Solubility of Piperine in Supercritical and Near Critical Carbon Dioxide*
2009-05-15KumoroHarcharanSinghandMasitahHasan
A.C. Kumoro, Harcharan Singh and Masitah Hasan
Solubility of Piperine in Supercritical and Near Critical Carbon Dioxide*
A.C. Kumoro1,**, Harcharan Singh2and Masitah Hasan3
1Department of Chemical Engineering, Faculty of Engineering Diponegoro University, SH Tembalang Semarang 50139, Indonesia2Mewah Oil Sdn Bhd, Lot 40, Section 4, Fasa 2A, Pulau Indah Industrial Park, Jalan Sungai Pinang 5/1, Pulau Indah, 42920 Pulau Indah, Selangor Darul Ehsan, Malaysia3Department of Chemical Engineering, Faculty of Engineering, University of Malaya 50603 Kuala Lumpur, Malaysia
Piperine is a member of the lipids family commonly found in peppercorn, ginger and other natural sources and is grouped as an alkaloid. The solubility of piperine has been determined in carbon dioxide at near critical and supercritical conditions in a dynamic extraction apparatus. The conditions studied were at pressures ranging from 10 to 20 MPa and temperatures at 293, 300, 313, 323 and 333 K. The results showed that piperine solubility increased with increasing pressure at all temperatures studied. The solubility of piperine in near critical conditions was slightly higher than that at supercritical conditions only at the low-pressure range. Two semi-empirical density dependent correlations, namely the Chrastil model and the Dilute Solution model, were also used to estimate the solubility data. Although both models showed good correlation with the solubility data, the Dilute Solution model performed better prediction than the Chrastil model.
solubility, piperine, supercritical, near critical, carbon dioxide
1 Introduction
A large variety of complex alkaloids are abundantly found in natural products. Piperine is an alkaloid commonly found in peppercorn and ginger. It is a solid substance essentially insoluble in water. It is a weak base that is tasteless at first, but leaves a burning after taste. Piperine is the-stereoisomer of 1-piperoylpiperidine. It is also known as (,)- 1-piperoylpiperidine and (,)-1-[5-(1, 3-benzodioxol- 5-yl)-1-oxo-2, 4-pentdienyl] piperidine. The molecular formula of piperine is C17H19NO3and its chemical structure is shown in Fig. 1 [1]. Piperine is found naturally in plants belonging to thefamily, such asL, commonly known as black pepper, andL, commonly known as long pepper. Piperine is the major pungent substance present in these plants and is isolated from the fruit of the black pepper and long pepper plants.
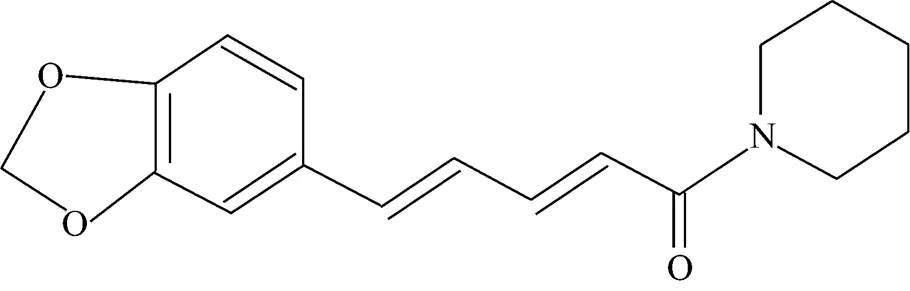
Figure 1 Molecular structure of piperine
The interest in this alkaloid compound is a result of recent medical studies, which have shown piperine to be very helpful in increasing the absorption of certain beneficial vitamins such as vitamin B andb-carotene, and trace element such as selenium. Piperine apparently has the ability to increase the natural thermogenic activities of the body. Thermogenesis is the process of generating energy in the cell. Piperine increases thermogenesis and in turn creates a demand for nutrients necessary for metabolism. This has so far been particularly helpful for patients who are sick or aged with defective intestinal lining [1]. Piperine does not only add a pinch of flavour to food but also to alcohol, for example, brandy, which is known for having a small amount of it. While piperine may sound like a pleasant spice, it can also be a strong repellent. It can be found in most insecticides, particularly those that kill the common housefly [2].
The work presented in this paper is a small part of a broader project to study the use of supercritical carbon dioxide to selectively extract piperine free pepper oil from black pepper corn. Previous researchers have reported that pepper oil is a mixture consisting of 90% hydrocarbons and 10% oxygenated terpenes and aromatic compound [3]. The hydrocarbon fraction is composed of monoterpenes (70%-80%) and sesquiterpenes (20%-30%), which appear to possess the desirable attributes of pepper flavour. Though oxygenatedterpenes are relatively minor constituents, they contribute to the characteristic odour of pepper oil [4].
The separation and isolation of piperine from its natural sources is very complex. Thus, it is not synthesised at a large scale in any chemical plant and is not commercially available [1]. Murga and co-workers reported the extraction of phenols from grape seeds using supercritical carbon dioxide and concluded that by adjusting variables such as temperature, pressure and co-solvent amount, a variety of phenolic compounds contained in grape seeds can be selectively extracted [5]. The designing of industrial scale processes for the extraction of piperine free black pepper oil at elevated pressure require a thorough investigation of the solubility data of pure piperine at near critical and supercritical carbon dioxide.
There are abundant of solubility data on solid-supercritical fluid equilibrium system available in the literature [6]. However, no solubility data has been reported for the piperine-supercritical carbon dioxide system. Estimation of solubility data using rigorous thermodynamic models is difficult due to the lack of literature critical property data resulting from the instability of high-molecular compounds at extreme conditions. In this work, two semiempirical models, namely Chrastil model [7] and Dilute Solution model [8] have been used to estimate the solubility data of piperine. The Chrastil model assumes a linear correlation between solid solubility and solvent density, whereas the Dilute Solution model is thermodynamically consistent and assumes dilute condition in the vapour phase. Although semiempirical models are not able to predict the equilibria in unknown phase, they are still widely used due to their simplicity and ability to correlate experimental data.

Table 1 Physical properties of piperine
①Obtained from Ref. [1];②Estimated using Fedors method [9];③Estimated using Edmister method [10].
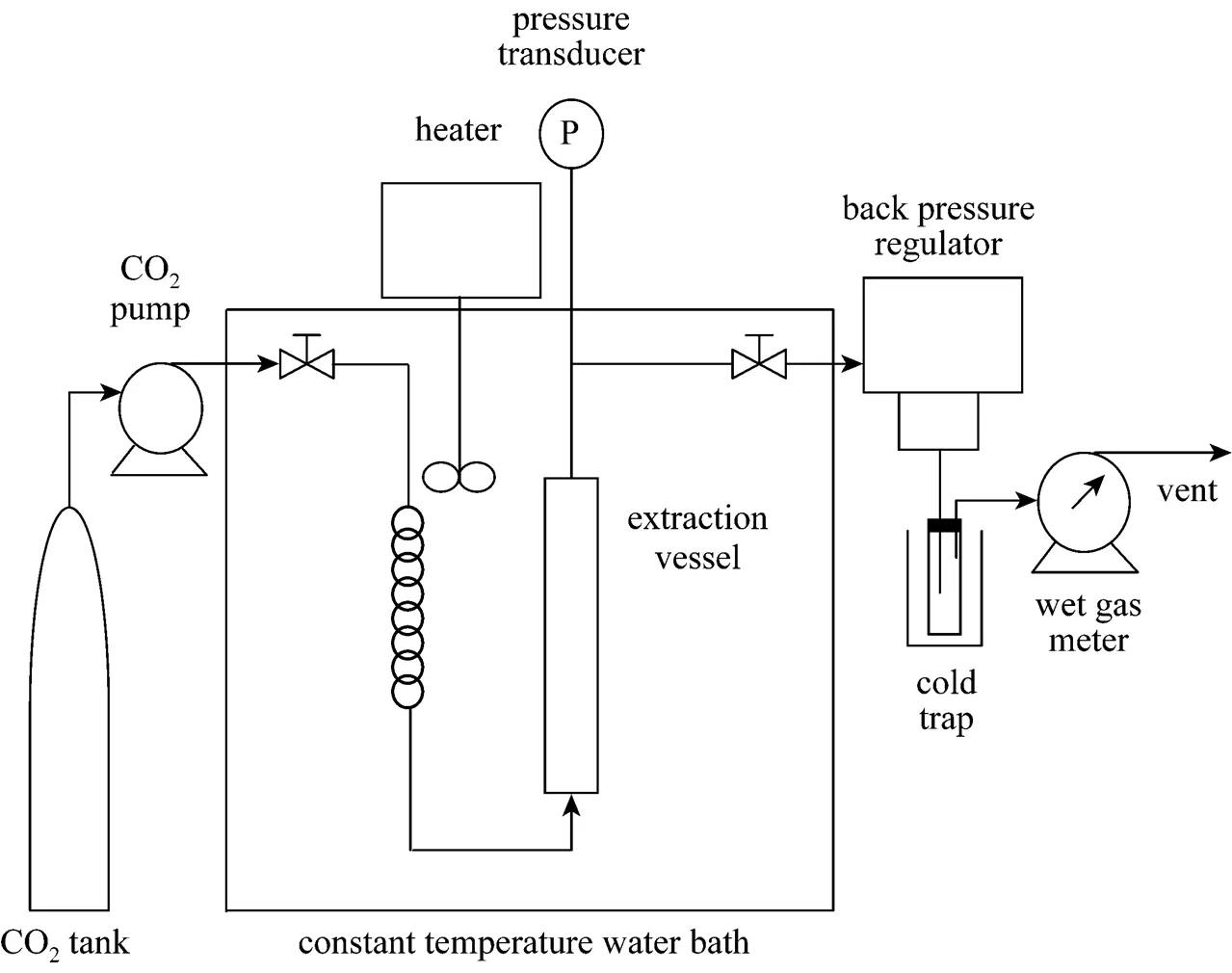
Figure 2 Schematic diagram of the solubility study apparatus
2 Experimental
2.1 Materials
Carbon dioxide with a purity of 99.7% was used as the solvent in this study and was supplied in the liquid form by Air Product (M) Berhad. The solute used was piperine with a purity of 97% and was obtained from Aldrich, Deisenhofen, Germany. Literature and estimated physical properties of piperine such as molecular weight (W), normal melting point (m), normal boiling points (b), critical temperature (c), critical pressure (c), critical volume (c) and acentric factor () are reported in Table 1. Theb,c,candcwere estimated using Fedors method [9] whereas the acentric factor was estimated using Edmister method [10].
2.2 Apparatus
The solubility of piperine was studied at 293, 300, 313, 323 and 333 K and pressures from 10 to 20 MPa using a dynamic analytical apparatus as shown in Fig. 2. It consists of a 16 cm3high-pressure stainless steel extraction vessel (10 mm inner diameter´200 mm length) immersed in a water bath with an electrical heater (Thermo Haake, model DC10) to control the temperature within±0.1 K. The system pressure was determined by a pressure transducer (Druck, model PDCR 961) with accuracy of±0.01 MPa. A Syringe pump (ISCO, model 260 DM) was used to compress the liquid CO2and to continuously deliver CO2at a volumetric flow rate of 1 ml·min-1. The CO2was depressurised by a back pressure regulator (Jasco, model 880-81) and the amount of CO2consumed was determined using a wet gas meter (Alaxander Wright & Co., model DM 3A). The solute collected was gravimetrically determined using a balance (Mettler Toledo, model AG 204) with an accuracy of±0.0001 g.
2.3 Method
Prior to solubility experiments, the extraction vessel was washed with ethanol, dried in an electric oven at 373 K for one hour and cooled in a desiccator. On the other hand, the tube lines, fittings and connections were cleaned up by flushing liquid CO2at 2 ml·min-1for about 15 min to totally remove dirt and solute.
In a typical run, the extraction vessel was first filled with 5 g of pure piperine crystal distributed in stainless steel balls in order to improve the piperine- solvent contact and to avoid channelling when CO2flowed through. The extraction vessel was then immersed in the water bath and the system was brought to the desired temperature. Liquid CO2was then fed into the extraction vessel until the desired pressure was achieved and the system was then left to equilibrate for 30 min before starting an experiment. The extracted solute was collected in a cold trap by depressurising the CO2at the back pressure regulator. The cold trap was a glass vial immersed in a pack of diced ice that was maintained at (277 K±2 K). The experiment was terminated after about 0.03 m3of CO2was consumed and the total amount of solute collected was weighed. For each condition studied, at least two experiments were carried out where the total amount of CO2that passed through the cell was slightly varied, hence varying the total amount of solute collected. The relative error obtained between the duplicate experiments was less than 5%. The solubility of piperine as a function of pressure and density at different temperatures was then evaluated.
3 Results and discussion
The temperature range for solubility determination at supercritical conditions was from 313 to 333 K and for near critical conditions was from 293 to 300 K, while the operating pressure for both conditions was varied from 10 to 20 MPa. The solubility of piperine in carbon dioxide at these temperatures and pressures is listed in Table 2 (for supercritical conditions) and Table 3 (for near critical conditions). The values for CO2density obtained from Ref. [11] and for both CO2viscosity and self-diffusivity obtained using HYSYS (version 3.0.1) at the conditions studied are also listed in these tables. The piperine solubility in supercritical conditions was varied between 0.78×10-5and 14.42´10-5mol·mol-1, whereas that for near critical conditions was between 2.93×10-5and 8.66´10-5mol·mol-1. These values were in the same order as those obtained for cholesterol [12, 13], ascorbic acid and propyl gallate [14], naproxen [15] and dyestuffs [16].
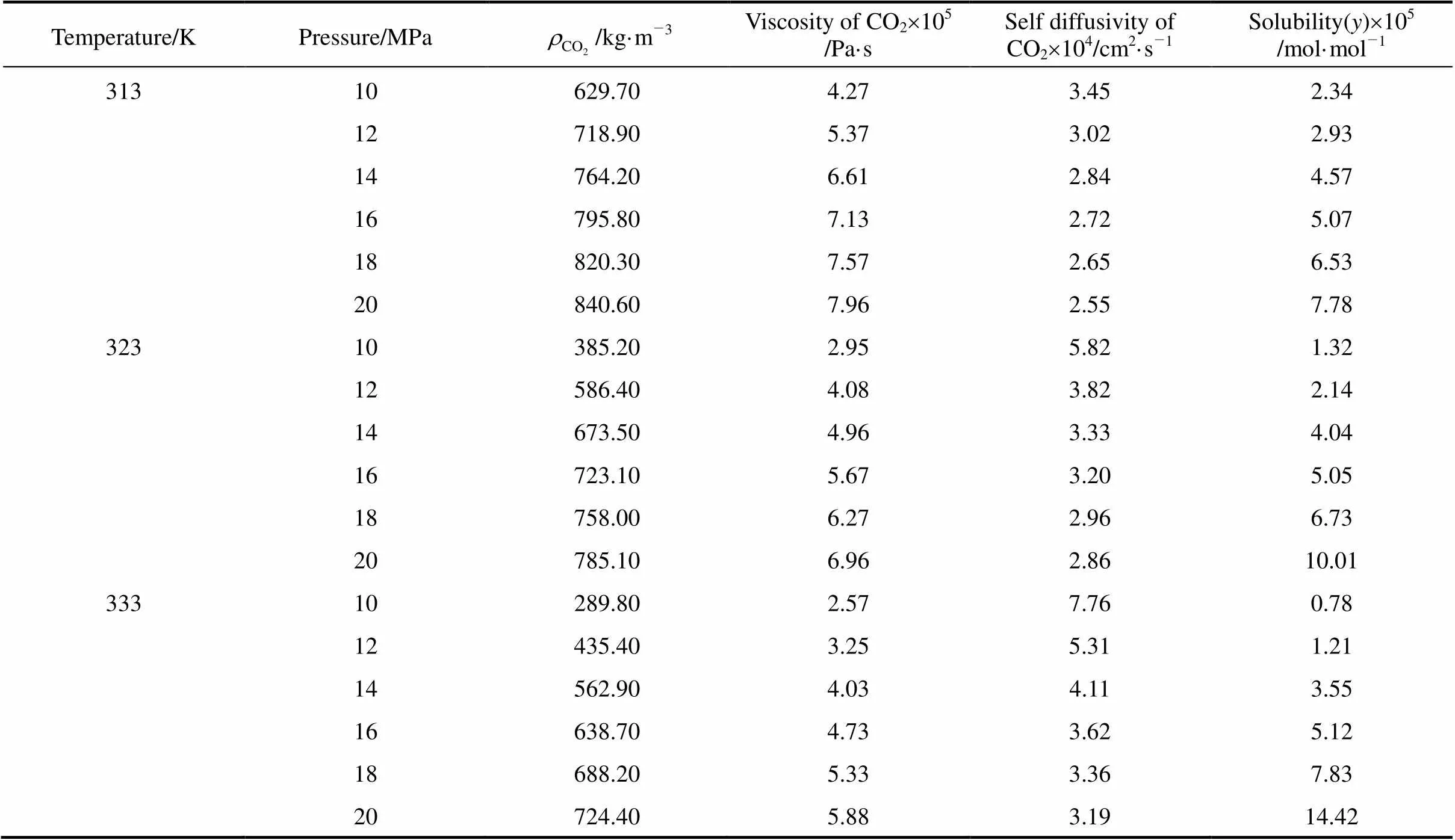
Table 2 Experimental solubility data of piperine in supercritical CO2

Table 3 Experimental solubility data of piperine in near critical CO2
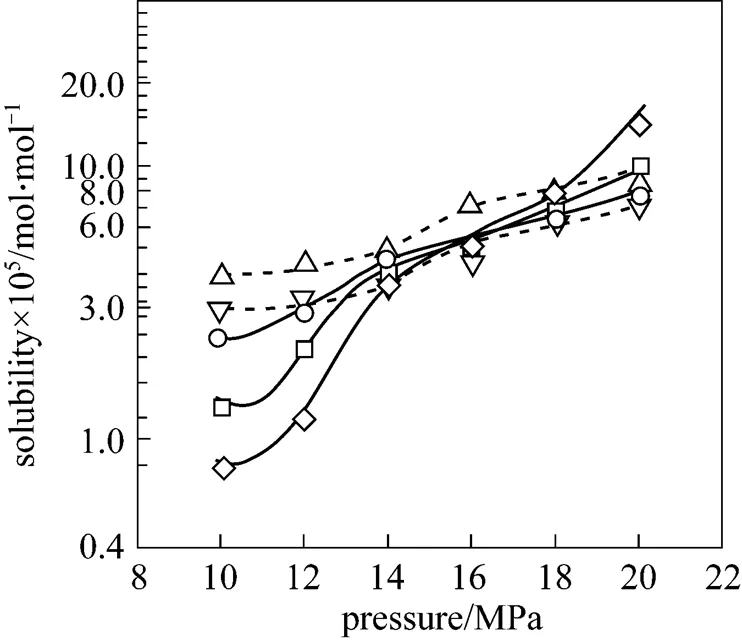
Figure 3 Solubility of piperine in supercritical and near critical CO2as a function of pressure
experimental data: △ 293 K;▽ 300 K;○ 313 K;□ 323 K;◇ 333 K

The solubility of piperine in near critical and supercritical CO2as a function of pressure is shown in Fig. 3. It can be seen that at all temperatures studied, the solubility of piperine increased with increasing pressure. This is expected since the density of CO2increases with an increase in pressure, causing a significant decrease of the intermolecular distances, which increases the solute-solvent interaction. The effect of temperature is more complex only for supercritical condition at about 16 MPa since a cross over of the isotherms was observed. Solubility studies of coumarin [17], naproxen [15], dyestuffs [16], phenolic compounds [18],-hydroxybenzoic acid [19], fatty acid esters [20], antioxidants [14] and cholesterol [12, 13] showed that the cross-over pressure occurred at conditions above the critical point of CO2as was the case in this study.
At pressures above the cross over pressure, the solubility increases with increasing temperature. On the other hand, at pressures below the cross over one, the solubility decreases with increasing temperature. At near critical conditions, there was no cross over of isotherms. This finding is similar to the solubility study of CHI3in ethane and carbon dioxide at near critical condition reported by other researchers [21, 22]. This is because the solvent density plays dominant role on solute solubility due to its solvating power at near critical condition rather than the role of solute vapour pressure [13]. Vapour pressure is a direct function of temperature. Thus for a given isobaric condition, the solvent density is higher at lower temperature resulting in higher solubility of the solute. The 300 K isotherm is very close to thecof CO2(304.15 K) where the compressibility is very large and a slight variation in the system can affect the results. This is due to the local density enhancement at near critical condition, which depends on both the fluid susceptibility (its isothermal compressibility or thermal expansivity) and the solute-solvent interactions [22].
The estimated values of CO2viscosity at near critical condition are higher than that of at supercritical condition as listed in Tables 2 and 3, indicating its flowability limitation. In addition, the estimated values of CO2self-diffusivity at near critical condition are lower than that at supercritical condition, indicating lower mass transfer rate. Thus, the high piperine solubility at near critical condition is mainly due to the contribution of high CO2density, which has a high solvating power.
Two semiempirical models were used to correlate the experimental solubility data. The Chrastil model assumes the formation of a solvato complex between molecules of the supercritical carbon dioxide solvent and the solute at equilibrium [7]. This model leads to the following linear relationship between solubility and CO2density for a given temperature:
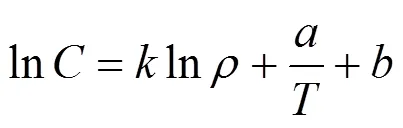
where the solubility,, is calculated by Eq. (2) as follows:
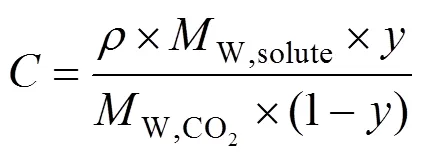
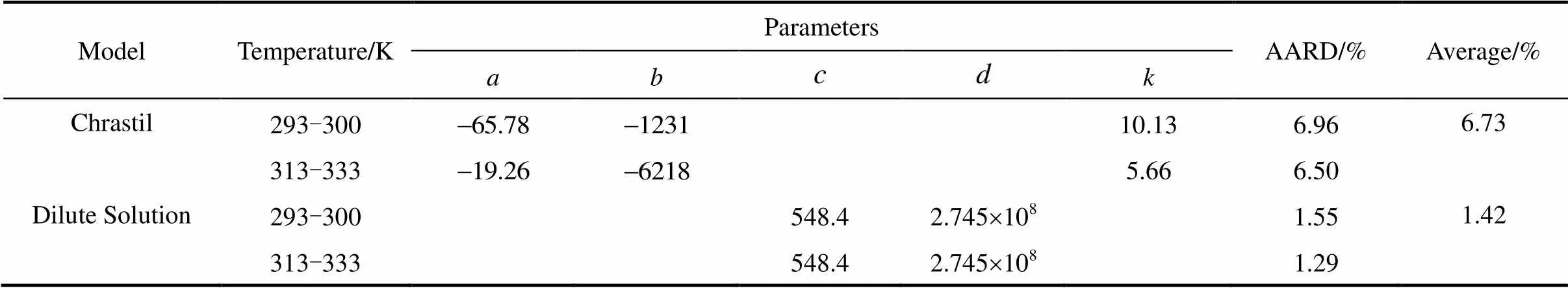
Table 4 Constants a, b and k of the Chrastil model, constants c and d of the Dilute Solution model and AARD obtained for estimation of solubility of piperine in supercritical CO2
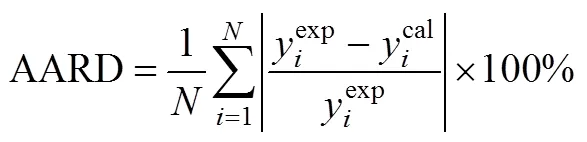
where in Eqs. (1) and (2)is the CO2density in g·L-1,is the solubility of the solute expressed as (g solute)/(L solvent),is the temperature in K,Wis the solute molecular weight,is the equilibrium mole fraction of the solute in the supercritical phase, and,andare adjustable parameters of the model. Constantis an association constant that represents the number of CO2molecules in the complex and depends on the vaporisation and solvation enthalpies of the solute, anddepends on the molecular weights of the solute and solvent. The optimum values for parameters,andwere obtained by double linear regression of the experimental solubility data.
The values for association constantand the average absolute relative deviation (AARD) for solubility of piperine obtained by using the Chrastil model at different temperatures in the supercritical region are shown in Table 4. The solubility data at 10 MPa was neglected because this condition is close to solvent critical condition and small changes in system conditions can cause a significant change in phase behaviour. Other researchers studying the solubility of phenolic compounds [18] have observed similar behaviour. Eq. (1) has the advantage of requiring only three parameters to fit all experimental data regardless of temperature. In addition, there is no need to estimate the critical properties of the solute.
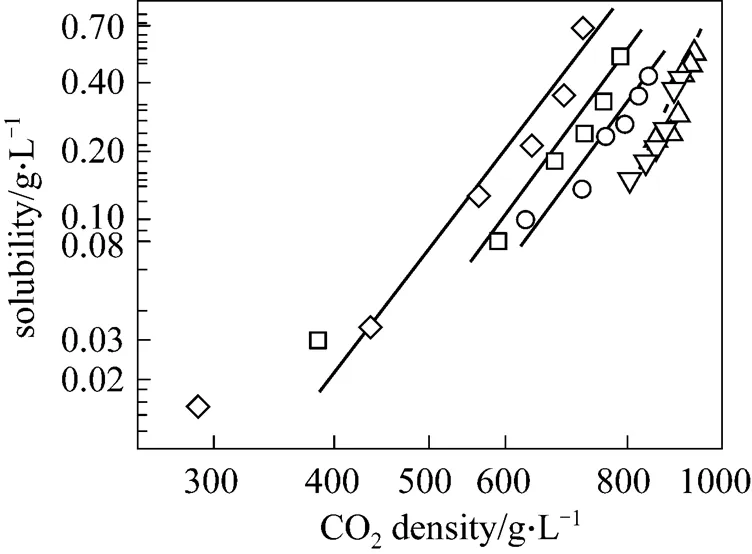
Figure 4 Solubility of piperine at supercritical and near critical conditions as a function of CO2density
experimental data: △ 293 K;▽ 300 K;○ 313 K;□ 323 K;◇ 333 K

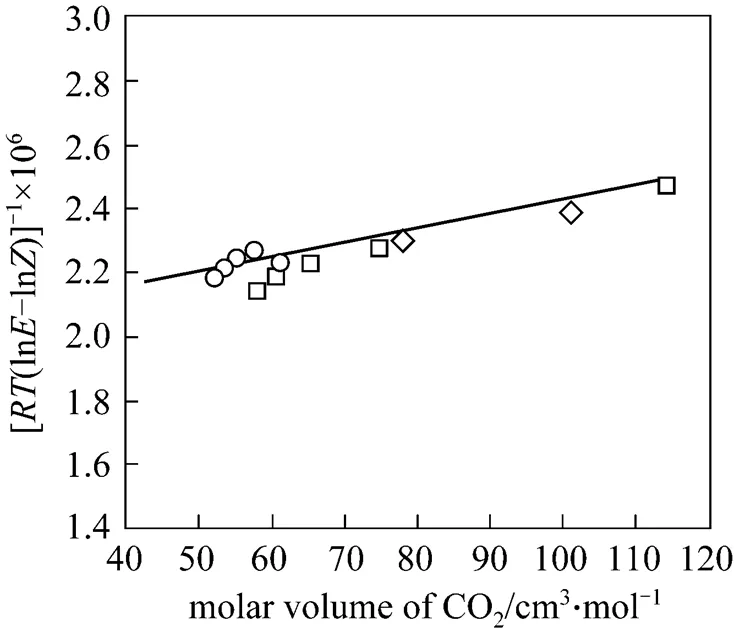
Figure 5 Solubility of piperine at supercritical condition as a function of CO2specific volume according to Dilute Solution model
○ 313 K;□ 323K;◇ 333 K
Figure 4 shows the linear correlation between piperine solubility and carbon dioxide density (both are expressed in g·L-1) at all conditions studied. The similar slopes of all isotherms at supercritical conditions confirm that the heat of solvation and heat of vaporisation of piperine are not significantly affected by temperature. In Table 4 at supercritical conditions, the values for association constantindicates that there are about six CO2molecules clustered around one molecule of piperine to form a quasi-solvato complex. However, at near critical conditions both isotherms (.. at 293 and 300 K) collapse on a single line and have a steeper slope. This implies that the sum of heat of solvation and heat of vapourisation of piperine is higher at lower temperatures. The high solvating power of CO2at near critical condition is due to its higher density at lower temperature as seen from the density values listed in Table 3. When CO2density is smaller than that at its triple point, attractive interactions are dominant and in dilute solutions, solvent-dipole-dipole interactions will be the dominant factor in determining the structure of the fluid. As the fluid density increases, the structure of the pure solvent does not provide an optimum orientation of solvent molecules around the solute particle to maximise interactions with the dipolar solute. At low density there is an inhomogeneous region in the vicinity of the solute molecule and the microinhomogeneity increases with the strength of the solute-solvent interaction [21].
The Dilute Solution model assumes a dilute condition in the supercritical phase. For sufficiently dilute solutions the solute molecules have almost zero opportunity to contact each other, whereby all the mutual interactions between solute molecules can be ignored. The effect of interactions and geometry can be ignored for gaseous mixtures at low pressure because these mixtures obey the ideal gas law. However, for compressed gaseous dilute solutions, which are of interest here, these systems must behave somewhere between ideal gas solutions and dilute liquid solutions. At high density and low temperature, the typical configuration in the gaseous solvent may be regarded as consisting of essentially isolated and randomly moving clusters of solvent molecules that are in dynamic equilibrium [8]. This model assumes that when a heavy solute (.. solid or liquid) is in equilibrium with a compressed gaseous solvent, the solubility of the solute seldom exceeds a few mole percent. The correlation for the Dilute Solution model is represented by the following linear equation:
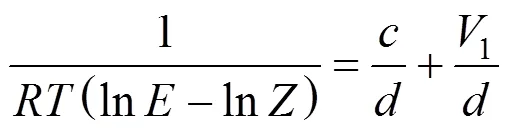
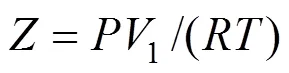
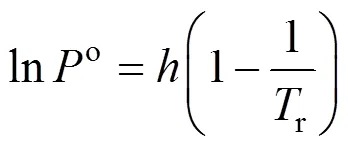
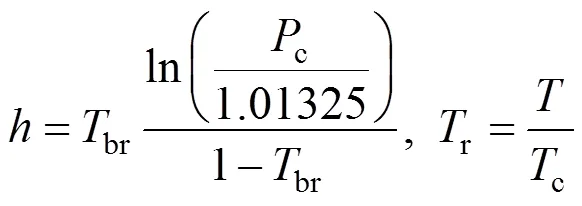
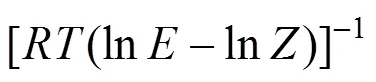
4 Conclusions
The solubility of piperine at supercritical and near critical conditions has been determined. The effect of pressure on solubility for piperine in supercritical CO2followed the expected trend of increasing solubility with an isothermal increase in pressure. The increase in piperine solubility is mainly due to the increase in CO2density with increasing pressure.
The solubility data measured at supercritical condition showed the typical trends of a solid/SCF system with a crossover pressure located at approximately 160 MPa. Solubility isotherms of piperine at near critical condition were found to be different compared to that at supercritical condition. At near critical conditions, there was no crossover of isotherms observed and both the solubility isotherms collapsed on a single line when plotted against density.
The Dilute Solution model based on statistical thermodynamics was able to better correlate the experimental solubility data and showed a more realistic description of the solute-solvent interactions in supercritical carbon dioxide compared to the phenomenological model proposed by Chrastil. The average AARD obtained using the Dilute Solution model was only about 1.42% as compared to about 6.73% obtained using the Chrastil model. The Chrastil model is truly not accurate for estimating solubility behaviour due to the dependence of the association constant term on temperature. Nevertheless, the Chrastil model is useful when vapour pressure data is not available.
1 Budavari, S., O’neil, M.J., Smith, A., Heckelman, E., Kinneary, J.F., The Merck Index: An Encyclopedia of Chemical, Drugs, and Biologicals, 12th edition, Merck & CO., Inc., New Jersey (1996).
2 Govindarajan, V.S., “Pepper chemistry, technology, and quality evaluation”,...., 9, 117-225 (1977).
3 Ferreira, S.R.S., Nikolov, Z.L., Doraiswamy, L.K., Meireles, M.A.A., Petenate, A.J., “Supercritical fluid extraction of black pepper (.) essential oil”,.., 14, 235-245 (1999).
4 Pino, J., Rodriguez-Feo, G., Rosado, A., “Chemical and sensory properties of black pepper oil”,, 34, 555 (1990).
5 Murga, R., Ruiz, R., Beltrán, S., Cabezas, J.L., “Extraction of natural complex phenols and tannins from grape seeds by using supercritical mixtures of carbon dioxide and alcohol”,..., 48 (8), 3408-3412 (2000).
6 Bartle, K.D., Clifford, A.A., Jaffar, S.A., Shilstone, G.F., “Solubility of solids and liquids of low volatility in supercritical carbon dioxide”,...., 20 (4), 713-756 (1991).
7 Chrastil, J., “Solubility of solids and liquids in supercritical gases”,..., 86, 3016-3021(1982).
8 Wang, X., Tavlarides, L.T., “Solubility of solutes in compressed gases: dilute solution theory”,...., 33, 724-729 (1994).
9 Fedors, R.F., “A method for estimating both the solubility parameters and molar volumes of liquids”,..., 14 (2), 147-154 (1979).
10 Reid, R.C., Prausnitz, J.M., Poling, B.E., The Properties of Gases and Liquids, 4th edition, McGraw-Hill International Edition, New York (1989).
11 Span, R., Wagner, W., “A new equation of state carbon dioxide covering the region from triple-point temperature to 1100 K at pressure up to 800 MPa”,...., 25 (6), 1509-1596 (1996).
12 Singh, H., Yun, S.L.J., Macnaughton, S.J., Tomasko, D.L. Foster, N.R., “Solubility of cholesterol in supercritical ethane and binary gas mixtures containing ethane”,...., 32, 2841-2848 (1993).
13 Yun, S.L.J, Liong, K.K., Gurdial, G., Foster, N.R., “The solubility of cholesterol in supercritical carbon dioxide”,...., 30, 2476-2482 (1991).
14 Cortesi, A., Kikic, I., Alessi, P., Turtoi, G., Garnier, S., “Effect of chemical structure on the solubility of antioxidants in supercritical carbon dioxide: experimental data and correlation”,.., 14, 139-144 (1999).
15 Ting, S.S.T., Tomasko, D.L., Foster, N.R., “Chemical-physical enterpretation of cosolvent effects in supercritical fluids”,...., 32, 1471-1481 (1993).
16 Tuma, D., Schneider, G.M., “Determination of the solubilities of dyestuffs in near- and supercritical fluids by a static method up to 180 MPa”,., 158-160, 743-757 (1999).
17 Yoo, K.P., Shin, H.Y., Noh, M.J., You, S.S., “Measurement and modelling solubility of bioactive coumarin and its derivatives in supercritical carbon dioxide”,..., 14 (5), 341-346 (1997).
18 Murga, R., Sanz, M.T., Beltrán, S., Cabezas, J.L., “Solubility of some phenolic compounds contained in grape seeds, in supercritical carbon dioxide”,., 23, 113-121 (2002).
19 Gurdial, G., Foster, N.R., “Solubilities of-hydroxybenzoic acid in supercritical carbon dioxide”,...., 10, 575-580 (1991).
20 Liong, K.K., Wells, P.A., Foster, N.R., “Diffusion coefficients of long chain esters in supercritical carbon dioxide”,...., 31, 400-404 (1992).
21 Gutkowski, K., Japas, M.L., Fernandez-Prini, R., “Solubility of solids in near-critical fluids (V) CHI3in ethane and in carbon dioxide”,..., 29, 1077-1086 (1997).
22 Fernandez, D.P., Hefter, G., Fernandez-Prini, R., “The solubility of solids in near-critical fluids (VI) CHI3in CO2revisited”,..., 33, 1309-1324 (2001).
23 del Valle, J.M., Aguilera, J.M., “An improved equation for predicting the solubility of vegetable oils in supercritical CO2”,...., 27, 1551-1553 (1988).
24 Sung, H.D S., J.J. Shim, J.J., “Solubility of C.I. disperse red 60 and C. I. disperse blue 60 in supercritical carbon dioxide”,..., 44, 985-989 (1999).
25 Jouyban, A., Chan, H.K., Foster, N.R., “Mathematical representation of solute solubility in supercritical carbon dioxide using empirical expressions”,.., 24 (1), 19-35 (2002).
2009-02-10,
2009-07-10.
the IRPA Project of the Ministry of Science, Technology and Innovation, Malaysia (09-02-03-0101-EA0001) and Fundamental Research Grant 2009 Directorate General of Higher Education, Ministry of National Education, the Republic of Indonesia.
** To whom correspondence should be addressed. E-mail: c.k.andrew@undip.ac.id
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Study on the Flow Field around Two Parallel Moving Bubbles andInteraction Between Bubbles Rising in CMC Solutions by PIV*
- Kinetic Rate Constant of Liquid Drainage from Colloidal Gas Aphrons*
- β-Diketones at Water/Supercritical CO2 Interface: A MolecularDynamics Simulation*
- Analysis of Sucrose Esters with Long Acyl Chain by Coupling ofHPLC-ELSD with ESI-MS System*
- Adsorptive Removal of Copper Ions from Aqueous Solution UsingCross-linked Magnetic Chitosan Beads
- Gas Flow in Unilateral Opening Pulse Tubes Based on Real Gas Equation of State
