Effect of Aminoaromatic Acids as Additives on the Activity andSelectivity of the Platinum-catalyzed Hydrosilylation of Alkenes*
2009-05-15BAIYing白赢PENGJiajian彭家建HUYingqian胡应乾LIJiayun厉嘉云QIUHuayu邱化玉andLAIGuoqiao来国桥
BAI Ying (白赢), PENG Jiajian (彭家建), HU Yingqian (胡应乾), LI Jiayun (厉嘉云), QIU Huayu(邱化玉) and LAI Guoqiao (来国桥)
Effect of Aminoaromatic Acids as Additives on the Activity andSelectivity of the Platinum-catalyzed Hydrosilylation of Alkenes*
BAI Ying (白赢), PENG Jiajian (彭家建), HU Yingqian (胡应乾), LI Jiayun (厉嘉云), QIU Huayu(邱化玉) and LAI Guoqiao (来国桥)**
Key Laboratory of Organosilicon Chemistry and Material Technology of the Ministry of Education, Hangzhou Normal University, Hangzhou 310012, China
A series of aromatic acids has been tested as additives for the platinum-catalyzed hydrosilylation of styrene with triethoxysilane. Both excellent conversion of styrene and selectivity in favor of the-adduct were achieved using aminobenzoic acids as additive. Moreover, the use of 4-aminobenzoic acid led to significantly superior enhancement in both catalytic activity and selectivity among the tested aminobenzoic acids. Indeed, 100% conversion of styrene and 98.4% selectivity in favor of the-adduct were obtained. Additionally, hydrosilylations of various alkenes with a variety of platinum catalysts have also been tested, and in each case the conversion of substrate and the selectivity of the-adduct were promoted by using 4-aminobenzoic acid as additive.
hydrosilylation, platinum catalyst, aminoaromatic acid, additive
1 INTRODUCTION

In this paper, we present the results of our study on the effect of aminoaromatic acids as promoter for the platinum-catalyzed hydrosilylation of alkenes (Scheme 1), and we compare their effect with those of aromatic acids and aromatic amines.
2 EXPERIMENTAL
GC-MS analysis was performed with a TRANCE DSQ spectrometer (Finnigan, USA) using a DB 530 m × 2.5 mm × 0.25 μm column; split: 50︰1; flow: 1 ml·min-1constant flow; inlet temp.: 260°C; column temp.: 50°C (hold for 1 min) then 15°C·min-1up to 260°C (held for 10 min).
1H-NMR spectra were recorded on a Bruker 400 MHz spectrometer using TMS as an internal standard in CDCl3.
General procedure: A certain amount of aminoaromatic acid, aromatic acid, or aromatic amine was mixed with catalyst solution H2PtCl6/THF (Pt content: 1.95×10-5mol·ml-1in THF) in a 10 ml flat-bottomed tube and the mixture was heated at 60°C for 10 min. Styrene (4.0 mmol) was then added, the reaction mixture was stirred for another 5 min, and then triethoxysilane (4.4 mmol) was added. After agitating at 60°C for 5 h, the reaction mixture was characterized by1H-NMR, GC, and GC-MS.
1H-NMR characterization of the-adduct from the hydrosilylation of styrene with triethoxysilane:2.66 (Ph-CH2),-adduct,3.40 (Ph-CH).
1H-NMR characterization of the-adduct from the hydrosilylation of 1-hexene with triethoxysilane:0.64 (Si-CH2).
1H-NMR characterization of the-adduct from the hydrosilylation of 1-octene with triethoxysilane:0.63 (Si-CH2).
1H-NMR characterization of the-adduct from the hydrosilylation of 1-undecene with triethoxysilane:0.62 (Si-CH2).
3 RESULTS and DISCUSSION
3.1 Effect of various additives on the hydrosilylation reaction
The results of hydrosilylations of styrene with triethoxysilane catalyzed by H2PtCl6in the presence of various additives are listed in Table 1.
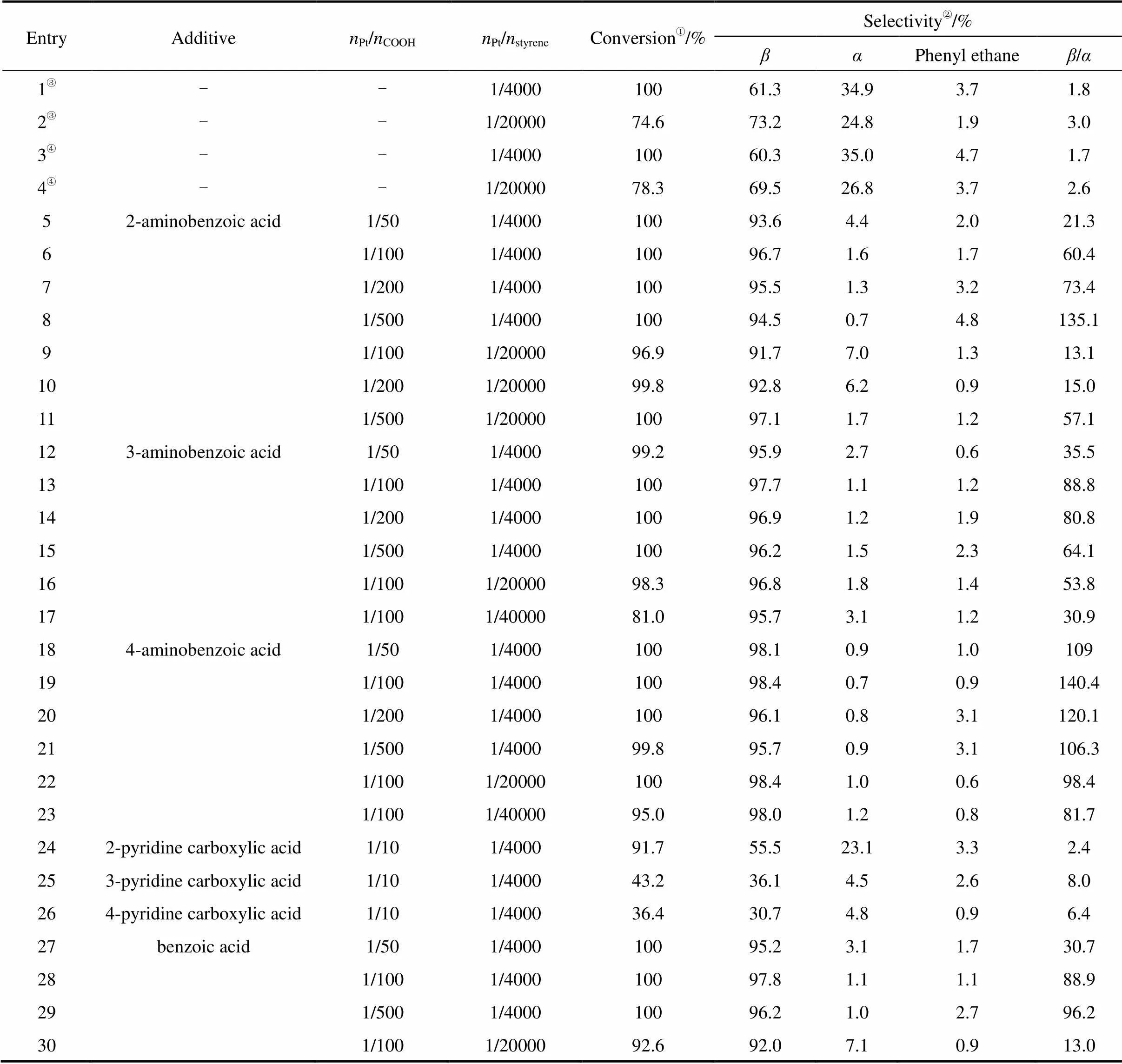
Table 1 Hydrosilylation of styrene with triethoxysilane catalyzed with H2PtCl6(styrene 4.0 mmol, triethoxysilane 4.4 mmol, 60°C, 5 h)
①Determined by GC analysis. ②Determined by GC-MS. ③Using H2PtCl6in THF solution as catalyst.④Using Speier’s catalyst.
Firstly, the hydrosilylation of styrene with triethoxysilane catalyzed by H2PtCl6/THF was conducted at various molar ratios ofPt/styrene. 100% conversion of styrene and 61.3% selectivity in favor of the-adduct were obtained (entry 1). When the molar ratio of platinum to styrene was decreased to 1/20000, the conversion decreased to 74.6% (entry 2). Similar results were obtained when Speier’s catalyst (1.95×10-5mol·ml-1in isopropanol solution) was used (entries 3 and 4).
Secondly, the hydrosilylation of styrene with triethoxysilane catalyzed by H2PtCl6/THF in the presence of aminobenzoic acids was tested. Surprisingly, the selectivity of the-adduct was significantly improved. Subsequently, a series of isomeric aminobenzoic acids, namely 2-aminobenzoic acid, 3-aminobenzoic acid, and 4-aminobenzoic acid, with various molar ratios ofPt/COOH, was tested under similar conditions (entries 5-23). The selectivity in favor of the-adduct relative to the-adduct (/) was seen to increase with increasing amounts of 2-aminobenzoic acid added (entries 5-8). Moreover, the conversion of styrene also increased upon decreasing the molar ratio ofPt/COOHfrom 1/100 to 1/500 (entries 9-11), and the best selectivity was obtained whenPt/COOHwas 1/500 (entry 8). The results indicated that 2-aminobenzoic acid enhanced both the catalytic activity and the selectivity in favor of the-adduct relative to the-adduct (/).
Under the same reaction conditions, 3-aminobenzoic acid and 4-aminobenzoic acid were likewise tested as additives. For both of these isomeric acids, the best selectivities were obtained whenPt/COOHwas 1/100 (entries 13 and 19).
As can be seen from Table 1, excellent conversions of styrene and selectivities of the-adduct were achieved by using aminobenzoic acids as additives in the platinum-catalyzed hydrosilylation of styrene with triethoxysilane. However, the use of 4-aminobenzoic acid led to superior enhancements of both the catalytic activity and selectivity compared to 2-aminobenzoic acid or 3-aminobenzoic acid.
Considering the coexistence of amino and carboxylic acid groups in aminoaromatic acids, the catalytic effect might be due to the interplay of these two groups. To verify this supposition, pyridine carboxylic acids were also tested as additives. However, each of the pyridine carboxylic acids, namely 2-, 3-, and 4-pyridine carboxylic acids, had a retarding effect on the catalytic activity (entries 24-26). For comparison purposes, benzoic acid was also used as an additive, and the results of entries 27-29 indicate a marked enhancement of the catalytic process.
In summary, among the aromatic acids tested, 4-aminobenzoic acid proved to be the best promoter of the platinum-catalyzed hydrosilylation reaction.
3.2 Effect of 4-aminobenzoic acid on the catalytic process
In order to obtain more information on the influence of 4-aminobenzoic acid on the hydrosilylation reaction, the reaction rate was investigated. As can be seen from Fig. 1 (a), the conversions of styrene with various catalysts proceeded at widely differing rates. The rate of hydrosilylation of styrene with triethoxysilane catalyzed by H2PtCl6/THF conforms to a normal second-order curve. As described above, the reaction rate is significantly promoted in an H2PtCl6/4- aminobenzoic acid system, and this rate enhancement in the hydrosilylation reaction was further confirmed by comparing the kinetics of the respective reactions with H2PtCl6/THF under the same conditions. The transformation of styrene reached completion in just 10 min when using the H2PtCl6/4-aminobenzoic acid catalyst system, while conversions of styrene of only 43.7% and 30% were obtained by using Karstedt’s catalyst and the H2PtCl6/THF catalyst, respectively. After 150 min, the conversion of styrene had reached 98.5% catalyzed by Karstedt’s catalyst. However, the selectivities in favor of the-adduct were 62.3% and 98.6%, respectively, when using the H2PtCl6/THF system and the H2PtCl6/4-aminobenzoic acid system [Fig. 1 (b)].
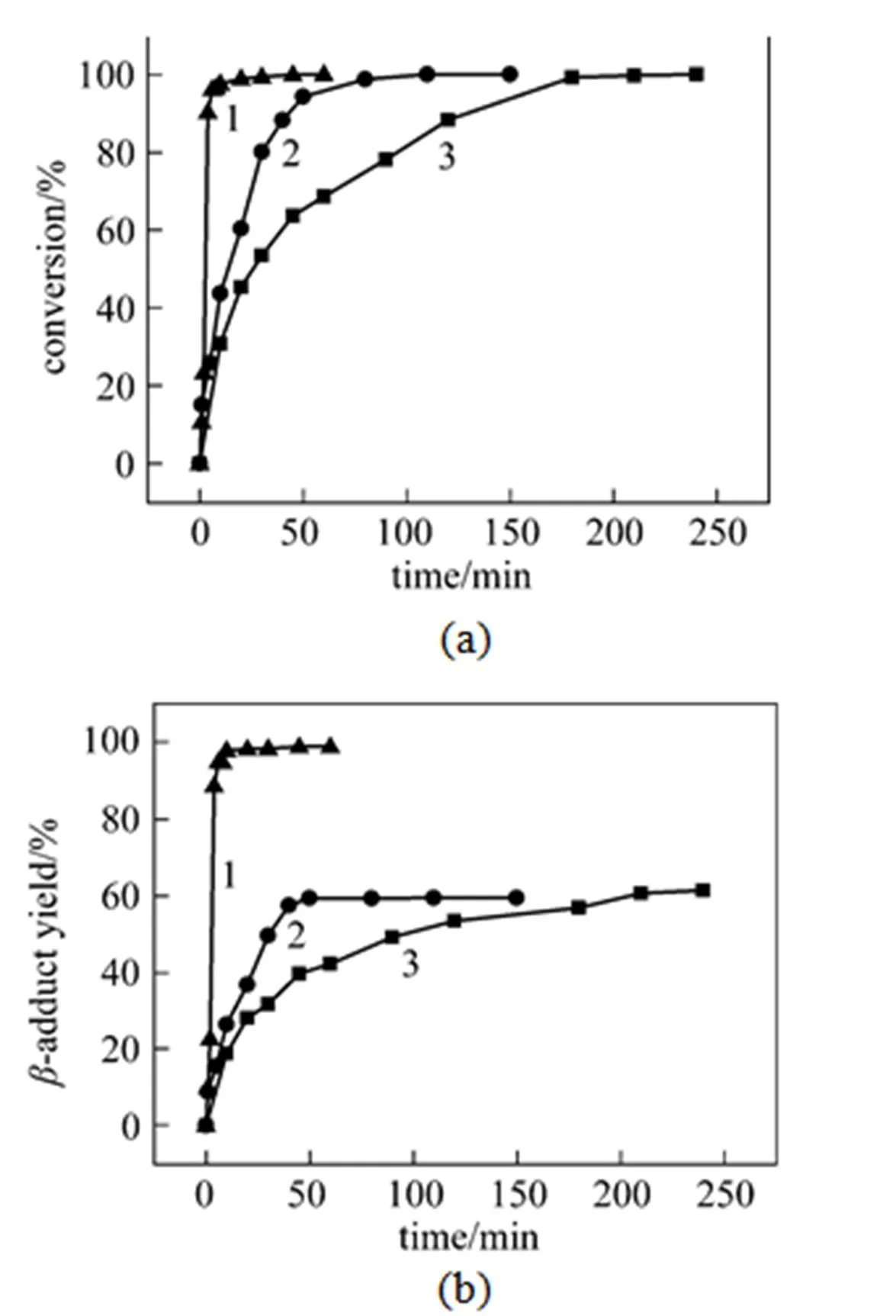
Figure 1 Model hydrosilylation of styrene with triethoxysilane with various platinum catalysts
catalyst:▲ H2PtCl6·THF/4-aminobenzoic acid;● Karstedt catalyst;■ H2PtCl6·THF
3.3 Effect of 4-aminobenzoic acid on the hydrosilylation of various alkenes
In order to investigate the scope of the reaction in terms of substrates, hydrosilylations of various alkenes with triethoxysilane under catalysis by H2PtCl6/4- aminobenzoic acid were tested under similar conditions. The results are summarized in Table 2.
Hydrosilylations of a variety of alkenes with triethoxysilane were conducted at 60°C using 0.025% (by mole) of H2PtCl6along with 4-aminobenzoic acid as an additive. Simultaneously, hydrosilylations of various alkenes using 0.025% (by mole) of H2PtCl6(in THF solution) without an additive were also tested. High selectivity was achieved by using the H2PtCl6/4- aminobenzoic acid system for the hydrosilylation of linear alkenes. As shown in Table 2, catalytic hydrosilylation reactions of 1-hexene, 1-octene, and 1-undecene, respectively, with triethoxysilane were carried out, and the corresponding hydrosilylation products were obtained in yields of 81%-88% with the H2PtCl6/THF catalyst system. Under the same conditions, but using the H2PtCl6/4-aminobenzoic acid catalyst system, hydrosilylation reactions of 1-hexene, 1-octene, and 1-undecene with triethoxysilane proceeded rapidly to give the corresponding adducts in yields of 94%-99%. An improvement in the activity and selectivity was thus observed. Unfortunately, none of the expected product could be detected when allylic chloride was used as the substrate.
Further studies are in progress, aimed at investigating the role of the aromatic acid in the catalytic system and the scope of the reaction in terms of substrates that can be applied. Mechanistic studies by both experimental and computational methods will also be carried out in due course.
1 Marciniec, B., Guliński, J., Urbaniak, W., Kornetka, Z.W., Comprehensive Handbook on Hydrosilylation, Pergamon Press, London (1992).
2 Sommer, L., Pietrusza, H.E.W., Whitmore, F.C., “Peroxide-catalyzed addition of trichlorosilane to 1-octene”,...., 69 (1), 188-189 (1947).
3 Speier, J.L., Machay, F.P., Steward, Q.W., Campbell, P.G., “The addition of silicon hydrides to alkeneic double bonds (II) The use of group VIII metal catalysts”,...., 79 (4), 974-979 (1957).
4 Ryan, J.W., Speier, J.L., “Notes-addition of silicon hydrides to alkeneic double bonds (IV) The addition to styrene and-methylstyrene”,..., 24 (12), 2052-2053 (1959).
5 Karstedt, B.D., Scotia, N.Y., “Platinum complexes of unsaturated siloxanes and platinum containing organopolysiloxanes”, US Pat., 3775452 (1973).
6 Marciniec, B., Kornetka, Z.M., Urbaniak, W., “Platinum and rhodium complexes supported on aminated silica as hydrosilylation catalysts”,..., 12 (2), 221-230 (1981).
7 Wang, L.Z., Jiang, Y.Y., “Catalytic activity and selectivity of poly--aminopropylsiloxane-Pt complex in hydrosilylation of unsaturated compounds”,..., 2 (3), 236-238 (1981). (in Chinese)
8 Wang, L.Z., Jiang, Y.Y., “An active and stable hydrosilylation catalyst, silica-poly--mercaptopropyl-siloxane-platinum complex”,.., 78-82 (1983). (in Chinese)
9 Hu, C.Y., Han, X.M., Jiang, Y.Y., “Hydrosilylation of acetylene catalyzed by a sulfur-containing polysiloxane platinum complex”,..., 35 (3), 329-333 (1986).
10 Chen, Y.M., Lu, X.R., Zhong, Z.L., “Synthesis of poly-4,7- dithianonylsesquisiloxane platinum complex and its catalyst activity on hydrosilylation of olefins with triethoxysilane”,.., 9 (3), 26-30 (1992). (in Chinese)
11 Liedtke, J., Loss, S., Widauer, C., Grützmacher, H., “Phosphiranes as ligands for platinum catalysed hydrosilylations”,, 56, 143-156 (2000).
12 Prignano, A.L., Trogler, W.C., “Silica-supported bis(trialkylphosphine) platinum oxalates. Photogenerated catalysts for hydrosilylation of alkenes”,...., 109 (12), 3586-3595 (1987).
13 Yamamoto, K., Hayashi, T., Zembayashi, M., Kumada, M., “Catalytic asymmetric hydrosilylation of alkenes (II) chiral phosphine-platinum (II) complexes as hydrosilylation catalysts”,..., 118 (2), 161-181 (1976).
14 Marciniec, B., Foltynowicz, Z., Urbaniak, W., “Catalysis of hydrosilylation (XV) A poly(phosphino-organosiloxanyl)silicate-supportedrhodium (I) catalyst for gas-phase hydrosilylation of acetylene”,..., 1 (5), 459-463 (1987).
15 Markó, I.E., Stérin, S., Buisine, O., Mignani, G., Branlard, P., Tinant, B., Declercq, J.P., “Selective and efficient platinum(0)-carbene complexes as hydrosilylation catalysts”,, 298, 204-206 (2002).
16 Bo, D.G., Berthon-Gelloz, G., Tinant, B., “Hydrosilylation of alkynes mediated by N-heterocyclic carbene platinum(0) complexes”,, 25 (8), 1881-1890 (2006).
17 MarkÓ, I.E., Stérin, S., Buisine, O., Berthon, G., Michaud, G., Tinant, B., Declercq, J.P., “Highly active and selective platinum(0)-carbene complexes: Efficient, catalytic hydrosilylation of functionalised alkenes”,..., 346 (12), 1429-1434 (2004).
19 Poyatos, M., Maisse-Francüois, A., Bellemin-Laponnaz, S., Gade, L.H., “Coordination chemistry of a modular N,C-chelating oxazole-carbene ligand and its applications in hydrosilylation catalysis”,, 25 (10), 2634-2641 (2006).
20 Mehta, K.R., “Process for the preparation of siloxane-oxyalkylene copolymers”, US Pat., 4847398 (1989).
21 Curtis, L., Schilling, Jr., “Method of highly efficient hydrosilylation”, CN 99122127.3 (1999).
22 Hamze, A., Provot, O., Brion, J.D., Alami, M., “Xphos ligand and platinum catalysts: A versatile catalyst for the synthesis of functionalized-(E)-vinylsilanes from terminal alkynes”,..., 693 (16), 2789-2797 (2008).
23 Hamze, A., Provot, O., Brion, J.D., Alami, M., “Platinum chloride/Xphos-catalyzed regioselective hydrosilylation of functionalized terminal arylalkynes”,., 49, 2429-2431 (2008).
24 Arsenyan, P., Oberte, K., Rubina, K., Belyakov, S., “Synthesis and application of a new selenoplatinum catalyst”,., 46, 1001-1003 (2005).
2009-04-27,
2009-09-28.
the National High Technology Research and Development Program of China (2006AA03A134) and Zhejiang Province Program (2008C14041).
** To whom correspondence should be addressed. E-mail: gqlai@hznu.edu.cn
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Study on the Flow Field around Two Parallel Moving Bubbles andInteraction Between Bubbles Rising in CMC Solutions by PIV*
- Kinetic Rate Constant of Liquid Drainage from Colloidal Gas Aphrons*
- β-Diketones at Water/Supercritical CO2 Interface: A MolecularDynamics Simulation*
- Analysis of Sucrose Esters with Long Acyl Chain by Coupling ofHPLC-ELSD with ESI-MS System*
- Adsorptive Removal of Copper Ions from Aqueous Solution UsingCross-linked Magnetic Chitosan Beads
- Gas Flow in Unilateral Opening Pulse Tubes Based on Real Gas Equation of State
