Adsorptive Removal of Copper Ions from Aqueous Solution UsingCross-linked Magnetic Chitosan Beads
2009-05-15HUANGGuolin黄国林YANGChuo杨婥ZHANGKai章凯andSHIJeffrey
HUANG Guolin (黄国林), YANG Chuo (杨婥), ZHANG Kai (章凯) and SHI Jeffrey
Adsorptive Removal of Copper Ions from Aqueous Solution UsingCross-linked Magnetic Chitosan Beads
HUANG Guolin (黄国林)1,*, YANG Chuo (杨婥)1, ZHANG Kai (章凯)1and SHI Jeffrey2
1Key Laboratory of Nuclear Resources and Environment of Ministry of Education, East China Institute of Technology, Jiangxi 344000, China2School of Chemical and Biomolecular Engineering, The University of Sydney, Sydney 2006, Australia
The performance of cross-linked magnetic chitosan, coated with magnetic fluids and cross-linked with epichlorohydrin, was investigated for the adsorption of copper (II) from aqueous solutions. Infrared spectra of chitosan before and after modification showed that the coating and cross-linking are effective. Experiments were performed at different pH of solution and contact time, and appropriate conditions for the adsorption of Cu(II) were determined. Experimental equilibrium data were correlated with Langmuir and Freundlich isotherms for determination of the adsorption potential. The results showed that the Langmuir isotherm was better compared with the Freundlich isotherm, and the uptake of Cu(II) was 78.13 mg·g-1. The kinetics of adsorption corresponded with the first-order Langergren rate equation, and Langergren rate constants were determined.
adsorption of copper (II), cross-linked magnetic chitosan, Langmuir isotherm, Langergren rate equation
1 INTRODUCTION
The presence of heavy metals in the environment has been of great concern because of their increased discharge, toxic nature and other adverse effects on receiving waters. The potential sources of copper in industrial effluents include metal cleaning and plating baths, pulp, paper and paper board mills, wood pulp production, fertilizer industry,[1]. Excessive intake of copper results in an accumulation in the liver. It is also toxic to aquatic organisms even at very small concentrations in natural water [2].
The conventional methods for heavy metal removal from water and wastewater include oxidation [3], reduction [4], electro-chemical precipitation [5], ion exchange [6], and membrane ultrafiltration [7]. Adsorptiontechniques have been shown to be a feasible option, both technically and economically [8]. It is reported that chitosan has become increasingly popular for adsorption of Cu(II) [9-11]. The amine groups and hydroxyl groups on the chitosan chain act as chelation sites for Cu(II) or other metal ions. Recent research interest has been focused on the modification of chitosan for enhanced adsorption performance [12, 13]. Coating chitosan with magnetic fluids is a typical modification method, which improves the surface area for adsorption and reduces the required dosage for the adsorption of metal ions [14, 15].
Glutaraldehyde, epichlorohydrin and ethylene glycol diglycidyl ether have been used to cross link the magnetic chitosan to improve the adsorption behaviour, since the cross-linking can change the crystalline nature of chitosan and enhance the adsorption ability [16]. Hsien. [17] prepared highly porous chitosan beads by dropwise adding an acidic chitosan solution into a precipitation bath of sodium hydroxide solution. The gelled chitosan beads were cross-linked with glutaraldehyde and then freeze-dried. Well-mixed batch adsorption experiments revealed that the cross-linked chitosan presented good adsorption ability for both metal and hydronium ions by a chelation mechanism. Li. [18] prepared a magnetic type of chitosan by coating chitosan with magnetite particles during the coprecipitation of Fe3+and Fe2+in alkaline solutions, and then cross-linking the resulting material with glutaraldehyde. The adsorption capacities of La3+, Nd3+, Eu3+and Lu3+ions on the magnetic chitosan were studied, and the adsorption behavior was fitted using the Langmuir equation.
In this study, a crosslinked magnetic chitosan (CMC) adsorbent is prepared. Infrared (IR) technique is used to study the surface properties of the CMC. The adsorption behavior of Cu(II) on the CMC is studied in a batch reactor for some adsorption parameters, including the pH value of the solution and the adsorption contact time, so that the adsorption process may be optimized. The Cu(II) adsorption isotherm and kinetics are to be determined. The information will be useful for further application in the treatment of waste effluents containing copper ions.
2 EXPERIMENTAL
2.1 Chemicals and reagents
Chitosan was purchased from China Chemical Agent Co. as a flaked material, with a deacetylation percentage of approximate 90% and an average molecular weight of 3.2´105. All other reagents were analytical grade, and distilled or double distilled water was used in the preparation of all solutions.
2.2 Preparation of Cu(II) solution
The Cu(II) stock solution with a concentration of 200 mg·L-1was prepared by dissolving (0.7812±0.0001) g of CuSO4×5H2O dried at 105°C for 2 h in a 1000 ml volumetric flask with deionized water. The experimental solutions were prepared at 50, 100 and 150 mg·L-1by serial dilution from the stock solution of 200 mg·L-1.
2.3 Preparation of cross-linked magnetic chitosan beads
A known volume of 8% (by mass) gelatin solution, 150 ml of double distilled water, 11.25 ml of 0.5 mol·L-1ferric sulfate solution and 10.00 ml of 1 mol·L-1Fe(NO3)3solution were added in a four-neck rounded bottom flask, with a dropper, a thermometer, a magnetic stirrer and a N2purge gas connected to the reaction flask. The mixture solution was purged with nitrogen and stirred in a water bath at 60°C for 30 min. The pH value of solution was adjusted to maintain at 8.0-9.0 by adding 25% (by volume) ammonium hydroxide solution during the reaction. A magnetic fluid was obtained for use in the chitosan coating.
0.5 g chitosan flake was dissolved in a 50 ml, 0.05 mol·L-1hydrochloric acid solution to give a final concentration of 1% (by mass). The chitosan solution was then dropped into 50 ml of the magnetic fluid in the flask through a dropper. After this step, 13 ml of pure epichlorohydrin was added into the reaction flask and mixed with the solution by stirring at 85°C for 3 hours, before the flask was cooled down to room temperature. The precipitate was first washed with distilled water until no Cl-(AgNO3method) was left in the effluents, and then washed with ethanol and ether and dried in a 50°C vacuum oven. The obtained product appears to be a deep yellow powder and is calledcross-linked magnetic chitosan (CMC) here. The CMC was then ground and sieved (< 250mm) before use.
2.4 Characterization of cross-linked magnetic chitosan beads
A swelling study for the CMC was carried out in distilled water at 25°C for a period of 24 h, and the amino content of the CMC was analyzed by the following procedure. 1 g of dry CMC was dissolved in a 20 ml, 0.3 mol·L-1hydrochloric acid solution. A sodium hydroxide (0.3 mol·L-1) solution was used for titration with methyl orange as indicator. The percentage swelling and the amino content of the CMC were calculated by using the following equations:

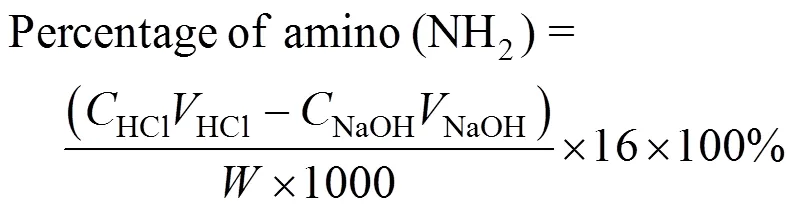
wheresandare the volume of swollen and dry beads in ml, respectively,HClandHClare the concentration and volume of hydrochloric acid standard solution added, in mol·L-1and ml, respectively, andNaOHandNaOHare the concentration and volume of sodium hydroxide standard solution used in the titration process, in mol·L-1and ml, respectively, andis the mass of dry beads in g.
The Fe content in the CMC was determined by using ICPE-9000 plasma emission spectrometry (Beijing, China). Infrared spectra of chitosan flake were recorded for the sample before and after the modification on a Vertex 7.0 IR spectrum (Bruker Co., Switzerland) spectrophotometer by using pressed KBr pellets. A scanning electron microscope (JSM-5900) photograph of the CMC samples was obtained. The sample was coated with carbon, and the SEM was operated at 10 keV.
2.5 Adsorption experiments
Batch adsorption experiments were carried out by using the CMC as the adsorbent. A series of conical flasks containing Cu(II) solutions with initial concentrations of 100 mg·L-1and a known dosage of the CMC were shaken in a SHA-C shaker (Changzhou, China) with a shaker speed of 100 r·min-1until the system reached equilibrium. The experiments indicated that two hours was adequate at ambient temperatures for the equilibrium to be reached. Before shaking, the pH value of the solution was adjusted with 0.5 mol·L-1H2SO4or 0.5 mol·L-1NaOH to cover a range from 2.0 to 9.0, which was measured using a PHS-3C pH meter (Hangzhou, China). After filtration, the concentration of Cu(II) in the supernatant was analysed by a standard atomic absorption spectroscopy method (China National Standards, GB/T 14638.1-1993). Three replicates were used at least to get each adsorption datum, and the standard deviations were less than 2% on the average. The adsorption amount was calculated based on the difference in the Cu(II) concentration in the aqueous solution before and after adsorption, according to the following equation:

where0andeare the initial and equilibrium concentrations of Cu(II), in mg·L-1, respectively,is the volume of Cu(II) solution, in L, andis the mass of the CMC used, in g.
The Cu(II) solution with initial concentration of 100 mg·L-1was used for adsorption isotherms, and three Cu(II) solutions with initial concentrations of 50, 100, and 150 mg·L-1were used for adsorption kinetics in our experiments.
2.6 Regeneration of adsorbent
For the purpose of reducing the operating cost and minimizing waste disposal, a practical recycling method of CMC was developed. 0.2 g Cu(II) saturated CMC was immersed in a 100 ml beaker with 20 ml of 0.1 mol·L-1hydrochloric acid solution for 24 h at room temperature. After filtration, the residual was washed with distilled water until no Cu(II) was left in the effluent. The CMC was then dried under vacuum.
3 RESULTS AND DISCUSSION
3.1 Characterization of CMC
It is found that the CMC obtained is insoluble in acidic and alkaline media as well as distilled water. Compared with the swelling behaviour of original chitosan flake, 37.50%, and its amino content, 10.20%, the corresponding values of the modified CMC were improved to 19.74% and 18.80%, respectively. The measurement was repeated twice. The amino group was recognized as an active binding site for the adsorption of heavy metal ions [19], and low swelling percentage was an important factor for use in a continuous adsorption column [20]. The Fe content in the CMC was 7.85% (by mass).

Figure 1 The IR spectrum of chitosan (a) and CMC (b)


Figure 2 The SEM appearance of CMC
3.2 Effect of pH on adsorption
The pH of the aqueous solution is an important controlling parameter in adsorption processes. The binding of metal ions by surface functional groups is strongly pH dependent. The effect of solution pH on the adsorption has been investigated over the range from 2.0 to 9.0, and the results are shown in Fig. 3. The uptake capacity of Cu(II) increases when the solution pH is increased from 2.0 to 6.0. The explanation may be that, at a lower pH, the amine groups on CMC surfaces are easily protonized, inducing an electrostatic repulsion of Cu(II) ions [12]. The maximum adsorption capacity occurs at pH 6.0 in the concentration range studied. The adsorption amount decreases significantly in the range of 7.0-9.0. Similar observations have been reported by Sankararamakrishnan. [23], Juang and Shao [19]. The optimal pH of 6.0 was selected for further study in the experiments.

Figure 3 The effect of pH on the adsorption amount
◇ 50 mg·L-1;□ 100 mg·L-1;△ 150 mg·L-1
3.3 Adsorption isotherm
In order to determine the adsorption potential, an adsorption isotherm is essential. The experimental data of Cu(II) on the CMC at ambient temperatures with the initial Cu(II) concentration of 100 mg·L-1are correlated with the Langmuir and Freundlich isotherms. The Langmuir isotherm is expressed as
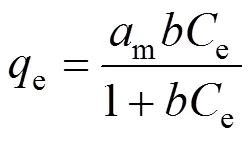
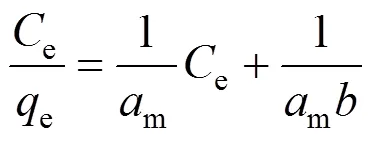
And the Freundlich isotherm is expressed as


whereeis the amount of adsorbed Cu(II) per gram of CMC andeis the equilibrium concentration of Cu(II) in the bulk solution, in mg·g-1and mg·L-1, respectively. The constantmandare characteristics of Langmuir equation, in mg·g-1and L·mg-1, respectively, whileandare the constants of Freundlich equation incorporating adsorption capacity and intensity, in L·g-1, respectively.
The Langmuir and Freundlich isotherms for the adsorption of Cu(II) from aqueous solution onto CMC are plotted in Figs. 4 and 5, and the parameters in two adsorption isotherms with the correlation coefficients are presented in Table 1. The linear relationships are statistically significant, which indicate the applicability of the two adsorption isotherms and the monolayer coverage of Cu(II) on the CMC surface. Adsorption capacity obtained from the Langmuir isotherm was 78.13 mg Cu(II) per gram CMC for Cu(II) concentration of 100 mg·L-1. In our previous study [24], the adsorption capacity was 82.37 mg Cu(II) per gram original chitosan. Although chitosan beads show higher adsorption capacity than the CMC beads, the CMC shows lower percentage of swelling, which is an important feature for an adsorbent, so that it can be used as a resin in ion-exchange chromatography columns.

Figure 4 The Langmuir plot
Figure 5 The Freundlich plot

Table 1 Constants in Langmuir and Freundlich isotherms
The Langmuir equation and the Freundlich equation for adsorption of Cu(II) are:

The essential characteristics of the Langmuir isotherm can be described by a separation factor, which is defined as [23]

The value ofLindicates the shape of Langmuir isotherm and the nature of adsorption process. It is considered as a favourable process when the value is within the range 0-1. In our study, the values ofLcalculated for the initial Cu(II) concentrations is 0.019, so that the adsorption of Cu(II) on CMC is a favourable process. The Freundlich constantsandare 53.06 L·g-1and 8.76, respectively. It is known thatvalue between 1 and 10 represents an appropriate adsorption.
3.4 Adsorption kinetics
The effect of contact time on the amount of adsorption has been investigated over the range from 10 to 80 min with three initial Cu(II) concentrations of 50, 100, and 150 mg·L-1, and the results are shown in Fig. 6. The uptake amount of Cu(II) increases with contact time but increases slightly after 60 min of contact time, indicating that the equilibrium is almost reached after 60 min. Therefore, the optimum contact time for adsorption of Cu(II) is about 60 min.

Figure 6 Effects of contact time on the adsorption behavior
◇ 50 mg·L-1;□ 100 mg·L-1;△ 150 mg·L-1
In order to examine the controlling mechanism for the adsorption process, kinetic models are used to assess the experimental data. The Langergren rate equation is one of the most widely used equations for the adsorption of a solute from a liquid solution [25].
The first order rate equation may be represented as follows
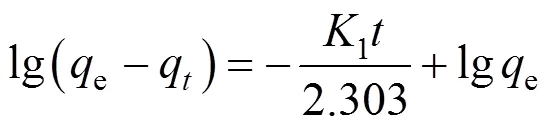
Similarly, the second-order rate equation is given by

whereeandqare the amounts of Cu(II) adsorbed at equilibrium and at time, in mg·g-1,1and2are rate constants, in L·min-1and g·mg-1×min-1, respectively.

Figure 7 The Langergren sorption diagram of Cu(II) on CMC (the first-order equation)
◇ 50 mg·L-1;□ 100 mg·L-1;△ 150 mg·L-1

Table 2 The parameters for the first-order and second-order adsorption rate
① The number of data points is 8.
Solid-liquid sorption processes are usually characterized by three consecutive steps, external diffusion, internal diffusion, and adsorption. The adsorption step is usually fast and may be neglected. In this study, the size of CMC was small (<250mm) and the mixing was appropriate with a shaker speed of 100 r·min-1, so that the rate-limiting steps may be the chemical adsorption and internal diffusion.
3.5 Desorption and reuse of CMC
The desorption of adsorbed Cu(II) from the CMC was investigated in a batch reactor. The adsorbent was recycled to adsorb Cu(II) from 100 ml of solution with an initial concentration of 100 mg·L-1under the same adsorption conditions. The recycling was repeated 10 times, and the results are shown in Fig. 9. The uptake amount of Cu(II) on the CMC decreases with increasing cycle number. The percentage adsorption maintains at 92.5% in the first four cycles, then decreases to some extent. At the end of the tenth cycles, the adsorption is 61.3% of the initial value. Therefore, the loaded CMC can be recycled for Cu(II) adsorption when regenerated with 0.1 mol·L-1hydrochloric acid solution. The mechanism of regeneration may be that in the first four cycles, both electrostatic and complexation reactions occur between the hydrochloric acid solution and metal ions [23].
4 CONCLUSIONS
Cross-linked magnetic chitosan coated by magnetic fluids and cross-linked with epichlorohydrin was prepared and characterized. The Cu(II) adsorption behavior on the CMC was studied at different solution pH values and adsorption contact time. The optimal adsorption conditions of Cu(II) on CMC were pH of solution 6.0 and adsorption time of 60 min.

Figure 8 The Langergren sorption diagram of Cu(II) on CMC (the second-order equation)
◇ 50 mg·L-1;□ 100 mg·L-1;△ 150 mg·L-1
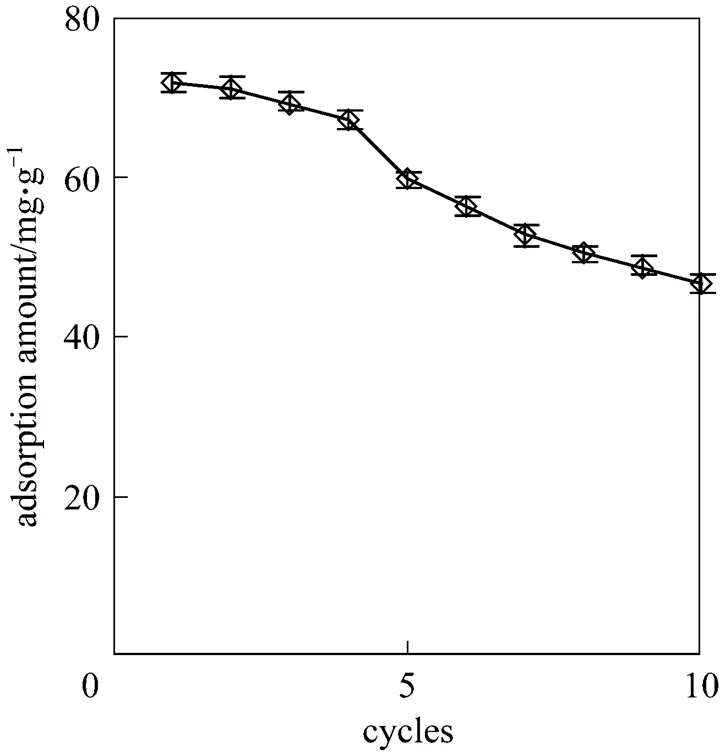
Figure 9 The effect of recycling on Cu(II) adsorption
The Langmuir model fitted the experimental equilibrium data well, with correlation coefficients (2) of 0.9951 for the initial Cu(II) concentrations of 100 mg·L-1. The experimental data for the kinetics of adsorption were correlated well by the first-order Langergren rate equation. The loaded CMC could be regenerated with 0.1 mol·L-1hydrochloric acid solution and reused repeatedly for Cu(II) adsorption for as many as ten cycles.
1 Solmaz, K., Abdülkerim, K., Adil, D., Yuda, Y., “ Batch removal of copper(II) and zinc(II) from aqueous solutions with low-rank Turkish coals”,..., 18 (3), 177-184 (2000).
2 Ngah, W.S., Endud, C.S., Mayanar, R., “Removal of copper (II) ions from aqueous solution onto chitosan and cross-linked chitosan beads”,..., 50 (5), 181-190 (2002).
3 Rao, M.M., Ramesh, A., Rao, G.P.C., Seshaiah, K., “Removal of copper and cadmium from the aqueous solutions by activated carbon derived from Ceiba pentandra hulls”,..., B129, 123-129 (2006).
4 Tian, Y., Li, Z.Q., Xu, H.F., Yang, F.L., “Comparison on electro-reduction of Cu(II) using polypyrrole and stainless steel electrodes”,..., 63 (2), 334-340 (2008).
5 Huang, C.H., Chen, L.K., Yang, C.L., “Effect of anions on electrochemical coagulation for cadmium removal”,..., 65 (2), 137-146 (2009).
6 Rengaraj, S., Yeon, K.H., Moon, S.H., “Removal of chromium from water and wastewater by ion exchange resins”,..., B87, 273-287 (2001).
7 Kamble, S.B., Marathe, K.V., “Membrane characteristics and fouling study in MEUF for the removal of chromate anions from aqueous streams”,..., 40 (12), 3051-3070 (2005).
8 Mesquita, J.P., Martelli, P.B., Gorgulho, H.F., “Characterization of copper adsorption on oxidized activated carbon”,...., 17 (6), 1133-1143 (2006).
9 Lima, I.S., Lazarin, A.M., Airoldi, C., “Favorable chitosan/cellulose film combinations for copper removal from aqueous solutions”,..., 36 (1/2), 79-83 (2005).
10 Abdel-Mohdy, F.A., Ibrahim, M.S., El-Sawy, S., “Metal removal by chitosan and some chitosan composite”,, 66 (5), 219-226 (2006).
11 Chu, K.H., “Removal of copper from aqueous solution by chitosan in prawn shell: adsorption equilibrium and kinetics”,..., 90 (1), 77-95 (2002).
12 Ngah, W.S., Ghani, S.A., Kamari, A., “Adsorption behaviour of Fe (II) and Fe (III) ions in aqueous solution on chitosan and cross-linked chitosan beads”,.., 96 (8), 443-450 (2005).
13 Cestari, A.R., Vieira, E.F.S., Oliveira, I.A., Bruns, R.E., “The removal of Cu (II) and Co (II) from aqueous solutions using cross-linked chitosan-Evaluation by the factorial design methodology”,..., 143 (1), 8-16 (2007).
14 Chang, Y.C., Chen, D.H., “Preparation and adsorption properties of monodisperse chitosan-bound Fe3O4magnetic nanoparticles for removal of Cu(II) ions”,.., 283, 446-451 (2005).
15 Chang, Y.C., Chang, S.W., Chen, D.H., “Magnetic chitosan nanoparticles: Studies on chitosan binding and adsorption of Co(II) ions”,..., 66 (3), 335-341 (2006).
16 Martinez, L., Agnely, F., Leclerc, B., Siepmann, J.,Cotte, M., Deiger, S., Couarraze, G., “Cross-linking of chitosan and chitosan poly(ethylene oxide)beads: A theoretical treatment”,..., 67 (6), 339-348 (2007).
17 Hsien, T.Y., Rorrer, G.L., Way, J.D., “Synthesis of porous-magnetic chitosan beads for removal of cadmium ions from wastewater”,...., 32 (14), 2170-2178 (1993).
18 Li, J.P., Song, L.M., Zhang, S.J., “Rare earth metal ion adsorption capacity on crosslinked magnetic chitosan”,, 20, 219-221 (2002). (in Chinese )
19 Juang, R.S., Shao, H.J., “A simplified equilibrium model for sorption of heavy metal ions from aqueous solutions on chitosan”,., 36 (12), 2999-3008 (2002).
20 Jeon, C., Holl, W.H., “Chemical modification of chitosan and equilibrium study for mercury ion removal”,., 37 (8), 4770-4780 (2003).
21 Yan, Y.C., Chen, B.R., Wang, R.X., “Studies of properties and preparation of chitosan resin cross-linked by formaldehyde and epichlorohydrin”,...., 20 (1), 53-57 (2004). (in Chinese)
22 Yu, J.H., Du, Y.M., Zheng, H., “Blend films of chitosan-gelation”,, 45 (5), 440-444 (1999). (in Chinese)
23 Sankararamakrishnan, N., Dixit, A., Iyengar, L., Sanghi, R., “Removal of hexavalent chromium using a novel cross linked xanthated chitosan”,.., 97 (9), 2377-2382 (2006).
24 Zhang, H.Y., “Removal of Cr (VI) from aqueous solution by using a crosslinked magnetic chitosan”, M.S. Thesis, East China Institute of Technology, Fuzhou (2006). (in Chinese)
25 Hameed, B.H., Din, A.T.M., Ahmad, A.L., “Adsorption of methylene blue onto bamboo-based activated carbon: Kinetics and equilibrium studies”,..., 14 (7), 819-825 (2007).
2009-02-18,
2009-04-21.
* To whom correspondence should be addressed. E-mail: guolinhuang@sina.com
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Study on the Flow Field around Two Parallel Moving Bubbles andInteraction Between Bubbles Rising in CMC Solutions by PIV*
- Kinetic Rate Constant of Liquid Drainage from Colloidal Gas Aphrons*
- β-Diketones at Water/Supercritical CO2 Interface: A MolecularDynamics Simulation*
- Analysis of Sucrose Esters with Long Acyl Chain by Coupling ofHPLC-ELSD with ESI-MS System*
- Gas Flow in Unilateral Opening Pulse Tubes Based on Real Gas Equation of State
- Solubility of Piperine in Supercritical and Near Critical Carbon Dioxide*
