陶瓷结合剂中各组分含量对其性能的影响
2024-12-06陈棋王春华栗正新张霖张国威周少杰夏学峰邵俊永
摘要"为探究R2O-Al2O3-B2O3-SiO2体系结合剂中各组分含量变化对其性能的影响,通过改变结合剂中Al2O3、B2O3和SiO2的含量,对各组结合剂的耐火度、流动性、热膨胀系数、抗折强度以及显微硬度进行测定。结果表明:当Al2O3和SiO2的质量分数分别达到最大25%和65%时,结合剂的耐火度最大,可达815℃;当B2O3的质量分数达到最大30%、Al2O3的质量分数达到最小10%时,结合剂的耐火度最小,为744℃;不同配方结合剂的流动性均为95%~135%;结合剂的热膨胀系数和抗折强度都会根据n(Al2O3+B2O3)/n(Na2O)的变化表现出不同的变化;各组分对结合剂显微硬度提高的影响为SiO2gt;B2O3gt;Al2O3。
关键词 陶瓷结合剂组分;三元相图;热膨胀系数;力学性能
中图分类号 TQ171 文献标志码 A
文章编号 1006-852X(2024)06-0761-08
DOI 码 10.13394/j.cnki.jgszz.2023.0126
收稿日期 2023-06-09 修回日期 2024-01-08
陶瓷结合剂磨具是磨料磨具行业的重要组成部分,相较于金属结合剂磨具与树脂结合剂磨具,其具有耐热性好、多孔结构易散热、易排屑、磨削弹性形变小、磨削精度高、自锐性好等优点[1],被广泛应用于各行各业。陶瓷结合剂磨具的性能很大程度上取决于陶瓷结合剂的性能,而陶瓷结合剂的性能与其组成成分和制备工艺相关。陶瓷结合剂中,不同的组分具有不同的作用。Al2O3不仅能增强结构键合强度,令玻璃网络结构更加聚合[2],还能促进特定体系结合剂中BaAl2Si2O8和γ-Li-AlSi2O6的形成,改善陶瓷结合剂立方氮化硼(cBN)磨具的耐热性能和力学性能[3]。B2O3的加入能够降低
陶瓷结合剂的烧结温度以及调整热膨胀系数[4],并且根据陶瓷结合剂中Na2O含量的不同,B2O3在陶瓷结合剂中会表现出不同的性能[5],随着B2O3含量的升高,陶瓷结合剂中会发生硼反常现象[6]。SiO2作为陶瓷结合剂的主要成分,主要构成陶瓷结合剂的玻璃网络结构,其含量的变化会直接影响结合剂中Al2O3[7-8]、Li2O[9]、B2O3[10]以及Na2O[11]的性能。碱金属氧化物[12-15]会向玻璃网络中提供自由氧,使网络结构致密度和结合剂耐火度降低、热膨胀系数升高。Li2O[16]的加入可以降低陶瓷结合剂的烧结温度,并且能够析出微晶相。在某些特定的陶瓷结合剂体系中,加入适量合适的添加剂对陶瓷结合剂的性能有很大的提升作用[17-20]。
金刚石是超硬材料行业常用的磨料,但其耐高温性能较差,因此其所需的结合剂应具有较低的烧结温度以及合适的流动性和热膨胀系数。本研究中探究了陶瓷结合剂中Al2O3、B2O3和SiO2的含量对其性能的影响,通过改变3种组分的含量,对比结合剂各性能的变化,以期得到更适合用于金刚石磨具的陶瓷结合剂。
1
实验
1.1
陶瓷结合剂的制备
根据文献[21]中R2O-Al2O3-B2O3-SiO2体系结合剂各组分的含量范围,确认结合剂配方如表1所示。三元相图中规定三组分质量分数之和为1,因此将配方中Al2O3、B2O3和SiO2的总质量分数设置为100%。为了降低结合剂的耐火度,再添加质量分数为25%的Na2O。其中B2O3用硼酸引入,Na2O用Na2CO3引入。根据总质量达到500g的试样设计,计算出各原料按照表1中质量分数所需的实际质量并精确称取,试样通过球磨机混合均匀后压制成块状,放入高温熔块炉中进行1400℃熔融。熔融后将其快速倒入冷水中水淬,水淬后取出玻璃料进行干燥,直至水分烘干,然后进行破碎、球磨,过74μm筛,得到16组结合剂。
1.2
样品的制备及检测
将各组结合剂通过质量分数为2.5%的糊精液黏结混合均匀,并在5MPa的压力下压制成5mm×6mm×30mm的试样条,将试样条在80℃下干燥12h。使用标准耐火锥测量各组结合剂的耐火度,采用平面流淌法测量结合剂的流动性,并对结合剂进行热膨胀系数的测定。检测各组结合剂的耐火度后,将每组结合剂以高于结合剂耐火度60℃的温度为烧结温度进行烧结,烧结过程中在400℃下保温0.5h,以除去糊精等挥发性杂质,烧结时间为2h,随炉冷却后取出。利用微机控制电子万能实验机,采用三点弯曲法,设定跨距为20mm,压头以(2±0.5)N/s的速度行进,直到试样断裂,测定试样的抗弯强度,每个试样测定3次取平均值。利用日本FM-ARS900显微硬度仪测量结合剂的显微硬度,并对结合剂进行X射线衍射(XRD)和微观形貌分析。
2
结果与讨论
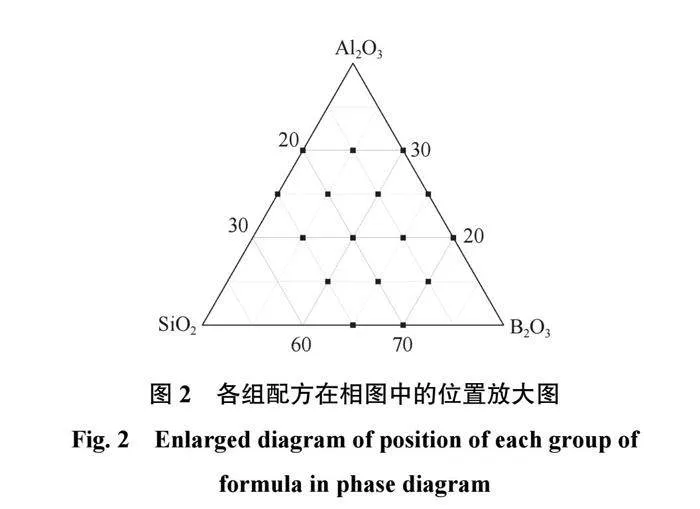
表1中各组陶瓷结合剂配方中的3种组分在相图中的位置如图1所示。图1中,线a中所有点的SiO2含量相同,线b中所有点的B2O3含量相同,线c中所有点的Al2O3含量相同。选取的结合剂各组分含量的范围在相图中仅有一小部分,截取后各配方在相图中的位置如图2所示,因此后续分析会截取相图中有效范围进行分析。2.1
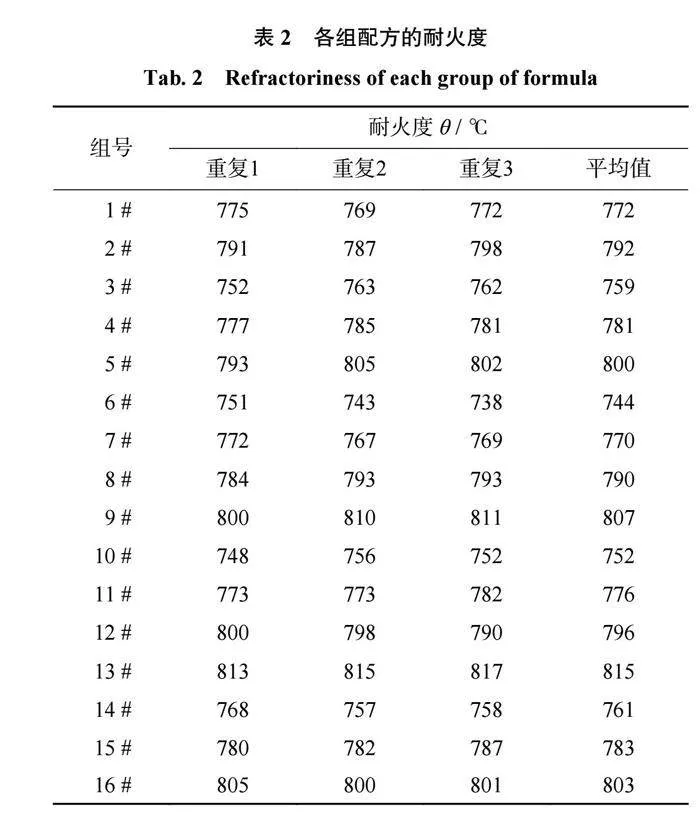
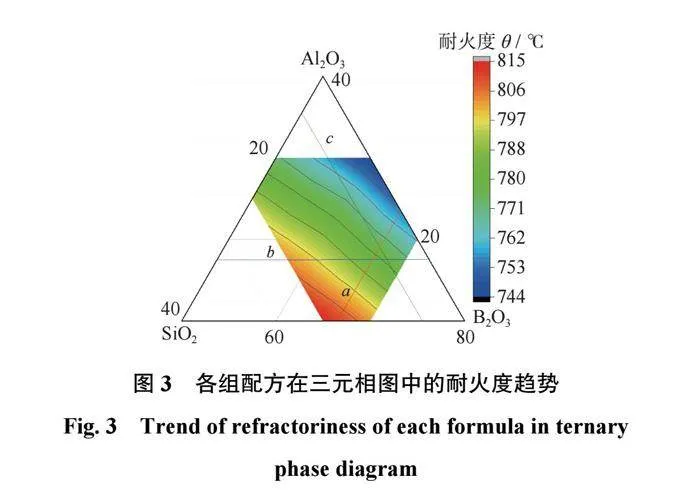
结合剂的耐火度分析结合剂的耐火度是直接影响磨具烧成温度的性能指标。各组配方的耐火度结果如表2所示,在三元相图中的耐火度趋势如图3所示,图3中的结果是3次测量结果的平均值。从表2和图3可以得出:当SiO2质量分数为60%时,该体系结合剂在B2O3质量分数为30%时具有最小的耐火度744℃,且随着B2O3质量分数的降低,耐火度逐渐增大;当SiO2质量分数为65%时,该体系结合剂在Al2O3质量分数为25%时具有最大的耐火度815℃,且随着Al2O3质量分数的降低,耐火度逐渐减小。当Al2O3质量分数为20%时,随着B2O3质量分数从10%提升至30%,结合剂的耐火度从803℃减小至772℃。当B2O3质量分数为20%时,随着Al2O3质量分数从10%升至25%,结合剂的耐火度从761℃增大至800℃。
因此可以推断出,B2O3在陶瓷结合剂中具有降低耐火度的作用,而SiO2和Al2O3在陶瓷结合剂中会使结合剂耐火度升高,且Al2O3对结合剂耐火度的影响要比SiO2的影响大。
2.2
结合剂的流动性分析
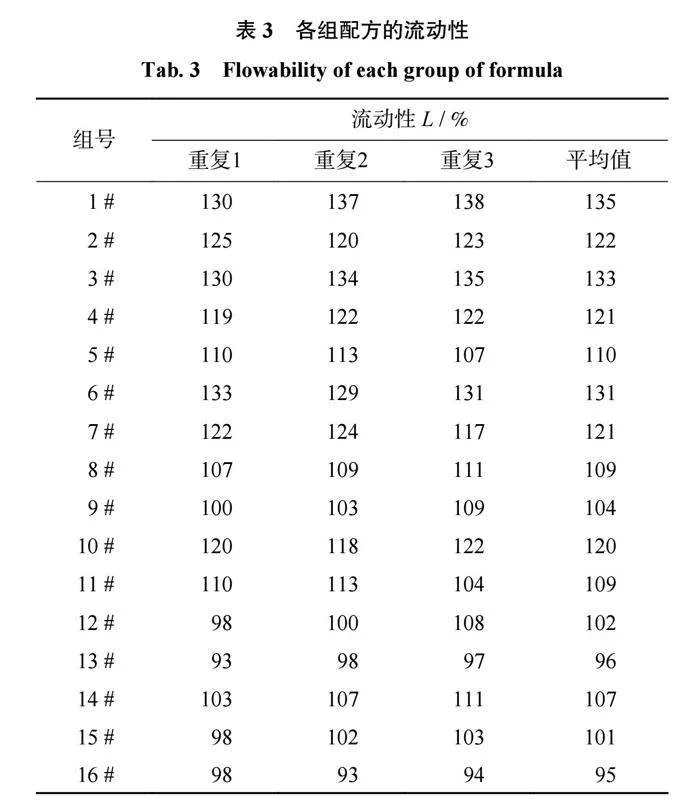
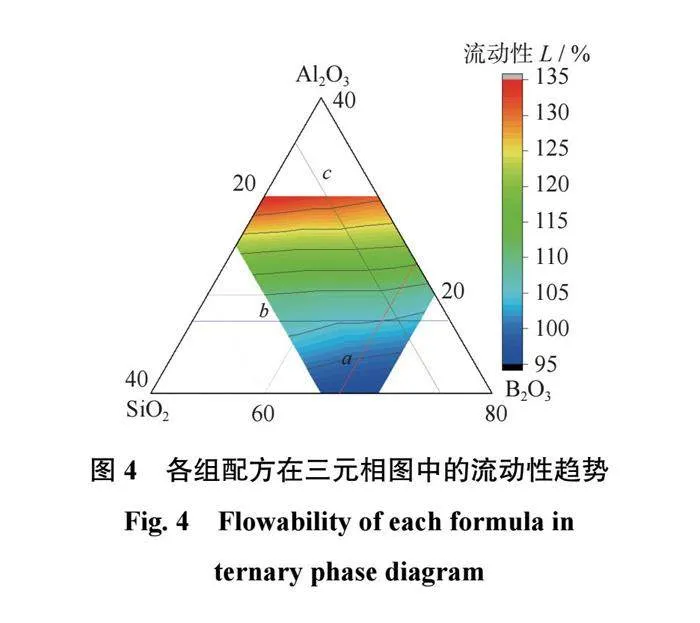
结合剂的流动性是反映结合剂在高温熔融状态下黏度变化的参数,结合剂的流动性越高,其与磨料的结合性能越好。各组配方的流动性检测结果如表3所示,在三元相图中的流动性趋势如图4所示。由表3和图4可以得出:当SiO2含量相同时,结合剂的流动性会随着B2O3含量的升高而升高;当Al2O3含量相同时,结合剂的流动性会随着B2O3含量的升高而升高;当B2O3含量相同时,SiO2含量的变化对结合剂流动性的影响不大。因此可以推断出,B2O3在陶瓷结合剂中具有提高结合剂流动性的作用,而Al2O3则会使陶瓷结合剂的流动性降低。
2.3
结合剂的热膨胀系数分析
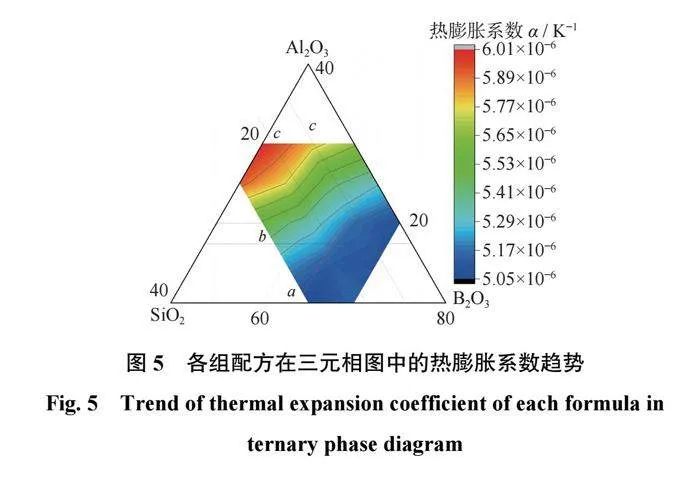
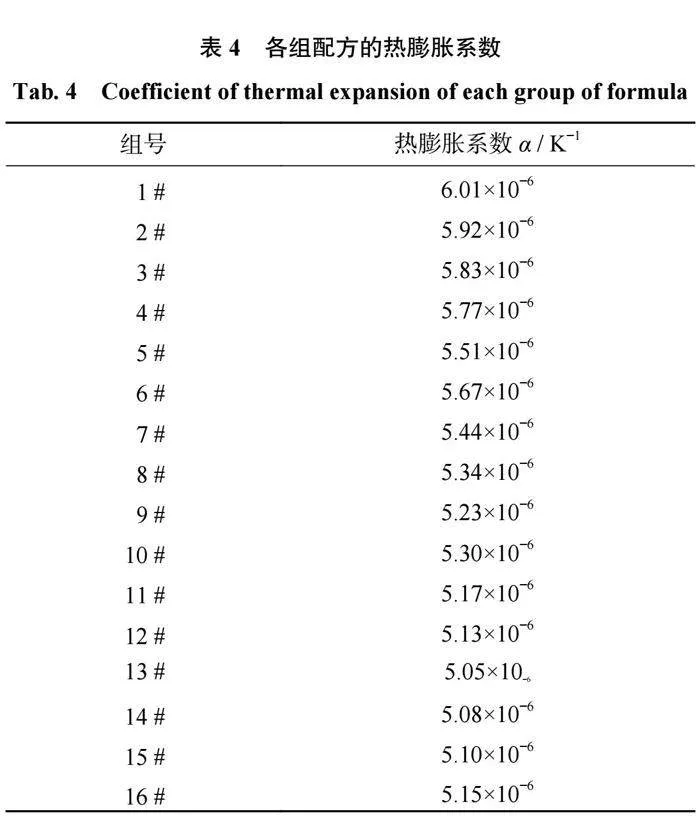
为了使烧结过程中陶瓷结合剂与磨料之间具有更好的把持力,应避免结合剂与磨料界面之间产生热应力,因此陶瓷结合剂的热膨胀系数需要与磨料的热膨胀系数相匹配。各组配方的热膨胀系数如表4所示,在三元相图中的热膨胀系数趋势如图5所示。从表4和图5可以得出:当SiO2含量相同时,结合剂的热膨胀系数会随着B2O3含量的升高而升高。当Al2O3质量分数为10%和15%时,结合剂的热膨胀系数会随着B2O3含量的升高而升高;当Al2O3质量分数为20%时,结合剂的热膨胀系数会随着B2O3含量的升高先降低后升高;当Al2O3质量分数为25%时,结合剂的热膨胀系数会随着B2O3含量的升高而降低。当B2O3含量相同时,结合剂的热膨胀系数会随着Al2O3含量的升高而升高。
之所以会出现图5所示的情况,是因为陶瓷结合剂的热膨胀系数是由各组分的含量及形成的网络结构决定的。当结合剂中Al2O3+B2O3和Na2O的摩尔比n(Al2O3+B2O3)/n(Na2O)<1时,Al2O3和B2O3会与Na2O中的氧离子结合形成[AlO4]和[BO4],参与到结合剂的网络结构中,令结合剂的网络结构致密化,进而降低结合剂的热膨胀系数;当n(Al2O3+B2O3)/n(Na2O)>1时,Na2O中的氧离子不足,Al2O3和B2O3形成[AlO3]和[BO3]三角体,使网络结构的致密性降低,导致热膨胀系数升高。
2.4
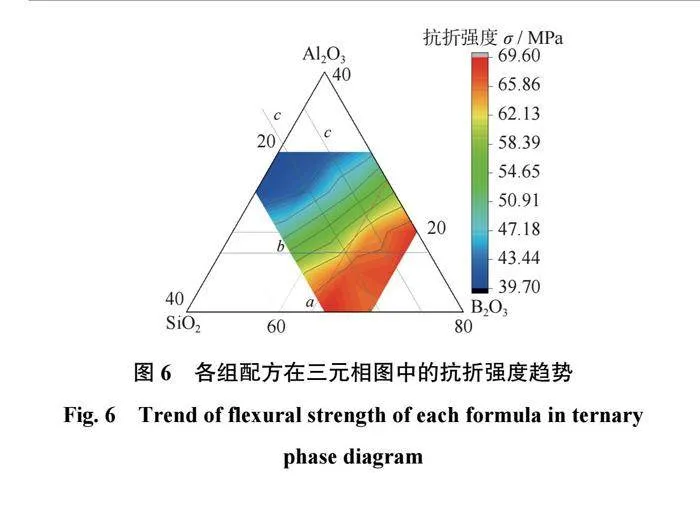
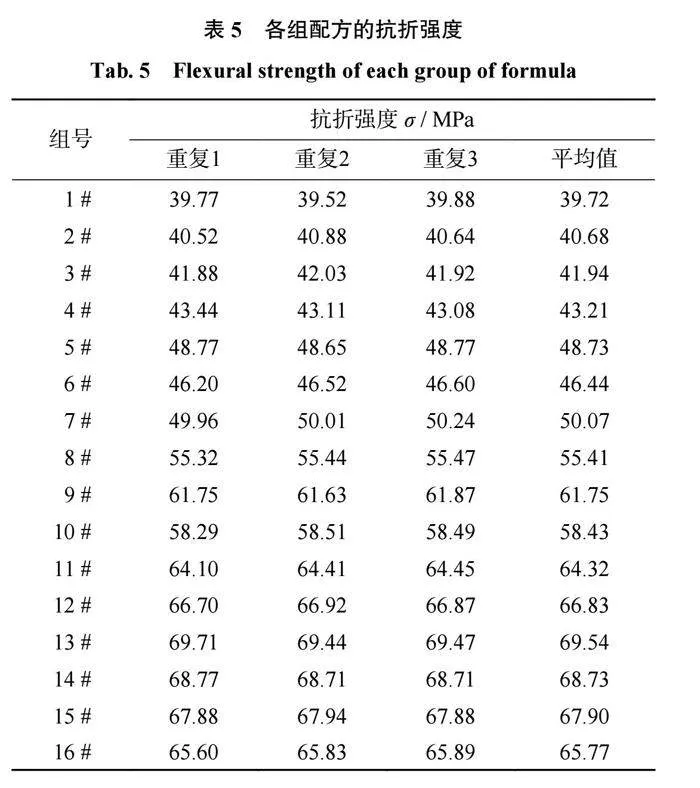
结合剂的抗折强度分析
各组配方的抗折强度如表5所示,在三元相图中的抗折强度趋势如图6所示。从表5和图6可以得出:当SiO2含量相同时,结合剂的抗折强度基本会随着Al2O3含量的升高而升高。当Al2O3质量分数为10%和15%时,结合剂的抗折强度会随着B2O3含量的升高而降低;当Al2O3质量分数为20%时,结合剂的抗折强度会随着B2O3含量的升高先升高后降低;当Al2O3质量分数为25%时,结合剂的抗折强度会随着B2O3含量的升高而升高。当B2O3含量相同时,结合剂的抗折强度会随着SiO2含量的升高而升高。其原因是当n(Al2O3+B2O3)/n(Na2O)<1时,结合剂的网络结构较致密,使得结合剂的抗折强度较高;当n(Al2O3+B2O3)/n(Na2O)>1时,结合剂的网络结构较为疏松,会产生较多的缺陷等,使得结合剂的抗折强度降低。
2.5
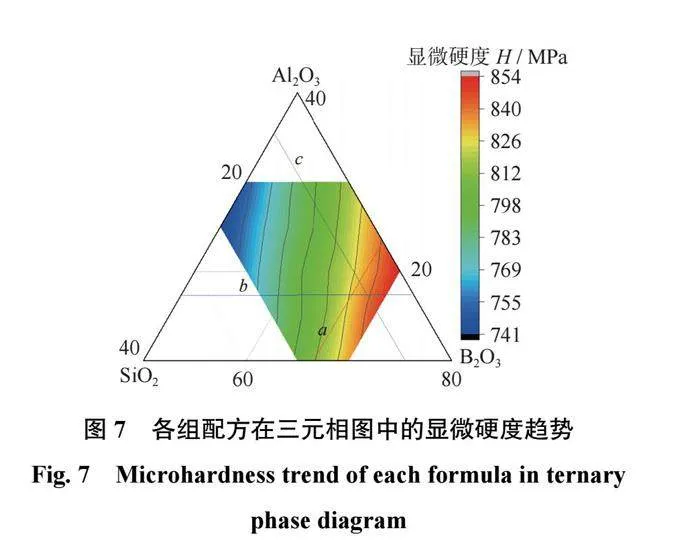
结合剂的显微硬度分析
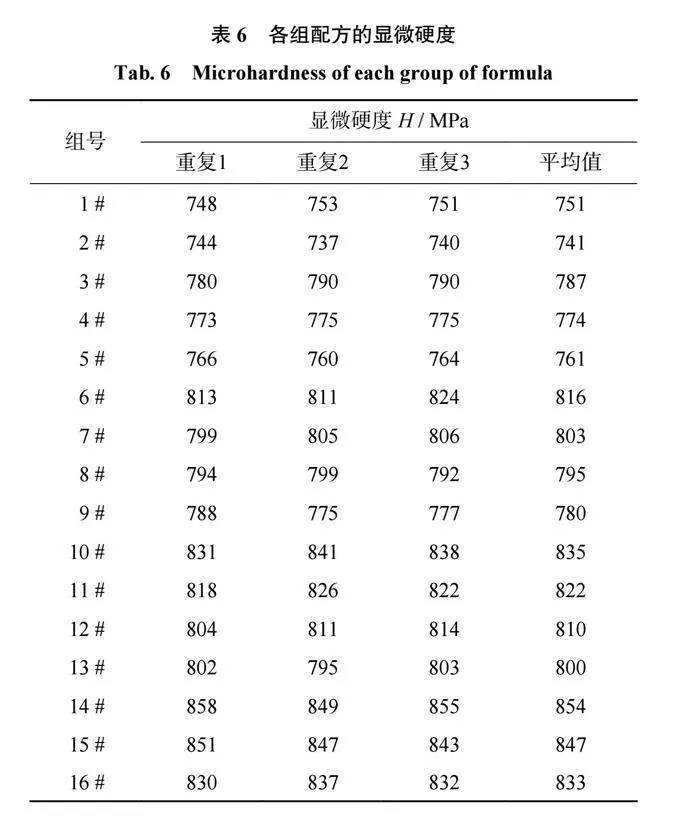
各组配方的显微硬度如表6所示,在三元相图中的显微硬度趋势如图7所示。结合表6和图7可以得出:当SiO2质量分数为65%时,结合剂中B2O3质量分数由10%上升至25%,结合剂的显微硬度上升35MPa;当Al2O3质量分数为15%时,结合剂中SiO2质量分数由15%上升至30%,结合剂的显微硬度上升60MPa;当B2O3质量分数为20%时,结合剂中SiO2质量分数由55%上升至70%,结合剂的显微硬度上升93MPa。因此可以推断出,各组分对结合剂显微硬度提高的影响为SiO2gt;B2O3gt;Al2O3。在结合剂的玻璃网络结构中,[SiO4]四面体为主体结构,其对结合剂显微硬度的影响最大,当SiO2含量较高时,n(Al2O3+B2O3)/n(Na2O)<1,Al2O3和B2O3大多以[AlO4]和[BO4]四面体的形式存在,使得玻璃网络结构较为致密,结合剂的显微硬度较大;当SiO2含量较低时,n(Al2O3+B2O3)/n(Na2O)>1,结合剂中除了有[AlO4]和[BO4]四面体,还有一部分[AlO3]和[BO3]三角体存在,导致网络结构的致密性降低,使结合剂的显微硬度降低。其中,Al−O键的键能小于B−O键的键能,破坏网络结构中[AlO4]四面体所需的能量小于破坏[BO4]四面体所需的能量,Al−O键的键长大于B−O键,[AlO3]三角体的体积较大,在网络结构中造成的缺陷更大。因此相对于[BO4]四面体和[BO3]三角体,[AlO4]四面体对网络结构致密化的影响程度较低,[AlO3]三角体对网络结构致密化的破坏较大,B2O3对结合剂显微硬度的影响要大于Al2O3。
2.6
结合剂的微观形貌分析
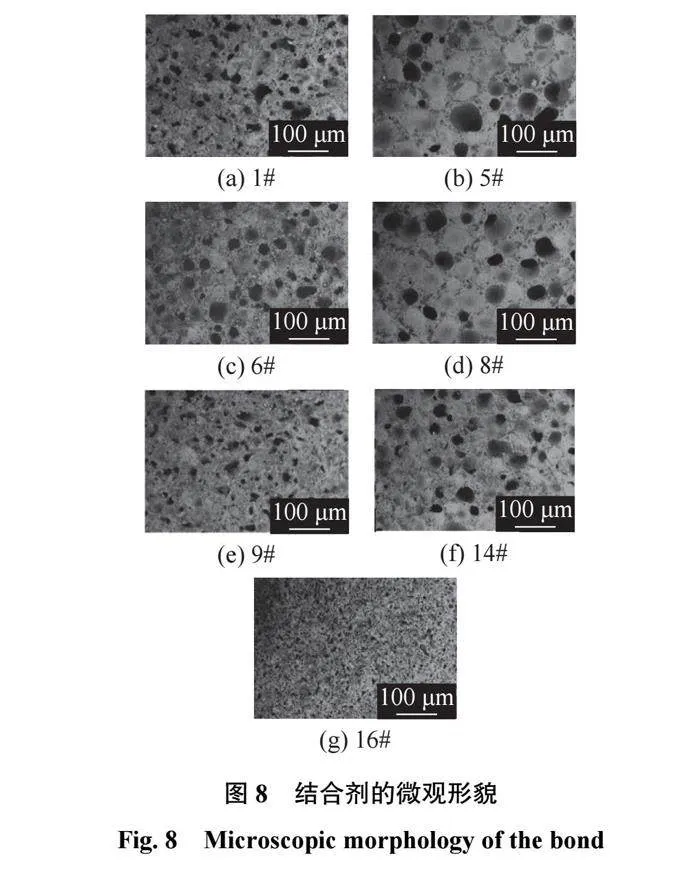
图8为结合剂的微观形貌图。对比图8b、图8d、图8f可知:当B2O3含量相同时,结合剂中的气孔数量和大小相差不大,说明Al2O3和SiO2在结合剂中都起到了使玻璃网络结构致密化的作用;对比图8a、图8d、图8g可知:当Al2O3含量相同,B2O3含量较高时,[BO3]三角体使玻璃网络结构疏松,出现较多的气孔,随着B2O3含量的降低,玻璃网络结构趋于致密,但B2O3含量过低则导致结合剂熔融较少,内部空气无法排除,产生较多的小气孔;对比图8c、图8d、图8e可知:当SiO2含量相同时,由于Al2O3的相对分子质量较大,随着Al2O3含量的升高,B2O3物质的量减少较多,但由于Al2O3和B2O3都会生成相应的三角体和四面体,三角体的总数量在减少,结合剂的结构更加趋于致密化。
3
结论
通过调整R2O-Al2O3-B2O3-SiO2体系结合剂中Al2O3、B2O3和SiO2的含量进行烧结,对结合剂的耐火度、流动性、热膨胀系数、抗折强度、显微硬度以及微观形貌进行了测定,将所得数据绘制在三元相图对应成分区域,依据数据分布特征得出以下结论:
(1)结合剂中SiO2和Al2O3的存在会提高结合剂的耐火度,且Al2O3对结合剂耐火度的影响比SiO2大,但Al2O3会降低结合剂的流动性;B2O3的存在会降低结合剂的耐火度并提高结合剂的流动性。
(2)结合剂的热膨胀系数和抗折强度都会因Al2O3和B2O3含量的变化而发生不同变化。当结合剂中Al2O3含量较高时,结合剂的热膨胀系数会随着B2O3含量的升高先降低再升高,抗折强度会随着B2O3含量的升高先升高再降低;当结合剂中Al2O3含量较低时,结合剂的热膨胀系数会随着B2O3含量的升高而升高,抗折强度会随着B2O3含量的升高而降低。当SiO2含量相同时,结合剂的热膨胀系数会随着B2O3含量升高而升高,抗折强度随着Al2O3含量的升高而升高;当B2O3含量相同时,结合剂的热膨胀系数会随着Al2O3含量的升高而升高,抗折强度随着B2O3含量的升高而升高。
(3)结合剂中各组分对结合剂显微硬度提高的影响为SiO2gt;B2O3gt;Al2O3。
参考文献:
[1]房佳斌. 陶瓷结合剂超硬材料制品的制备与性能研究 [D]. 西安: 西安建筑科技大学, 2018.
FANG Jiabin. Preparation and properties of vitrified bond superhard
abrasive composites [D]. Xi'an: Xi'an University of Architecture and
Technology, 2018.
[2]韩娟. 低温高强陶瓷结合剂的研究与制备 [D]. 武汉: 武汉理工大学,2016.
HAN Juan. Research and preparation of low temperature high strength
vitrified bond [D]. Wuhan: Wuhan University of Technology, 2016.
[3]MENG X L, XIAO B, WANG S Y. Effects of Al 2 O 3 addition on sintering
behaviour, microstructure, and physical properties of Al 2 O 3 /vitrified bond
CBN composite [J]. Ceramics International,2023,49(6):9173-9184.
[4]王照, 徐三魁, 黄威, 等. B 2 O 3 含量对 SiO 2 –B 2 O 3 –Al 2 O 3 –Na 2 O 系陶瓷结
合剂结构与性能的影响 [J]. 金刚石与磨料磨具工程,2022,42(5):552-559.
WANG Zhao, XU Sankui, HUANG Wei, et al. Effect of B 2 O 3 content on
the structure and properties of SiO 2 -B 2 O 3 -Al 2 O 3 -Na 2 O system ceramic
binder [J]. Diamond amp; Abrasives Engineering,2022,42(5):552-559.
[5]HU X L, CUI L, LIU T Y,et al. Crystallization and properties of B 2 O 3
doped LZAS vitrified bond for diamond grinding tools [J]. Journal of
Non-Crystalline Solids,2015,427:69-75.
[6]陆佩文. 无机材料科学基础: 硅酸盐物理化学 [M]. 武汉: 武汉理工大学出版社, 2005.
LU Peiwen. Fundamentals of inorganic materials science: physical
chemistry of silicates [M]. Wuhan: Wuhan University of Technology Press, 2005.
[7]赵光涛. 新型陶瓷刚玉磨具低温陶瓷结合剂的研究与制备 [D]. 天津:天津大学, 2012.
ZHAO Guangtao. Research on the preparation and properties of low
temperature vitrified bond for new ceramic alumina abrasive tool [D].
Tianjin: Tianjin University , 2012.
[8]肖攀. 普通磨具低温陶瓷结合剂的制备与性能研究 [D]. 天津: 天津大学, 2010.
XIAO Pan. Research on the preparation and properties of low
temperature ceramic bond for ordinary abrasive tools [D]. Tianjin:
Tianjin University, 2010.
[9]SHI J, HE F, XIE J L, et al. Exploring the influences of Li 2 O / SiO 2 ratio
on Li 2 O–Al 2 O 3 –SiO 2 –B 2 O 3 –BaO glass-ceramic bonds for vitrified cBN
abrasives [J]. Ceramics International,2019,45(12):15358-15365.
[10]HE F, ZHOU Q, XIE J L, et al. Characterization of low sintering
temperature and high strength SiO 2 -B 2 O 3 -CaO vitrified bonds for
diamond abrasive tools [J]. Ceramics International,2015,41(3):3449-3455.
[11]梅涛, 黄启忠, 王绍斌, 等. 碱金属氧化物 Na 2 O 对陶瓷结合剂金刚石
磨具性能的影响 [J]. 硅酸盐通报,2021,40(3):978-983.
MEI Tao, HUANG Qizhong, WANG Shaobin, et al. Effect of alkali
metal oxide Na 2 O on performance of vitrified bond diamond abrasive
tools [J]. Bulletin of the Chinese Ceramic Society,2021,40(3):978-983.
[12]刘小磐. 陶瓷结合剂金刚石砂轮的制备及磨削性能研究 [D]. 长沙: 湖南大学, 2012.
LIU Xiaopan. Study on the preparation and grinding properties of
vitrified bonded diamond wheel [D]. Changsha: Hunan University, 2012.
[13]SHI J, HE F, XIE J L, et al. Effects of Na 2 O / BaO ratio on the structure
and the physical properties of low-temperature glass-ceramic vitrified
bonds [J]. Ceramics International,2018,44(9):10871-10877.
[14]赵仕敬, 王海龙, 李剑, 等. Na 2 O 含量对金刚石砂轮用 Na 2 O-SiO 2 -
Al 2 O 3 -B 2 O 3 系陶瓷结合剂性能的影响 [J]. 机械工程材料,2018,42(4):1-6, 39.
ZHAO Shijing, WANG Hailong, LI Jian, et al. Effect of Na 2 O content on
properties of Na 2 O-SiO 2 -Al 2 O 3 -B 2 O 3 system vitrified bond for diamond
grinding wheel [J]. Materials for Mechanical Engineering,2018,42(4):1-6, 39.
[15]万明. RO 及 R 2 O 对金刚石砂轮陶瓷结合剂性能的影响 [D]. 秦皇岛:燕山大学, 2018.
WAN Ming. Effect of RO and R 2 O on the propeities of vitrified bond of
diamond wheel [D]. Qinhuangdao: Yanshan University, 2018.
[16]GUO Z, HE F, LI Z, et al. Effect of Li 2 O on structure and properties of
glass-ceramic bonds [J]. Ceramics-Silikáty,2021,65(2):198-205.
[17]CHEN S, LIU X, WAN L, et al. Effect of V 2 O 5 addition on the
wettability of vitrified bond to diamond abrasive and grinding
performance of diamond wheels [J]. Diamond and Related Materials,
2020,102:107672.
[18]HAN J, HE F, WANG L L, et al. Effect of WO 3 on the structure and
properties of low sintering temperature and high strength vitrified bonds
[J]. Journal of Alloys and Compounds,2016,679:54-58.
[19]LIANG Y X, SUN K, LI Z H , et al. Performance evaluation of vitrified /
diamond composites by adding ZnF 2 [J]. Diamond and Related Materials,2020,108:107910.
[20]CHUANG T K, TSAI Y T, LIN K H. Effects of added nano titanium on
the microstructure and mechanical properties of vitrified bond diamond
tools [J]. International Journal of Refractory Metals and Hard Materials,2018,74:107-113.
[21]侯永改. 纳米氮化铝改性低温陶瓷结合剂金刚石磨具的组织与性能控制 [D]. 秦皇岛: 燕山大学, 2012.
HOU Yonggai. Control of the microstructure and performance of nano-
AlN modified low temperature vitrified diamond tools [D]. Qinhuangdao:Yanshan University, 2012.
作者简介
通信作者: 栗正新,男,1964 年生,教授。主要研究方向:磨料磨具、超硬材料及制品,计算机在材料科学中的应用。
E-mail: zhengxin_li@haut.edu.cn
(编辑:王洁)
Effect"of"composition"and"content"on"properties"of"vitrified"bond
CHEN Qi
1 , WANG Chunhua 1 , LI Zhengxin 1 , ZHANG Lin 2 , ZHANG Guowei 2 ,
ZHOU Shaojie
2 , XIA Xuefeng 3 , SHAO Junyong 4
(1. School of Materials Science and Engineering, Henan University of Technology, Zhengzhou 450001, China)
(2. White Dove Abrasives and Grinding Co., Ltd., Zhengzhou 450199, China)
(3. SINOMACH-INT Co., Ltd., Zhengzhou 450018, China)
(4. Chengdu Tool Research Institute Co., Ltd., Chengdu 610500, China)
Abstract
Objectives: Vitrified bonded diamond grinding tools are widely used in the machining industry, but the high-temperature resistance of diamond is poor. Therefore, there are high requirements on sintering temperature, flowability,and thermal expansion coefficient of vitrified bond materials. The influence of the content of Al 2 O 3 , B 2 O 3 , and SiO 2 invitrified bond on its properties is investigated. By changing the content of these three components and comparing thechanges in the properties of the bonds, a more suitable vitrified bond for diamond grinding tools is obtained. Methods:Using a ternary phase diagram, the content of Al 2 O 3 , B 2 O 3 , and SiO 2 in the R 2 O-Al 2 O 3 -B 2 O 3 -SiO 2 bond system is adjus-ted. Sixteen different formulas were designed to prepare 5 mm × 6 mm × 30 mm sample strips under a pressure of 5MPa, and dried at 80 ℃ for 12 hours. The refractoriness of each group of bonds was measured using a standard refract-ory cone, the flowability of the bonds was measured using the plane flow method, and the thermal expansion coefficientof the bonds was determined. According to the refractoriness data determined by each formula, sintering was carried outat a temperature 60 ℃ higher than the refractoriness of the bond. A microcomputer-controlled electronic universal test- ing machine was used to determine the flexural strength of the bond using the three-point bending method. The micro-hardness of the bond was measured using a microhardness tester, and the microstructure of the bond was analyzed. Res-ults:"From the analysis of the measured performance data, it can be concluded that: (1) B 2 O 3 has the effect of reducingthe refractoriness in vitrified bonds, while SiO 2 and Al 2 O 3 increase the refractoriness of the bonds. Al 2 O 3 has a greaterimpact on the refractoriness of the bonds than SiO 2 . (2) B 2 O 3 has the effect of improving the flowability of bonds, whileAl 2 O 3 reduces the flowability of bonds. The thermal expansion coefficient and the flexural strength of the bond will varydepending on the content of Al 2 O 3 and B 2 O 3 . When the Al 2 O 3 content in the bond is high, the thermal expansion coeffi-cient of the bond will first decrease and then increase with the increase of B 2 O 3 content, and the flexural strength willfirst increase and then decrease with the increase of B 2 O 3 content. When the Al 2 O 3 content in the bond is low, thethermal expansion coefficient of the bond will increase with the increase of B 2 O 3 content, and the flexural strength willdecrease with the increase of B 2 O 3 content. When the SiO 2 content is fixed, the thermal expansion coefficient of thebond will increase with the increase of B 2 O 3 content, and the flexural strength will increase with the increase of Al 2 O 3content. When the B 2 O 3 content is fixed, the thermal expansion coefficient of the bond will increase with the increase ofAl 2 O 3 content, and the flexural strength will increase with the increase of B 2 O 3 content. The influence of each compon-ent in the bond on the microhardness change of the bond is SiO 2 gt;B 2 O 3 gt;Al 2 O 3 . When the molar ratio of Al 2 O 3 +B 2 O 3 toNa 2 O in the bond is less than 1, Al 2 O 3 and B 2 O 3 will combine with oxygen ions in Na 2 O to form [AlO 4 ] and [BO 4 ],which participate in the network structure of the bond and densify it. Breaking the dense network structure requireshigher energy. Therefore, densification of the network structure in the bond can reduce its thermal expansion coefficientand improve its flexural strength and microhardness. When n(Al 2 O 3 +B 2 O 3 )/n(Na 2 O)gt;1, the oxygen ions in Na 2 O are in-sufficient, and Al 2 O 3 and B 2 O 3 form [AlO 3 ] and [BO 3 ] triangles, reducing the density of the network structure. The fluffystructure makes the network structure more sensitive to energy, increasing the thermal expansion coefficient of the bondand reducing its flexural strength and microhardness. Conclusions: A ternary phase diagram based on the content ofAl 2 O 3 , B 2 O 3 , and SiO 2 in the R 2 O-Al 2 O 3 -B 2 O 3 -SiO 2 system bond can intuitively reflect the synergistic effect of the threecomponents during sintering. The three components will exhibit different effects in bonds with different contents, andtheir impact on the performance of the bond will also be different. When designing the formula for vitrified bonds, it isnecessary to consider the roles of different components in the bond and their interactions with other components.
Key"words
vitrified bond components;ternary phase diagram;thermal expansion coefficient;mechanical property
