flask. The mixture was dispersed with ultrasonic for half an hour. After deoxygenating by taking out air with a vacuum pump and filling nitrogen gas alternately for four times, the flask containing the mixture was heated up to 80 ℃. Then,0.25 g benzoyl peroxide(BPO) as initiator and 25 g styrene as monomer were added simultaneously into the mixture.The polymerization reaction would last 5 h under the protection of nitrogen gas and continuously stirring at the temperature of 80 ℃. Afterwards, the resultant suspension was treated by vacuum filtration, the precipitate was purified in a centrifuge by dispersing in toluene and following centrifugal separation for six times to eliminate homopolymer. Finally, the precipitate was dried under vacuum at 50℃ for 12 h to obtain α-alumina-gpolystyrene(α-Al2O3-g-PS) powder with 27.6% grafting ratio. By adopting different weight percent of styrene versus α-Al2O3, α-Al2O3-g-PS composite particles with different grafting ratio were prepared.
2.1.3 Sulfonation
3 g Ag2SO4and 500 mL sulfuric acid were charged into a 1 000 mL three-necked flask, and then heated up to 98−99 ℃. Subsequently, the prepared α-Al2O3-g-PS powder was added into it slowly. The reaction would last 4 h under the protection of drier and continuously stirring at the above temperature. After the solution cooled to room temperature, it was poured into DI water under incessant stirring to precipitate, and the precipitate was washed with DI water for six times to remove remanent sulfuric acid.Finally, the precipitate was dried under vacuum at 80 ℃to obtain α-Al2O3-g-PSS powder.
2.1.4 Preparation of the slurry
2.5 wt.% α-Al2O3-g-PSS particles and 1 wt.%polyoxyethylene carboxyl acid as dispersant were added into DI water in a container under continuously stirring.Then the mixture was milled for 2 h in a vibrator containing ZrO2balls as abrasives. Finally, the mixture was filtrated with a 30.8 μm pore strainer to obtain slurry containing α-Al2O3-g-PSS abrasive.
2.2 Characterization of α-Al2O3-g-PSS abrasive
The purifiedα-Al2O3-g-PSS composite particle was characterized by Fourier transform infrared spectroscopy(FTIR), X-ray photoelectron spectroscopy(XPS), time-offlight secondary ion mass spectroscopy(TOF-SIMS), and scanning electron microscopy(SEM), respectively.
FTIR spectra were obtained on a Nicolet AVATAR 370 FTIR spectrometer. Samples for measurements were mixed with carefully dried KBr solid, and the solid mixture was then made into pellets using a 13 mm die and a hydraulic press.
从提出增强文化自信、价值观自信〔2〕到将文化自信同道路自信、理论自信和制度自信并列构成“四个自信”,〔3〕再到十九大报告中强调文化自信基础性、根本性的地位和作用,指出文化自信是更基本、更深沉、更持久的力量。〔4〕并将文化自信提升到国家发展战略的高度,明确指出坚定文化自信,推动社会主义文化繁荣兴盛。〔5〕在习近平总书记的讲话中,能够感受到他对中华文化的自信与坚定,感受到他对人民坚定文化自信的期望。现有研究成果表明,文化自信的提出具有历史必然性,同时具有深刻的现实依据。
XPS spectrum was obtained on a KRATOS XSAM 800 electron spectrometer by using the Mg Kα line with pass energy of 12.5 kV×18 mA. The binding energy of C1s(284.6 eV) was used as reference.
TOF-SIMS analyses were performed with a Physical Electronics TRIFT II instrument using a pulsed gallium ion beam with the energy of 15 kV. The analytical region was 200 μm×200 μm, and post acceleration was 5 kV/5 kV(+/−).
SEM analyses were accomplished by using a JEOL JSM-6700F field emission scanning electron microscope with voltage of 10 kV.
The graft ratio was determined by calcination under 700 ℃ for 6 h, grafted organic compounds could be decomposed into gases (CO2, H2O, etc) and volatilized away while inorganic α-Al2O3particles remained and didn't change. The weight loss after calcining divided by initial sample weight gave graft ratio.
2.3 Polishing tests
Polishing tests were conducted with a SPEEDFAM-16B-4M CMP equipment (SPEEDFAM Co. LTD) by using the following polishing conditions: down force of 7 kPa,rotating speed of 23 r/min and slurry supplying rate of 1 L/min. Workpieces were φ170 mm sodium-calcium glass substrates with an average roughness (Ra) of about 520 nm. The polishing pad was a Rodel porous polyurethane pad.
After being polished, the substrates were washed with ultrasonic in a cleaning solution containing 0.5 wt.%surfactant in DI water. Finally, they were dried by a multi-functional drying system.
2.4 Examination of the polished surfaces
The polished surface roughness (Ra) was measured to evaluate the polishing effects of different slurries. Rawas measured by using a Wyko optical profiler (WYKO NT 9800, VEECO) with scan area of 480 μm×736 μm. The polished surface topography was measured by using a DI D-300 atomic force microscope (Digital Instrument Corp.,USA) with the resolution of 0.01 nm in vertical direction and 0.1 nm in horizontal direction. The AFM operating mode was tapping mode with scan area of 5 μm×5 μm.
3 Results and Discussions
3.1 Structure and dispersibility of α-Al2O3-g-PSS composite abrasive
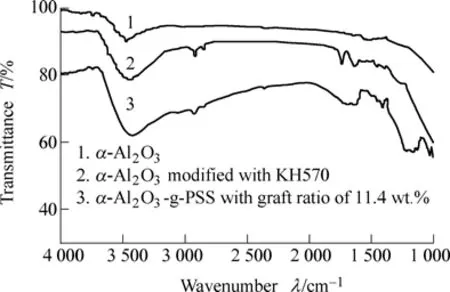
Fig. 1. FTIR spectra of α-Al2O3 particles before and after modification
Further, XPS and TOF-SIMS analyses were conducted to confirm the structure of α-Al2O3-g-PSS particles. In XPS analysis, it is found that element S appears on the surface of the composite particles. As shown in Fig. 2, the binding energy of S2p occurs at 175.3 eV, which should be attributed to the sulfur in sulfonated polystyrene.TOF-SIMS is an effective means for element analysis because of its high sensitivity, mass and space resolution[25].Fig. 3 shows the TOF-SIMS analysis of the composite particles. Horizontal axis is ion mass, and vertical axis is number of ions counted. It is found that the peaks of S and Al ions appear in the anions and cations spectra,respectively. The introduction of element S on the alumina surface indicates that sulfonated polystyrene was successfully grafted on the surface of α-Al2O3particles,which is consistent with the result of XPS. Based on the above results, the structure of the prepared composite particles may be deduced: a core/shell structure with α-Al2O3as core and sulfonated polystyrene chain as shell.
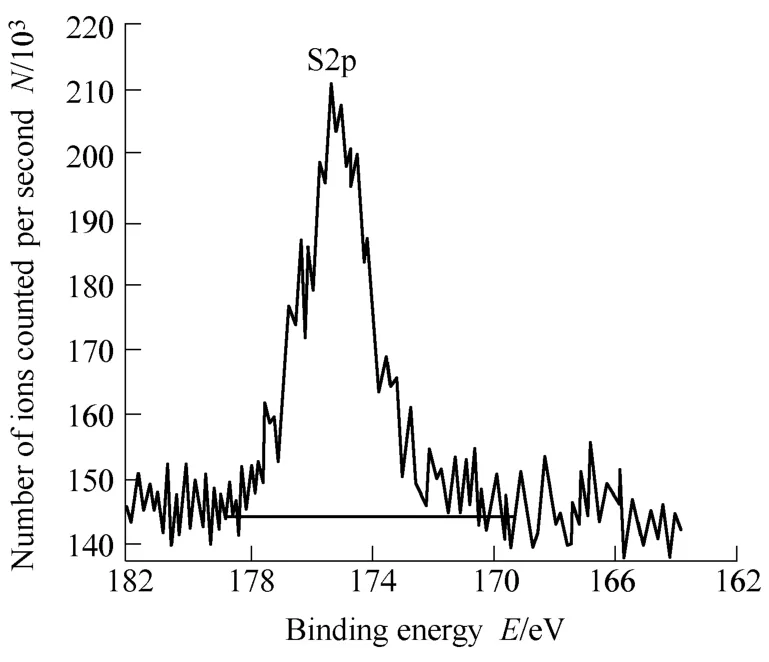
Fig. 2. S2p XPS spectrum of α-Al2O3-g-PSS particles with graft ratio of 11.4 wt.%
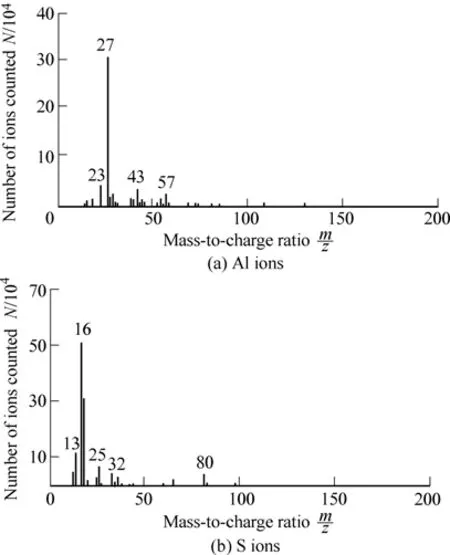
Fig. 3. TOF-SIMS spectra of α-Al2O3-g-PSS particles with graft ratio of 11.4 wt.%
In order to analyze the dispersibility of alumina particles before and after surface modification, the morphology of the α-Al2O3particles before and after surface modification was analyzed by SEM, and the results are shown in Fig. 4.It is found that the α-Al2O3-PSS composite particles have better dispersibility while obvious agglomeration is observed for pure α-Al2O3particles. The improvement of the dispersibility of the composite particles can be attributed to the presence of polystyrene sulfonic acid chain.The water-solubility of sulfonated polystyrene chain may endue the composite particles with better hydrophilicity and solvability in aqueous medium, and the sulfonated polystyrene long chain can provide the composite particles with stronger steric hindrance effect, which in turn prevents the agglomeration among the α-Al2O3particles. In order to investigate the dispersion mechanism, zeta potential was measured. As shown in Table 1, negative surface charge of alumina particles was increased through grafting with polystyrene sulfonic acid(PSS) chain. The higher zeta potential is, the better thermodynamic dispersion stability will be. In other words, the thermodynamic dispersion stability of alumina particles was improved by coating PSS on the surfaces. The increasing of zeta potential may account for why the α-Al2O3-PSS particles have better dispersibility than pure α-Al2O3particles.

Fig. 4. SEM images of α-Al2O3 particles before and after grafting
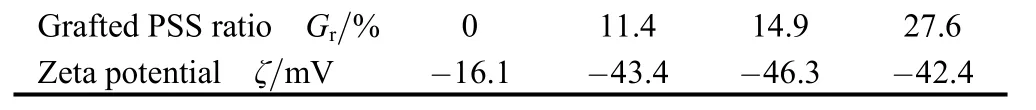
Table 1. Zeta potential of Al2O3-g-PSS composite abrasive with different grafting ratio
3.2 CMP behavior of α-Al2O3-g-PSS composite abrasive
Fig. 5 shows the influence of polishing time on Rawith α-Al2O3-PSS composite abrasive of 27.6 wt.% grafting ratio. With the increase of polishing time, the number of rough peaks on the surface decreases, which leads to the surface roughness to decrease. The surface average roughness shows almost no change when polishing time is from 60 min to 180 min. In other words, polishing time of 60 min is enough for glass polishing with the prepared abrasive. Prolonging the polishing time cannot improve surface quality any more.
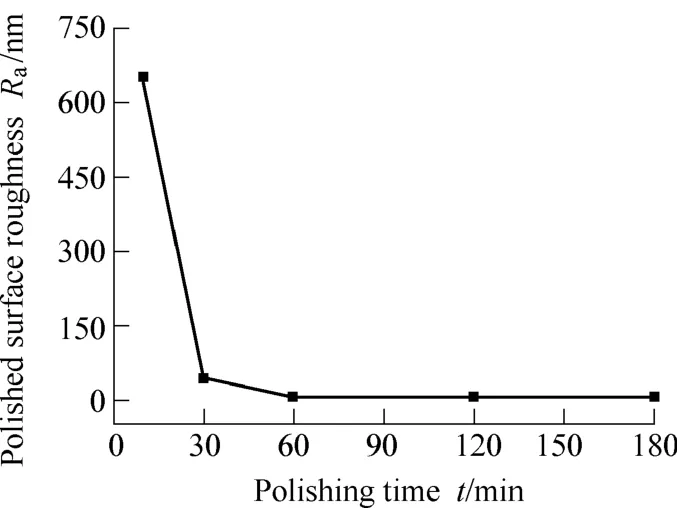
Fig. 5. Influence of polishing time on Ra(measured by WYKO profiler)
Table 2 shows the effect of grafting ratio on Raof the polished surface (polishing time is 60 min). It is indicated that the prepared α-Al2O3-g-PSS abrasives give lower Ra values than pure α-Al2O3abrasive. And with the increasing of grafting ratio, the Ravalue decreases. In other words, the high grafting ratio helps to reach the high surface planarization.
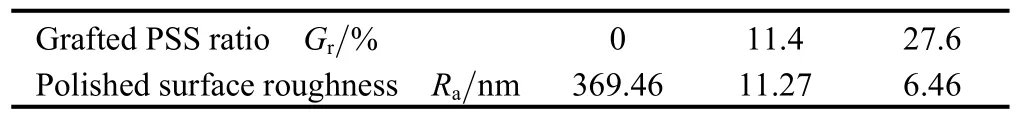
Table 2. Effect of the grafted PSS content on Ra(measured by WYKO profiler)
Further, in order to investigate the difference in polishing performances between the prepared α-Al2O3-g-PSS abrasive and pure α-Al2O3abrasive, the topographical micrographs of polished glass substrate surfaces were analyzed by AFM, and the result is shown in Fig. 6. By comparison with pure α-Al2O3abrasive, the prepared α-Al2O3-g-PSS core-shell abrasive gives lower topographical variations as well as less and shallower scratches.And it is also found that the prepared α-Al2O3-g-PSS core-shell abrasive gives Raof 0.583 nm while pure α-Al2O3abrasive gives Raof 0.835 nm. The lower Ravalue means the higher surface planarization. In other words, the prepared α-Al2O3-g-PSS abrasive possesses higher surface planarization than pure alumina abrasive.
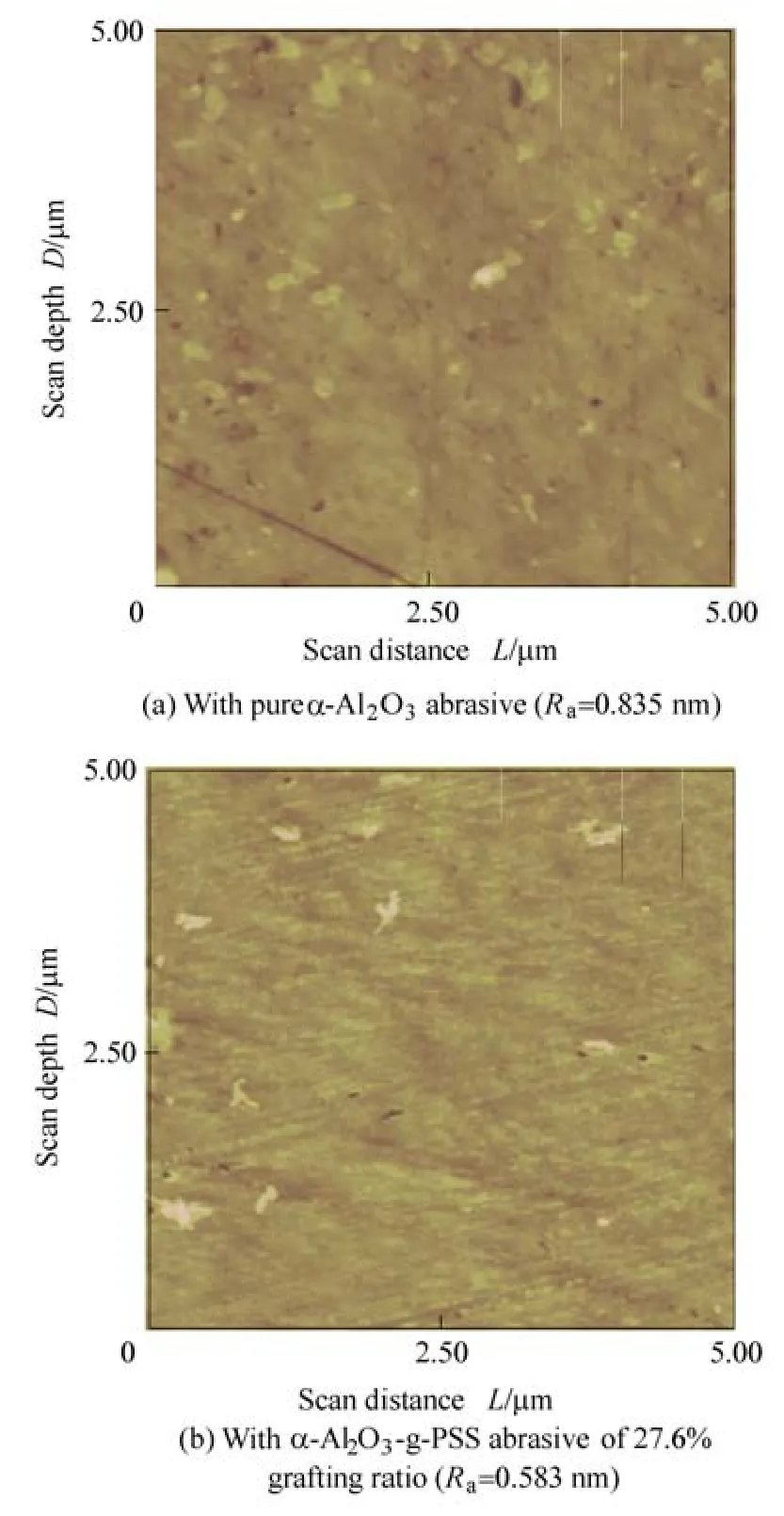
Fig. 6. Surface profiles of glass substrates polished in slurries containing different abrasives
The improvement in CMP performances of the α-Al2O3-g-PSS abrasive may be attributed to its core-shell structure. The grafted polystyrene sulfonic acid chain as shell on the hard α-Al2O3core is softer than α-Al2O3substrate, which can reduce the hardness of α-Al2O3particles and smooth the appearance of sharp particles.Thus polystyrene sulfonic acid shell may behave as a cushion during CMP, which can decrease the hard impacts and excessive mechanical damage caused by α-Al2O3particles, and accordingly the scratching level and surface roughness were reduced.
In addition, through the grafting of water-soluble polystyrene sulfonic acid chain on the α-Al2O3surface, the dispersibility of α-Al2O3particles in aqueous medium was improved and large particles were eliminated. The elimination of the agglomeration of α-Al2O3particles may in turn lead to the reduction of surface scratch and the improvement of surface planarization.
4 Conclusions
(1) Novel α-Al2O3-g-PSS core-shell abrasive was prepared via a three-step process: surface activation,followed by graft polymerization and sulfonation,respectively.
(2) FTIR, XPS, TOF-SIMS and SEM analyses indicate that modified α-Al2O3abrasive has better dispersion stability than pure α-Al2O3.
(3) The slurry containing α-Al2O3-g-PSS composition abrasive exhibits better surface planarization and less scratch in the CMP of glass substrate surface. The improvement of surface qualities may be attributed to the cushioning effect of its core-shell structure and the elimination of the agglomeration among the α-Al2O3particles.
[1] PATRICK W J, GUTHRIE W L, STANDLEY C L. Application of chemical mechanical polishing to the fabrication of VLSI circuit interconnections[J]. Journal of the Electrochemical Society, 1991,138(6): 1 778–1 784.
[2] LEI Hong, LUO Jianbin. CMP of hard disk substrate using a colloidal SiO2slurry: preliminary experimental investigation[J].Wear, 2004, 257(5–6): 461–470.
[3] LEI Hong, ZHANG Pengzhen, LU Haishen. Sub-nanometer precision polishing of glass substrate with a colloidal SiO2slurry[J].Lubrication Engineering, 2006, 1(1): 31–34. (in Chinese)
[4] SOROOSHIAN A, ASHWANI R, CHOI H K. Effect of particle interaction on agglomeration of silica-based CMP slurries[C]//Materials Research Society Symposium Proceedings, San Francisco,CA, United States, April 13–15, 2004: 125–131.
[5] ZHOU Chunhong, SHAN Lei, HIGHT R J, et al. Influence of colloidal abrasive size on material removal rate and surface finish in SiO2chemical mechanical polishing[J]. Tribology Transactions,2002, 45(2): 232–238.
[6] BASIM G B, ADLER J J, MAHAJAN U. Effect of particle size of chemical mechanical polishing slurries for enhanced polishing with minimal defects[J]. Journal of the Electrochemical Society, 2000,147(9): 3 523–3 528.
[7] LEI Hong, ZHANG Pengzhen. Preparation of alumina/silica core-shell abrasives and their CMP behavior[J]. Applied Surface Science, 2007, 253(21): 8 754–8 761.
[8] LEI Hong, LUO Jianbin, LU XinChun. Two-step chemicalmechanical polishing of rigid disk substrate to get atom-scale planarization surface[J]. Chinese Journal of Mechanical Engineering, 2006, 19(4): 496–499.
[9] LEI Hong, CHU Yuliang, TU Xifu. Preparation of ultra-fined Al2O3slurry and its polishing properties on disk CMP[J]. Journal of Functional Materials, 2005, 36(9): 1 425–1 428. (in Chinese)
[10] ZHANG Pengzhen, LEI Hong, ZHANG Jianping. Preparation of nano-sized CeO2and its polishing performances[J]. Optical Technique, 2006, 32(5): 682–684. (in Chinese)
[11] FENG Xiandong, HER Y S, ZHANG W L, et al. CeO2particles for chemical mechanical planarization[C]//Materials Research Society Symposium Proceedings, San Francisco, CA, United States, April 12–24, 2003, 767: 173–183.
[12] XU Jin, LUO Jianbin, WANG Liangliang, et al. The crystallographic change in subsurface layer of the silicon single crystal polished by chemical mechanical polishing[J]. Tribology International, 2007, 40(2): 285–289.
[13] XU Jin, LUO Jianbin, ZHANG ChaoHui, et al. Nano-deformation of a Ni-P coating surface after nanoparticle impacts[J]. Applied Surface Science, 2006, 252(16): 5 846–5 854.
[14] XU Jin, LUO Jianbin, LU XinChun, et al. Atomic scale deformation in the solid surface induced by nanoparticle impacts[J].Nanotechnology, 2005, 16(6): 859–864.
[15] LUO Jianbin, XU Xifu, JING Yang, et al. Movements and collisions of nanoparticles in two phase flow[C]//Proceedings of the World Tribology Congress III-2005, Washington D.C., United States, September 12–16, 2005: 355–356.
[16] ZHANG Zefang, LEI Hong. Preparation of α-alumina/polymethacrylic acid composite abrasive and its CMP performance on glass substrate[J]. Microelectronic Engineering, 2008, 85(4):714–720.
[17] SINGH R K, BAJAJ R. Advances in chemical-mechanical planarization[J]. MRS Bulletin, 2002, 27(10): 743.
[18] YANO H, MATSUI Y, MINAMIHABA G, et al.High-performance CMP slurry with inorganic/resin abrasive for Al/low-k damascene[C]//Materials Research Society Symposium Proceedings, San Francisco, CA, United States, April 18–20, 2001,671: M2.4.1–M2.4.5.
[19] SHIHO H, MANABE Y, KAWAHASHI N. Magnetic compounds as coatings on polymer particles and magnetic properties of the composite particles[J]. Journal of Material Chemistry, 2000, 10:333–336.
[20] KAWAHASHI N, HATTORI M. An evaluation on the effects of newly designed abrasives in CMP slurry[C]//Materials Research Society Symposium Proceedings, San Francisco, CA, United States,April 18–20, 2001, 671: M2.2.1–M2.2.8.
[21] SHIHO H, KAWAHASHI N. Iron compounds as coatings on polystyrene latex and as hollow spheres[J]. Journal of Colloid and Interface Science, 2000, 226(1): 91–97.
[22] SILVIA A, VALENTINA T, KAREN M. Composite nanoparticles for defectivity reduction during CMP[C]//AIChE Annual Meeting,Conference Proceedings, Austin, TX, United States, November 7–12, 2004: 2 473–2 484.
[23] LEI Hong, LU Haishen, LUO Jianbin, et al. Preparation of α-alumina-g-polyacrylamide composite abrasive and chemical mechanical polishing behavior[J]. Thin Solid Films, 2008, 516(10):3 005–3 008.
[24] CECIL A COUTINHO A, SUBRHAMANYA R MUDHIVARTHI,et al. Novel ceria-polymer microcomposites for chemical mechanical polishing[J]. Applied Surface Science, 2008, 255(4):3 090–3 096.
[25] DAUCHOT G, CASTRO E D, REPOUX M, et al. Application of TOF-SIMS surface analysis to tribochemistry in metal forming processes[J]. Wear, 2006, 260(3): 296–304.
猜你喜欢
杂志排行
Chinese Journal of Mechanical Engineering的其它文章
- Precision Grinding of Reaction Bonded Silicon Carbide Using Coarse Grain Size Diamond Wheels
- Fretting Wear Behavior of Medium Carbon Steel Modified by Low Temperature Gas Multi-component Thermo-chemical Treatment
- Auditory-model-based Feature Extraction Method for Mechanical Faults Diagnosis
- Discrete Stress-strength Interference Model of Reliability Analysis under Multi-operating Conditions
