白藜芦醇通过ESR1-PI3K信号通路抑制3T3-L1细胞脂滴形成
2024-10-15王倩杨豹李银蒋钦杨黄艳娜





摘要:【目的】探究白藜芦醇(RES)与雌激素受体1(ESR1)对3T3-L1细胞脂滴形成的影响及调控机制,为RES抑制脂肪沉积潜在机制的研究提供理论依据。【方法】以3T3-L1细胞为试验材料,设计并合成针对ESR1基因的小干扰RNA(siRNA),在3T3-L1细胞培养基中添加RES,以添加等量二甲基亚砜(DMSO)为对照,利用油红O染色、实时荧光定量PCR检测、Western blotting检测等分析RES与ESR1对3T3-L1细胞分化的影响。【结果】与对照组相比,RES能显著(P<0.05)减少3T3-L1细胞脂滴的生成,极显著(P<0.001)上调脂肪分解关键基因ATGL相对表达量,极显著下调(P<0.001)脂肪合成关键基因CEBPα、PPARγ及FAS相对表达量。RES能极显著(P<0.001)增加ESR1基因相对表达量,极显著(P<0.01)增加ESR1蛋白相对表达水平;同时,RES能极显著(P<0.001)下调PI3K信号通路中PI3K和AKT基因相对表达量,极显著(P<0.001)上调FOXO1基因相对表达量。干扰ESR1基因可极显著(P<0.01)增加3T3-L1细胞脂滴的生成,极显著(P<0.001)下调ATGL基因相对表达量,极显著(P<0.001)上调CEBPα、PPARγ及FAS基因相对表达量;加入RES后,干扰ESR1基因的3T3-L1细胞脂滴与未加入RES相比无显著差异(P>0.05,下同),ATGL、CEBPα、PPARγ及FAS基因相对表达量也均无显著差异。干扰ESR1基因能极显著(P<0.001)上调PI3K信号通路中PI3K和AKT基因相对表达量,极显著(P<0.001)下调FOXO1基因相对表达量;加入RES后,PI3K、AKT及FOXO1基因相对表达量与未加入RES相比均无显著差异。【结论】RES能通过ESR1-PI3K信号通路,上调脂肪分解关键基因ATGL相对表达量,下调脂肪合成关键基因CEBPα、PPARγ及FAS相对表达量,从而抑制3T3-L1细胞脂滴形成。
关键词:白藜芦醇;雌激素受体1(ESR1);PI3K信号通路;脂滴形成
中图分类号:S816.7文献标志码:A文章编号:2095-1191(2024)07-1916-09
Resveratrol inhibits lipid droplet formation in 3T3-L1 cells via ESR1-PI3K signaling pathway
WANG Qian YANG Bao LIYin JIANG Qin-yang HUANG Yan-na *
(1College of Animal Science and Technology,Guangxi University,Nanning,Guangxi 530004,China;2GuangxiKey Laboratory of Animal Breeding&Disease Control,Nannimg,Guangxi 530004,China)
Abstract:【Objective】To investigate the effects of resveratrol(RES)and estrogen receptor 1(ESR1)on the forma-tion of lipid droplets in 3T3-L1 cells and their regulatory mechanisms,and to provide theoretical basis for study of the po-tential mechanism of RES to inhibit fat deposition.【Method】Using 3T3-L1 cells as test materials,small interfering RNA(siRNA)targeting ESR1 gene was designed and synthesized.RES was added to 3T3-L1 cell culture medium,with the ad-dition of equal amounts of dimethyl sulfoxide(DMSO)as control,the effects of RES and ESR1 on the differentiation of 3T3-L1 cells were analyzed using oil red O staining,real-time fluorescence quantitative PCR assay and Western blotting assay.【Result】Compared with the control group,RES significantly(P<0.05)reduced the production of lipid droplets in 3T3-L1 cells,extremely significantly(P<0.001)up-regulated the relative expression of ATGL,a key gene forlipolysis,and extremely significantly(P<0.001)down-regulated the relative expression of CEBPα,PPARγand FAS,key genes for adipogenesis.RES extremely significantly(P<0.001)increased the relative expression of ESR1 gene expression,and ex-tremely significantly(P<0.01)increased the relative expression level of ESR1 protein;at the same time,RES extremely significantly(P<0.001)down-regulated the relative expression of PI3K and AKT genes in the PI3K signaling pathway,and extremely significantly(P<0.001)up-regulated the relative expression of FOXO1 gene.Interference with ESR1 gene extremely significantly(P<0.01)increased the generation of lipid droplets in 3T3-L1 cells,extremely significantly(P<0.001)down-regulated the relative expression of ATGL gene,and extremely significantly(P<0.001)up-regulated the relative expression of CEBPα,PPARγand FAS genes;after the addition of RES,the lipid droplets of 3T3-L1 cells inter‐fering with ESR1 gene were not significantly different(P>0.05,the same below)compared with those without RES.There was no significant difference in the relative expression of ATGL,CEBPα,PPARγand FAS genes.Interfering with ESR1 gene could extremely significantly(P<0.001)up-regulate the relative expression of PI3K and AKT genes in the PI3K signaling pathway,and extremely significantly(P<0.001)down-regulate the relative expression of FOXO1 gene;the relative expression of PI3K,AKT and FOXO1 genes were not significantly different when RES was added compared with that of not adding RES.【Conclusion】Through the ESR1-PI3K signaling pathway,RES can up-regulate the relative expression of ATGL,the key gene of lipolysis,and down-regulate the relative expression of CEBPα,PPARγand FAS,the key genes of fat synthesis,thereby inhibiting the formation of lipid droplets in 3T3-L1 cells.
Key words:resveratrol;estrogen receptor 1(ESR1);PI3K signaling pathway;lipid droplet formation
Foundation items:National Natural Science Foundation of China(32360839,31760672);Guangxi Natural Science Foundation(2022XNSFAA035525)
0引言
【研究意义】脂肪沉积是脂肪合成和分解相互协调,直至达成平衡的复杂生化过程。脂肪组织分为内脏脂肪组织和皮下脂肪组织,对能量维持和代谢稳态至关重要(Chouchani and Kajimura,2019;Ho-ward and Margolis,2020)。皮下脂肪是分布于动物皮下的一种脂肪组织,有隔热、保护和储存能量的功能(Ji et al.,2020),占动物全身脂肪的大部分(Schu-macheretal.,2022)。皮下脂肪积累不仅会导致畜禽肉品质下降及饲料利用效率降低,还会引起心血管疾病、糖尿病等健康问题,导致动物生产性能下降(Patel and Abate,2013;Li et al.,2022;Xiong et al.,2023)。因此,减少畜禽皮下脂肪沉积对提高畜禽的瘦肉率、生产性能和肉品质有重要意义。【前人研究进展】研究发现,共轭亚油酸和甜菜碱在一定条件下会逆转胰岛素引起的脂解异常,从而增加脂肪分解,减少皮下脂肪沉积(Fernández-Fígares et al.,2019);在饲粮中添加不饱和脂肪酸能提高猪的生长性能和肉品质,并降低猪的皮下脂肪沉积(Nong et al.,2020;Fanallietal.,2022);甘草酸单铵能抑制脂质代谢基因和细胞凋亡基因的表达,从而减少犊牛的皮下脂肪沉积(Zhang et al.,2022b);胆汁酸通过下调脂肪生成相关基因,减少羔羊皮下脂肪沉积(Zhang et al.,2022a)。白藜芦醇(RES)是一种非黄酮类的天然植物多酚化合物,广泛存在于葡萄、蔓越莓、蓝莓、花生等植物中,具有抗癌、抗糖尿病、保护神经和心脏等作用(Ramírez-Garza et al.,2018)。RES可通过调节细胞脂肪因子调控脂质代谢(van Andel etal.,2019;Wei et al.,2021;田艳杰等,2023;Wang et al.,2023)。雌激素受体1(Estrogen receptor ESR1)属于核受体家族转录因子之一(Lung et al.,2020)。ESR1可通过与靶基因结合或调节脂肪分化关键基因的转录因子抑制其磷酸化,从而减少脂质形成以抑制脂肪沉积(Palmisano etal.,2017)。研究表明,脂肪组织中ESR1的表达与肥胖呈正相关,与胰岛素敏感性呈负相关(徐宁和张薇,2019;Zhou et al.,2020)。ESR1基因缺失的小鼠比正常小鼠更肥胖(Ribas et al.,2010),在白色脂肪组织中,敲除ESR1基因导致编码线粒体DNA聚合酶γ催化亚基的Polg1基因表达相应减少,导致脂肪沉积增加(Zhou et al.,2020);同时,ESR1可通过调节线粒体功能和胰岛素抵抗影响脂肪沉积(Ribas et al.,2016)。相关研究发现,RES对ESR1存在亲和力,可选择性激活ESR1从而影响其转录活性(Bowers et al.,2000;Ratz-Łyko and Arct,2019)。【本研究切入点】ESR1对脂肪生成有重要调节作用,RES可影响其转录活性,但RES通过ESR1影响脂肪沉积的机制尚未明确。【拟解决的关键问题】以3T3-L1前体脂肪细胞为试验材料,通过小干扰RNA(siRNA)干扰ESR1基因表达,在细胞培养基中添加RES,通过油红O染色检测细胞脂滴的生成,检测ESR1基因及ESR1蛋白的表达情况,检测与脂肪分化相关的脂肪甘油三酯脂肪酶基因ATGL、CCAAT/增强子结合蛋白α基因CEBPα、过氧化酶体增殖物激活受体γ基因PPARγ、脂肪酸合酶基因FAS的表达情况,检测与PI3K信号通路相关的磷脂酰肌醇3激酶基因PI3K、蛋白激酶B基因AKT、叉头框蛋白O1基因FOXO1的表达情况,探究RES与ESR1对3T3-L1细胞脂滴形成的影响及其调控机制,为RES抑制脂肪沉积潜在机制的研究提供理论依据。
1材料与方法
1.1试验材料
3T3-L1细胞由广西大学动物科学技术学院保存提供;3T3-L1细胞专用培养基购自苏州海星生物科技有限公司;胰蛋白酶、PBS均购自美国Gibco公司;胰岛素、地塞米松、IBMX、RIPA、PMSF、BCA蛋白浓度测定试剂盒均购自上海碧云天生物技术股份有限公司;RES(纯度99%)购自陕西慈缘生物技术有限公司;油红O染色试剂盒、二甲基亚砜(DMSO)均购自北京索莱宝科技有限公司;siRNA-NC购自广州市锐博生物科技有限公司;Lipofectamine RNAiMAX购自赛默飞世尔科技公司;反转录试剂盒(PrimeScriptTM RT reagent Kit)购自日本TaKaRa公司;ChamQ Universal SYBR qPCR Master Mix购自南京诺唯赞生物科技股份有限公司;兔抗GAPDH多克隆抗体、兔抗ESR1单克隆抗体、HRP标记羊抗兔IgG均购自武汉爱博泰克生物科技有限公司;无水乙醇、多聚甲醛、氯仿和异丙醇等均购自广西卓一生物技术有限公司。
1.2试验方法
1.2.1 ESR1基因siRNA设计与合成根据NCBI已公布的ESR1基因编码区(CDS)序列,设计3条干扰序列(siRNA-ESR1-1、siRNA-ESR1-2、siRNA-ESR1-3)并委托广州市锐博生物科技有限公司合成,siRNA序列信息如表1所示。
1.2.2 RES处理待3T3-L1细胞长满后,分为RES组与对照组。参照Zhang等(2023)的方法,配制RES组诱导培养基:在3T3-L1细胞专用培养基(10%FBS+90%DMEM)中加入30μmol/L RES、10 mg/mL胰岛素、10μmol/L地塞米松及0.5 mmol/L IBMX;配制RES组分化培养基:在3T3-L1细胞专用培养基(10%FBS+90%DMEM)中加入30μmol/L RES、10 mg/mL胰岛素。对照组将相应培养基中的RES更换为等量DMSO。用诱导培养基培养3T3-L1细胞2 d后,更换为分化培养基培养8 d,共诱导分化10 d。
1.2.3 siRNA转染待3T3-L1细胞生长汇合度达60%~70%后,根据Lipofectamine RNAiMAX说明以siRNA-ESR1-1、siRNA-ESR1-2、siRNA-ESR1-3及siRNA-NC(对照)分别转染3T3-L1细胞,待6~8 h后更换为完全培养基,培养48h。筛选出干扰效果最好的siRNA,以siRNA-NC与筛选出的siRNA分别转染3T3-L1细胞,培养后分为siRNA-NC组、siRNA-ESR1组和siRNA-ESR1+RES组,siRNA-NC组和siRNA-ESR1组用添加DMSO的培养基诱导细胞分化,siRNA-ESR1+RES组用添加RES的培养基诱导细胞分化,方法同1.2.2。
1.2.4油红O染色待3T3-L1细胞分化至脂滴成熟后用PBS清洗3次,用4%多聚甲醛固定30 min后,使用60%异丙醇浸洗5min,按照油红O储备液∶PBS=3∶2比例配制油红O工作液,过滤并预热,加入细胞培养板并避光孵育30min,用60%异丙醇清洗干净后置于荧光显微镜下观察记录,图片采用Image J进行量化分析。
1.2.5 RNA提取、cDNA合成和实时荧光定量PCR检测待3T3-L1细胞长满后加入TRIzol、氯仿提取RNA,12000 r/min离心15 min后取上清液,并依次加入异丙醇、75%乙醇、无水乙醇洗涤后12000r/min离心10 min,加入适量RNase free H2O溶解并检测其浓度。以总RNA为模板,使用PrimeScriptTM RT reagent Kit反转录合成cDNA。采用实时荧光定量PCR检测RES处理和siRNA干扰对ESR1基因、脂肪分化相关基因(ATGL、CEBP“、PPARγ、FAS)、PI3K信号通路相关基因(PI3K、AKT、FOXO1)表达的影响。根据NCBI已公布的小鼠基因序列设计引物并送至南宁捷尼斯生物科技有限公司合成,引物信息见表2。实时荧光定量PCR反应体系10.00μL:cDNA模板2.50μL,10μmol/L上、下游引物各0.25μL,ChamQ Universal SYBR qPCR Master Mix 5.00μL,RNase free H2O 2.00μL。扩增程序:95℃预变性30 s;95℃5 s,60℃30 s,进行30~35个循环。内参基因为18S rRNA,基因相对表达量采用2-ΔΔCt法计算。
1.2.6 Western blotting检测待3T3-L1细胞分化至脂滴成熟后,按照RIPA∶PMSF=100∶1比例提取ESR1蛋白,采用BCA蛋白浓度测定试剂盒测定蛋白浓度,取适量蛋白样品进行SDS-PAGE凝胶电泳,转移至PVDF膜上进行封闭、一抗及二抗孵育,采用Gel Doc XR凝胶成像系统(美国Bio-Rad公司)进行检测并拍照记录。
1.3统计分析
试验数据采用GraphPad Prism 8.0进行单因素方差分析(One-way ANOVA)和t检验并绘图,结果以平均值±标准误差表示。
2结果与分析
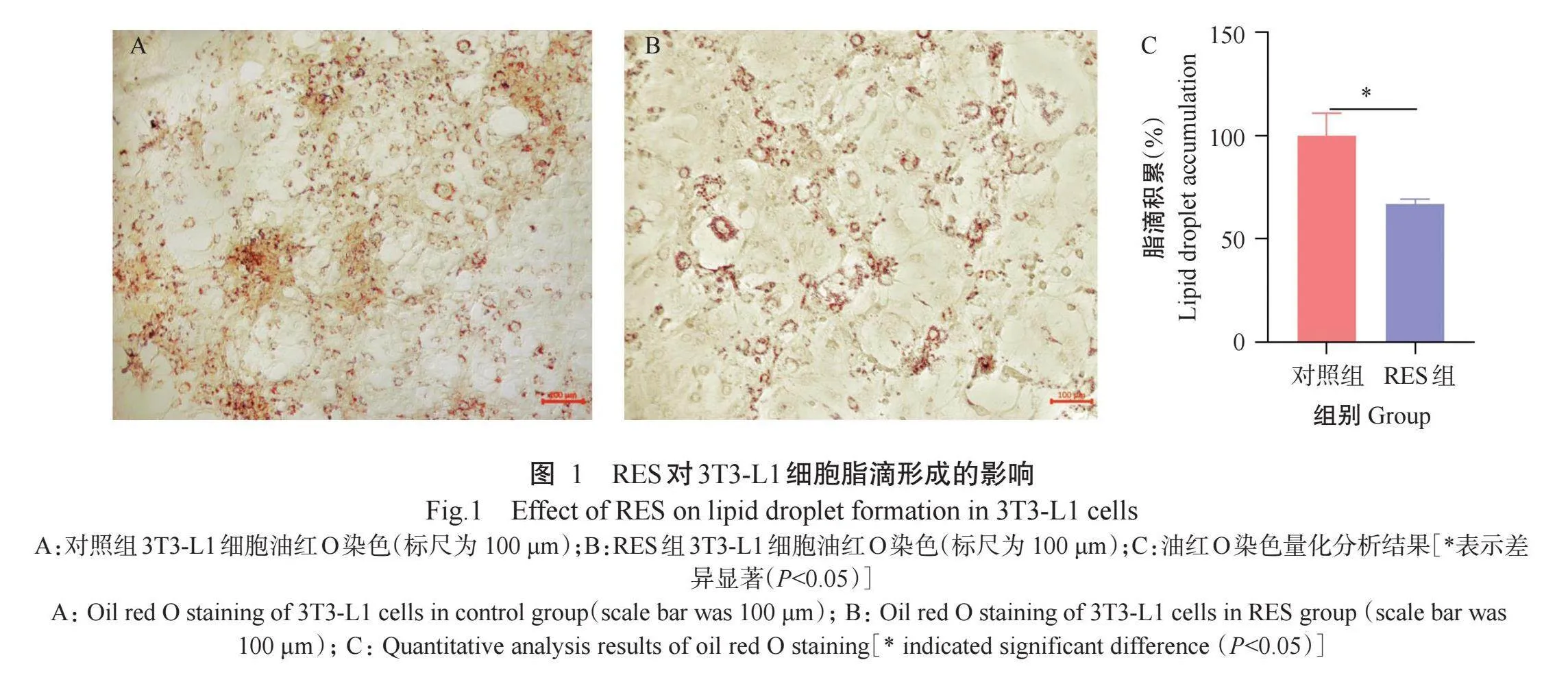
2.1 RES对3T3-L1细胞脂滴形成的影响
3T3-L1细胞诱导分化10 d后,油红O染色结果如图1所示,与对照组相比,RES组3T3-L1细胞脂滴明显减少,进一步利用Image J对油红O染色结果进行量化分析(图1-C),发现RES显著(P<0.05,下同)减少3T3-L1细胞脂滴的生成。
2.2 RES对3T3-L1细胞脂肪分化相关基因表达的影响
3T3-L1细胞诱导分化10 d后,提取RNA通过实时荧光定量PCR检测脂肪分化基因的相对表达量,结果如图2所示,与对照组相比,RES组ATGL基因相对表达量极显著增加(P<0.001),CEBPα、PPARγ及FAS基因相对表达量极显著降低(P<0.001)。结果表明RES能通过上调脂肪分解关键基因ATGL相对表达量,下调脂肪合成关键基因CEBPα、PPARγ及FAS相对表达量,影响3T3-L1细胞脂滴形成。
2.3 RES对3T3-L1细胞分化过程中ESR1表达的影响
在3T3-L1细胞诱导分化过程中,ESR1基因的相对表达量变化趋势如图3-A所示,在分化第6、8和10 d,与对照组相比,RES组ESR1基因相对表达量极显著升高(P<0.001)。3T3-L1细胞诱导分化10 d后,ESR1蛋白相对表达水平如图3-B所示,与对照组相比,RES组ESR1蛋白相对表达水平极显著升高(P<0.01)。综上所述,RES能增加3T3-L1细胞分化过程中ESR1的表达。
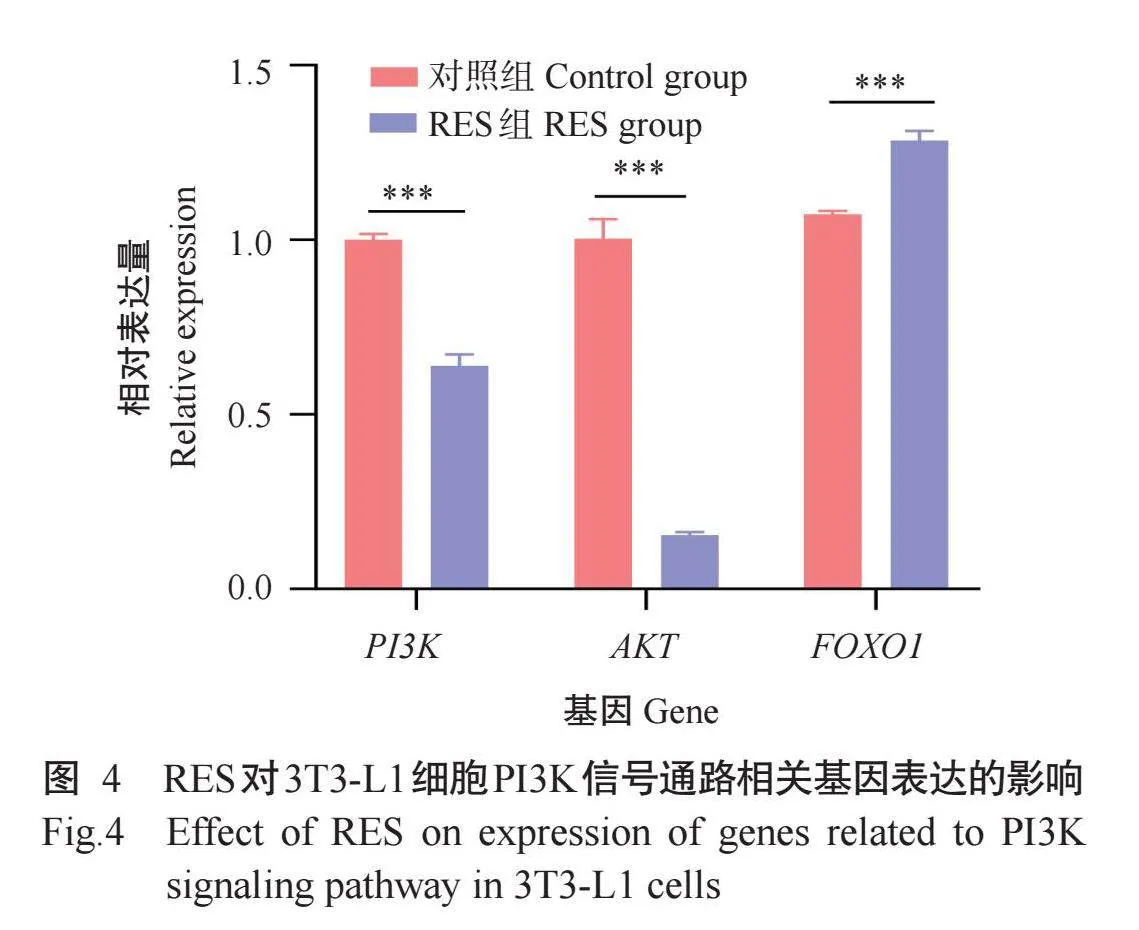
2.4 RES对3T3-L1细胞PI3K信号通路相关基因表达的影响
3T3-L1细胞诱导分化10 d后,提取RNA通过实时荧光定量PCR检测PI3K信号通路相关基因的相对表达量,结果如图4所示,与对照组相比,RES组FOXO1基因相对表达量极显著增加(P<0.001),PI3K、AKT基因相对表达量极显著降低(P<0.001)。结果表明RES能调节ESR1-PI3K信号通路,从而抑制3T3-L1细胞脂滴形成。
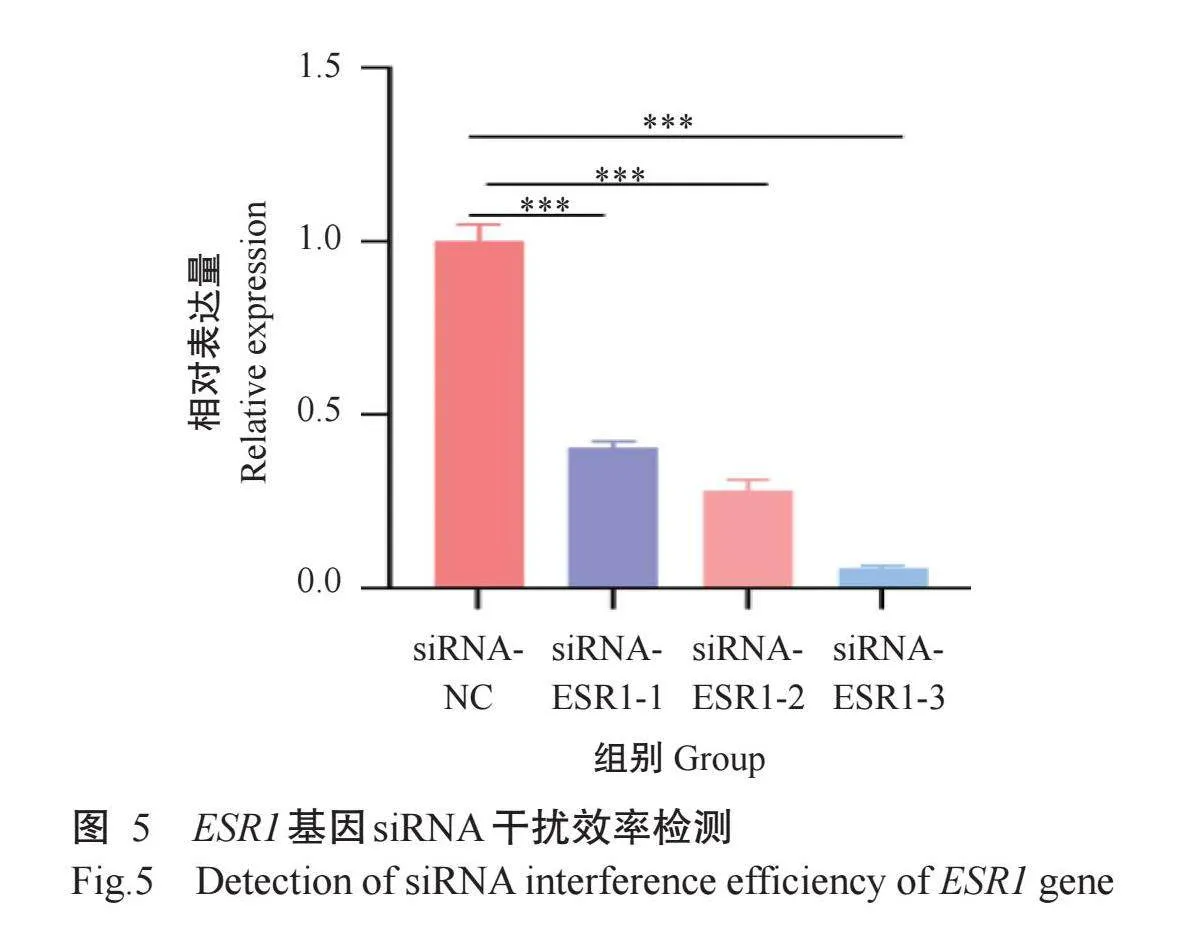
2.5 ESR1基因siRNA干扰效率检测与筛选
以siRNA-ESR1-1、siRNA-ESR1-2、siRNA-ESR1-3及siRNA-NC分别转染3T3-L1细胞,48 h后提取RNA通过实时荧光定量PCR检测ESR1基因相对表达量,结果(图5)显示,siRNA-ESR1-1、siRNA-ESR1-2、siRNA-ESR1-3干扰目的基因ESR1的效率依次为60%、80%、90%,均与siRNA-NC差异极显著(P<0.001)。siRNA-ESR1-3的干扰效果最好,故选择siRNA-ESR1-3进行后续试验。
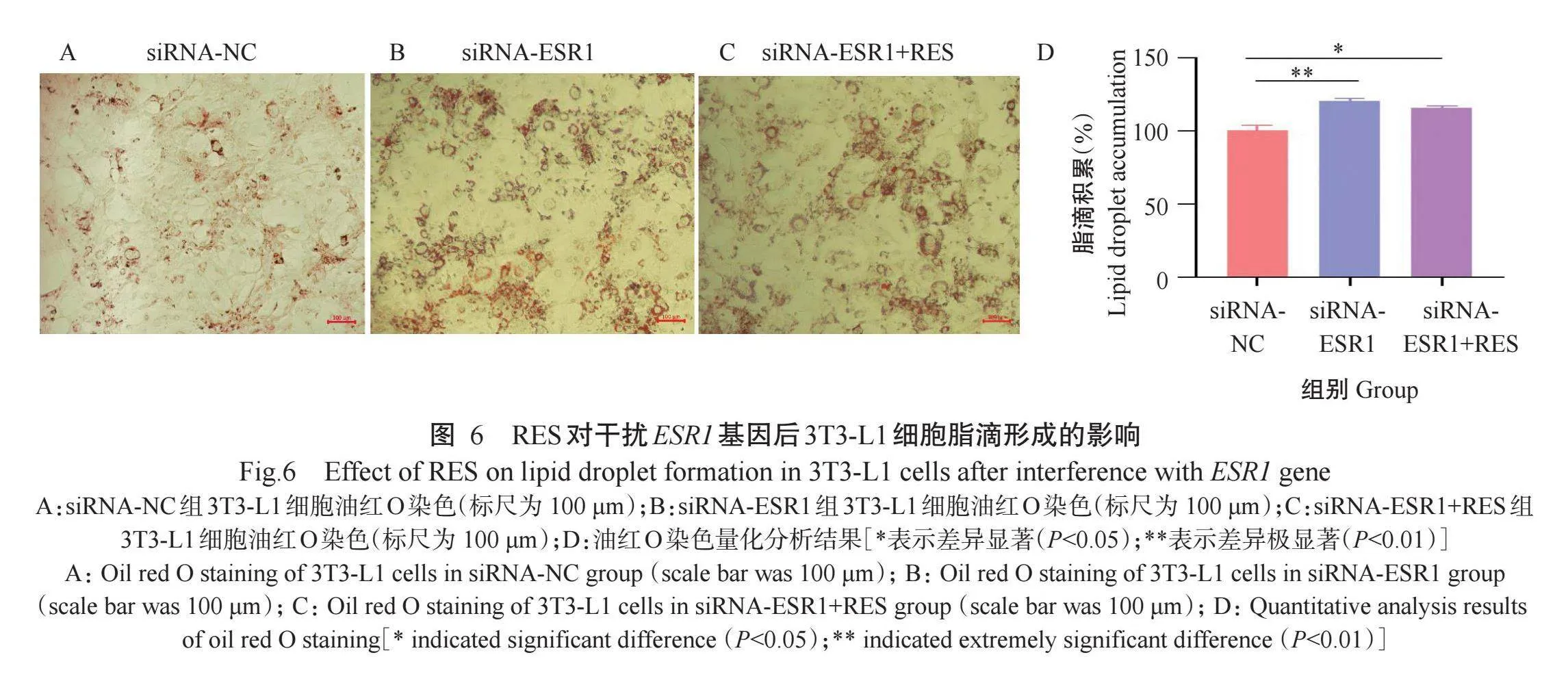
2.6 RES对干扰ESR1基因后3T3-L1细胞脂滴形成的影响
以siRNA-ESR1-3(标记为siRNA-ESR1)与siRNA-NC分别转染3T3-L1细胞,siRNA-ESR1+RES组用加入RES的培养基诱导细胞分化,siRNA-NC组和siRNA-ESR1组用添加DMSO的培养基诱导细胞分化,至诱导分化第10 d进行油红O染色,结果(图6)显示,与siRNA-NC组相比,siRNA-ESR1组和siRNA-ESR1+RES组3T3-L1细胞脂滴明显增多,但siRNA-ESR1组与siRNA-ESR1+RES组无明显差异。采用Image J对油红O染色结果进行量化分析发现,干扰ESR1基因极显著增加3T3-L1细胞脂滴的生成(P<0.01),RES处理后,干扰ESR1基因的3T3-L1细胞脂滴形成减少,但与未加入RES相比无显著差异(P>0.05,下同)。
2.7 RES对干扰ESR1基因后3T3-L1细胞脂肪分化相关基因表达的影响
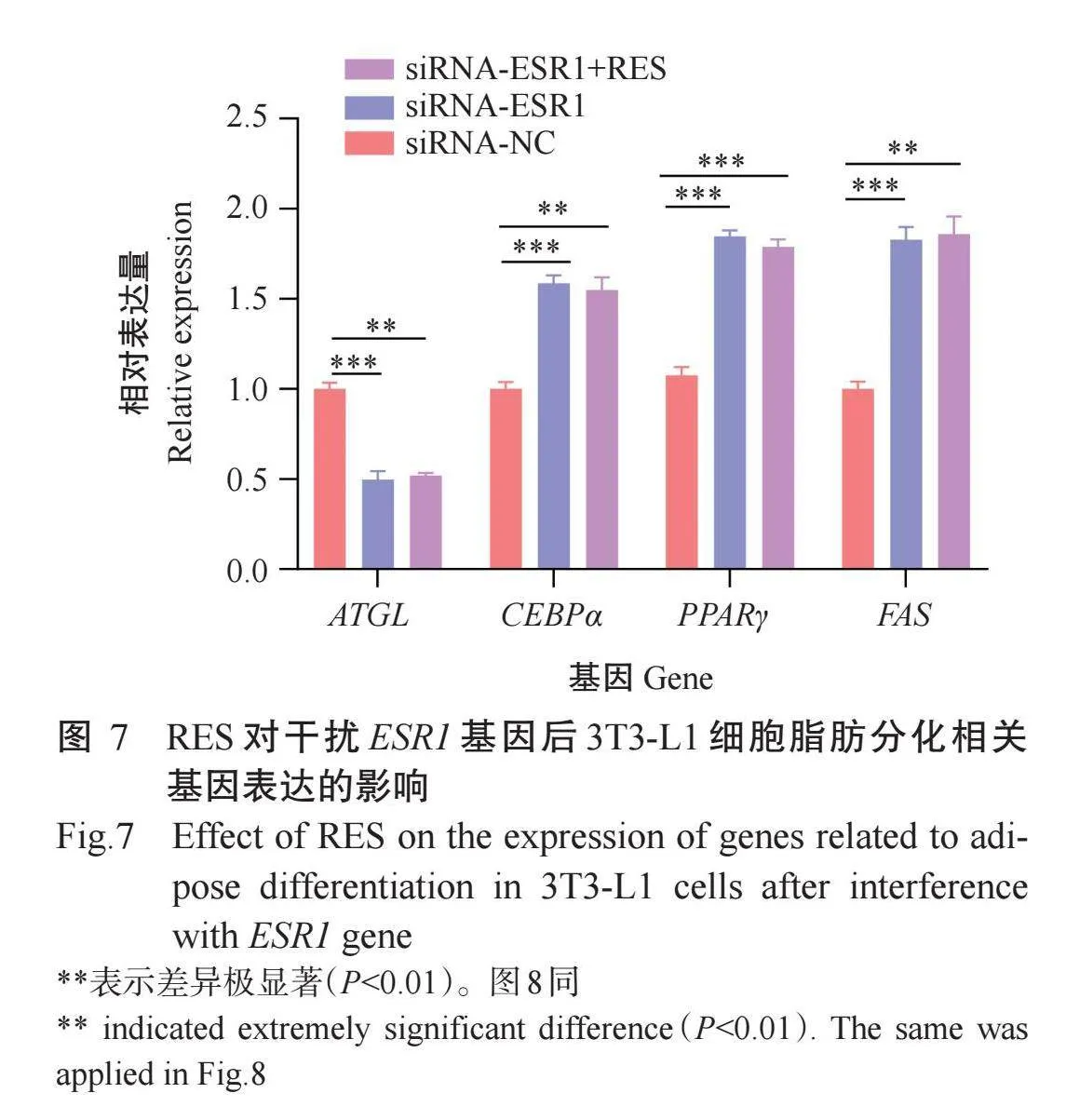
以siRNA-ESR1-3及siRNA-NC分别转染3T3-L1细胞,诱导细胞分化10 d后,提取RNA通过实时荧光定量PCR检测脂肪分化相关基因的相对表达量,结果(图7)显示,与siRNA-NC组相比,siRNA-ESR1组ATGL基因相对表达量极显著降低(P<0.001),CEBPα、PPARγ及FAS基因相对表达量极显著增加(P<0.001);与siRNA-NC组相比,siRNA-ESR1+RES组ATGL基因相对表达量极显著降低(P<0.01),CEBPα、PPARγ及FAS基因相对表达量极显著增加(P<0.01或P<0.001);但siRNA-ESR1+RES组ATGL、CEBPα、PPARγ及FAS基因相对表达量与siRNA-ESR1组无显著差异。结果表明干扰ESR1基因的表达能下调脂肪分解关键基因ATGL相对表达量,上调脂肪合成关键基因CEBPα、PPARγ及FAS相对表达量,从而影响3T3-L1细胞脂滴形成。
2.8 RES对干扰ESR1基因后3T3-L1细胞PI3K信号通路相关基因表达的影响
以siRNA-ESR1-3及siRNA-NC分别转染3T3-L1细胞,诱导细胞分化10 d,提取RNA通过实时荧光定量PCR检测PI3K信号通路相关基因的相对表达量,结果(图8)显示,与siRNA-NC组相比,siRNA-ESR1组ESR1基因表达受干扰,PI3K和AKT基因相对表达量极显著增加(P<0.001),FOXO1基因相对表达量极显著降低(P<0.001);与siRNA-NC组相比,siRNA-ESR1+RES组PI3K和AKT基因相对表达量极显著增加(P<0.01),FOXO1基因相对表达量极显著降低(P<0.001);但siRNA-ESR1+RES组PI3K、AKT和FOXO1基因相对表达量与siRNA-ESR1组无显著差异,表明RES通过ESR1-PI3K信号通路抑制3T3-L1细胞脂滴形成。
3讨论
RES是一种具有广泛生物活性的天然化合物,大量研究表明RES对脂肪沉积具有重要作用(郑新杰等,2017;Zhang et al.,2021),RES能通过脂联素信号通路促进脂肪酸分解,从而抑制脂肪沉积(Zhang et al.,2023),RES还能通过PKA/LKB1/AMPK信号通路逆转线粒体功能障碍和氧化应激以调控脂肪沉积(Huang et al.,2019),同时RES能通过mTOR信号通路减少3T3-L1细胞脂滴生成(Liu et al.,2020)。ESR1作为转录因子在脂肪生成过程中起重要调节作用(Ribas et al.,2010),然而RES对其的影响尚不明确,本研究发现RES能显著抑制3T3-L1细胞脂滴形成,同时增加ESR1基因相对表达量及ESR1蛋白相对表达水平,RES还能通过PI3K信号通路,上调脂肪分解关键基因ATGL相对表达量,下调脂肪合成关键基因CEBP“、PPARγ及FAS相对表达量,表明RES能通过ESR1-PI3K信号通路抑制3T3-L1细胞脂滴形成。
ATGL是由细胞内质网分泌的脂肪酶,能水解脂蛋白中的中性脂肪(Li etal.,2022),将甘油三酯水解为游离脂肪酸和甘油,为脂肪代谢提供能量和底物,对脂肪分解起关键作用(Cerk et al.,2018;Sch-reiberet al.,2019)。CEBPα在调节脂肪生成中起正向调控作用(Lourenço and Coffer,2017;岳永起等,2021)。PPARγ能正向调节脂肪的分化(朱磊等,2019;Li etal.,2020)。FAS能通过增加从头合成的脂质提高肝脏脂肪储存和甘油三酯含量,并能直接与葡萄糖生成基因结合并抑制其转录,从而导致脂质积累(Qiu etal.,2017)。本研究中,油红O染色结果表明,siRNA-ESR1组细胞脂滴明显多于siRNA-NC组,而siRNA-ESR1+RES组细胞脂滴较siRNA-ESR1组减少,但差异不明显;同时,干扰ESR1基因后,3T3-L1细胞的脂肪分解相关基因ATGL相对表达量极显著降低(P<0.001),脂肪合成相关基因CEBPα、PPARγ和FAS相对表达量极显著增加(P<0.001),但加入RES后的干扰ESR1基因3T3-L1细胞与未加入RES相比,上述基因的相对表达量无显著差异。说明RES能通过ESR1上调脂肪分解关键基因ATGL表达量,下调脂肪合成关键基因CEBPα、PPARγ及FAS相对表达量,从而抑制3T3-L1细胞脂滴形成。
PI3K信号通路作为经典胰岛素信号通路能调节机体脂质代谢(Zhao et al.,2021)。PI3K/AKT轴是胰岛素途径的中枢环节,能调节肝糖原合成、糖苷生成和脂质合成(钟佳琳等,2019)。研究发现,在3T3-L1细胞中激活PI3K/AKT轴会增加其胰岛素敏感性,从而调节机体脂代谢(Zhang et al.,2022c)。ESR1作为转录因子可激活PI3K/AKT轴,调控其表达(Stanimirovic et al.,2018);ESR1能与PI3K相互作用,从而增强细胞内磷酸肌醇的产生,激活PI3K下游通路调节细胞增殖与存活(Khatpe et al.,2021;Sirohietal.,2022;Clusanetal.,2023)。本研究中,干扰ESR1基因后,3T3-L1细胞的PI3K和AKT基因相对表达量极显著提高(P<0.001),FOXO1基因相对表达量极显著降低(P<0.001),加入RES后,PI3K、AKT及FOXO1基因相对表达量与未加入RES相比均无显著差异,表明RES能通过ESR1-PI3K信号通路调控3T3-L1细胞脂滴形成。
4结论
RES能通过ESR1-PI3K信号通路,上调脂肪分解关键基因ATGL相对表达量,下调脂肪合成关键基因CEBPα、PPARγ及FAS相对表达量,从而抑制3T3-L1细胞脂滴形成。
参考文献(References):
田艳杰,石爱民,刘红芝,代蕾,刘哲,王强.2023.白藜芦醇的生物活性及其运载体系研究进展[J].食品科学,44(1):371-379.[Tian Y J,ShiA M,Liu H Z,Dai L,Liu Z,Wang Q.2023.Progress in research on biological activities and delivery systems of resveratrol[J].Food Science,44(1):371-379.]doi:10.7506/spkx 1002-6630-20220308-104.
徐宁,张薇.2019.妊娠期糖尿病患者脂肪组织ERα表达水平对胰岛素抵抗的影响[J].医学研究杂志,48(11):109-113.[Xu N,Zhang W.2019.Effect of ERαexpression in adipose tissue of gestational diabetes mellitus on insulin resistance[J].Journal of Medical Research,48(11):109-113.]doi:10.11969/j.issn.1673-548X.2019.11.025.
岳永起,华永琳,贾逸格,李键,熊燕,熊显荣.2021.牦牛CEBPα基因克隆及在脂肪细胞中的表达模式[J].华北农学报,36(6):228-234.[Yue Y Q,Hua Y L,Jia Y G,Li J,Xiong Y,Xiong X R.2021.Cloning of yak CEBPαgene and its expression pattern in yak adipocytes[J].Acta AgriculturaeBoreali-Sinica,36(6):228-234.]doi:10.7668/hbnxb.20192454.
郑新杰,刘子惠,廖维瑶,李青蓉,郑琳,罗晓林,冯翔.2017.白藜芦醇对高脂诱导肥胖小鼠脂肪组织分布的影响及其机制[J].中国热带医学,17(12):1oppa0xvjPGmOpTfpEJbBhg==176-1180.[Zheng X J,Liu Z H,Liao W Y,Li Q R,Zheng L,Luo X L,Feng X.2017.Effects and mechanism of resveratrol on adipose tis-sue distribution of mice fed a high-fat diet[J].China Tropi-cal Medicine,17(12):1176-1180.]doi:10.13604/j.cnki.46-1064/r.2017.12.03.
钟佳琳,郑立,贺花,王健,何盼,宋成创,徐天扬,张禹,曹修凯,雷初朝,陈宏,黄永震.2019.PI3K/Akt信号通路相关的生物学调控机制研究进展[J].基因组学与应用生物学,38(1):143-147.[Zhong J L,Zheng L,He H,Wang J,He P,Song C C,Xu T Y,Zhang Y,Cao X K,Lei C C,Chen H,Huang Y Z.2019.Research progress of biologicalregulatory mechanism related to PI3K/Akt signal pathway[J].Genomics and Applied Biology,38(1):143-147.]doi:10.13417/j.gab.038.000143.
朱磊,路瑛丽,冯连世,张素娴.2019.低氧训练诱导miR-27/PPARγ调控肥胖大鼠腓肠肌脂肪酸代谢的研究[J].体育科学,39(6):55-61.[Zhu L,Lu Y L,Feng L S,Zhang S X.2019.Effects of hypoxia exercise induced miR-27/PPARγon fatty acids metabolism in gastrocnemius of obese rat[J].China Sport Science,39(6):55-61.]doi:10.16469/j.css.201906007.
Bowers J L,Tyulmenkov V V,Jernigan S C,Klinge C M.2000.Resveratrol acts as a mixed agonist/antagonist for estrogen receptorsαandβ[J].Endocrinology,141(10):3657-3667.doi:10.1210/endo.141.10.7721.
Cerk I K,Wechselberger L,Oberer M.2018.Adipose triglyce-ride lipase regulation:An overview[J].Current Protein&Peptide Science,19(2):221-233.doi:10.2174/13892037 18666170918160110.
Chouchani E T,Kajimura S.2019.Metabolic adaptation and maladaptation in adipose tissue[J].Nature Metabolism,1(2):189-200.doi:10.1038/s42255-018-0021-8.
Clusan L,Ferrière F,Flouriot G,Pakdel F.2023.A basicreview on estrogen receptor signaling pathways in breast cancer[J].International Journal of Molecular Sciences,24(7):6834.doi:10.3390/ijms24076834.
Fanalli S L,da Silva B P M,Petry B,Santana M H A,Polizel G H G,Antunes R C,de Almeida V V,Moreira G C M,Luchiari Filho A,Coutinho L L,Balieiro J Cc,Reecy J M,Koltes J,Koltes D,Cesar A S M.2022.Dietary fatty acids applied to pig production and their relation to the biologi-cal processes:A review[J].Livestock Science,265:105092.doi:10.1016/j.livsci.2022.105092.
Fernández-Fígares I,Lachica M,Martínez-Pérez M,Ramsay T G.2019.Conjugated linoleic acid and betaine affect lipoly-sis in pig adipose tissue explants[J].Animal,13(12):2840-2846.doi:10.1017/s 1751731119001186.
Howard E E,Margolis L M.2020.Intramuscular mechanisms mediating adaptation to low-carbohydrate,high-fat diets during exercise training[J].Nutrients,12(9):2496.doi:10.3390/nu 12092496.
Huang Y J,Zhu X H,Chen K,Lang H D,Zhang Y,Hou P F,Ran L,Zhou M,Zheng J W,Yi L,Mi M T,Zhang Q Y.2019.Resveratrol prevents sarcopenic obesity by reversingmitochondrial dysfunction and oxidative stress via the PKA/LKB1/AMPK pathway[J].Aging,11(8):2217-2240.doi:10.18632/aging.101910.
Ji M,Xu K,Zhang D W,Chen T T,Shen L C,Wu W J,Zhang J.2020.Adipose-tissue-specific expression of pig ApoR protects mice from diet-induced obesity[J].Journal of Agricultural and Food Chemistry,68(7):2256-2262.doi:10.1021/acs.jafc.9b06995.
Khatpe A S,Adebayo A K,Herodotou C A,Kumar B,Nakshatri H.2021.Nexus between PI3K/AKT and estro-gen receptor signaling in breast cancer[J].Cancers,13(3):369.doi:10.3390/cancers 13030369.
Li L,Fu J Q,Liu D,Sun J,Hou YY,Chen C J,Shao J B,Wang L L,Wang X,Zhao R,Wang H H,Andersen M E,Zhang Q,Xu Y Y,Pi J B.2020.Hepatocyte-specific Nrf2 defi-ciency mitigates high-fat diet-induced hepatic steatosis:Involvement of reduced PPARγexpression[J].Redox Bio-logy,30:101412.doi:10.1016/j.redox.2019.101412.
Li T J,Guo W,Zhou Z X.2022.Adipose triglyceride lipase in hepatic physiology and pathophysiology[J].Biomolecu-les,12(1):57.doi:10.3390/biom 12010057.
Liu Z H,Liao W Y,Yin X H,Zheng X J,Li Q R,Zhang H M,Zheng L,Feng X.2020.Resveratrol-induced brown fat-like phenotype in 3T3-L1 adipocytes partly via mTOR pathway[J].Food&Nutrition Research,64:3656.doi:10.29219/fnr.v64.3656.
Lourenço A R,Coffer P J.2017.A tumor suppressor role for C/EBPαin solid tumors:More than fat and blood[J].Onco-gene,36(37):5221-5230.doi:10.1038/onc.2017.151.
Lung D K,Reese R M,Alarid E T.2020.Intrinsic and extrinsicfactors governing the transcriptional regulation of ESR1[J].Hormones and Cancer,11(3-4):129-147.doi:10.1007/s12672-020-00388-0.
Nong Q Y,Wang L Y,Zhou Y B,Sun Y,Chen W T,Xie J T,Zhu X D,Shan T Z.2020.Low dietary n-6/n-3 PUFA ratio regulates meat quality,reduces triglyceride content,and improves fatty acid composition of meat in Heigai pigs[J].Animals,10(9):1543.doi:10.3390/ani 10091543.
Palmisano B T,Zhu L,Stafford J M.2017.Role of estrogens in the regulation of liver lipid metabolism[M]//Mauvais-Jarvis F.Sex and gender factors affecting metabolic homeostasis,diabetes and obesity.Cham:Springer:227-256.doi:10.1007/978-3-319-70178-3_12.
Patel P,Abate N.2013.Role of subcutaneous adipose tissue in the pathogenesis of insulin resistance[J].Journal of Obe-sity,2013(1):489187.doi:10.1155/2013/489187.
Qiu S Q,Vazquez J T,Boulger E,Liu H Y,Xue P,Hussain M A,Wolfe A.2017.Hepatic estrogen receptorαis critical for regulation of gluconeogenesis and lipid metabolism in males[J].Scientific Reports,7(1):1661.doi:10.1038/s41598-017-01937-4.
Ramírez-Garza S L,Laveriano-Santos E P,Marhuenda-Muñoz M,Storniolo C E,Tresserra-Rimbau A,Vallverdú-Queralt A,Lamuela-Raventós R M.2018.Health effects of resve-ratrol:Results from human intervention trials[J].Nutrients,10(12):1892.doi:10.3390/nu 10121892.
Ratz-Łyko A,Arct J.2019.Resveratrol as an active ingredientfor cosmetic and dermatological applications:A review[J].Journal of Cosmetic and Laser Therapy,21(2):84-90.doi:10.1080/14764172.2018.1469767.
Ribas V,Drew B G,Zhou Z Q,Phun J,Kalajian N Y,Soley‐mani T,Daraei P,Widjaja K,Wanagat J,de Aguiar Vallim T Q,Fluitt A H,Bensinger S,Le T,Radu C,Whitelegge J P,Beaven S W,Tontonoz P,Lusis A J,Parks B W,Vergnes L,Reue K,Singh H,Bopassa J C,Toro L,Stefani E,Watt M J,Schenk S,Akerstrom T,Kelly M,Pedersen B K,Hewitt S C,Korach K S,Hevener A L.2016.Skeletal muscle action of estrogen receptorαis critical for the maintenance of mitochondrial function and metabolic homeostasis in females[J].Science Translational Medi‐cine,8(334):334ra54.doi:10.1126/scitranslmed.aad3815.
Ribas V,Nguyen M T,Henstridge D C,Nguyen A K,Beaven S W,Watt M J,Hevener A L.2010.Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERα-deficient mice[J].American Journal of Physiology-Endocrinology and Metabolism,298(2):E304-E319.doi:10.1152/ajpendo.00504.2009.
Schreiber R,Xie H,Schweiger M.2019.Of mice and men:The physiological role of adipose triglyceride lipase(ATGL)[J].Biochimica et Biophysica Acta-Molecular and Cell Biology of Lipids,1864(6):880-899.doi:10.1016/j.bbalip.2018.10.008.
Schumacher M,DelCurto-Wyffels H,Thomson J,Boles J.2022.Fat deposition and fat effects on meat quality—A review[J].Animals,12(12):1550.doi:10.3390/ani 1212 1550.
Sirohi V K,Medrano T I,Mesa A M,Kannan A,Bagchi I C,Cooke P S.2022.Regulation of AKT Signaling in mouse uterus[J].Endocrinology,163(1):bqab233.doi:10.1210/endocr/bqab233.
Stanimirovic J,Obradovic M,Panic A,Petrovic V,Alavantic D,Melih I,Isenovic E R.2018.Regulation of hepatic Na+/K+-ATPase in obese female and male rats:Involvement of ERK1/ AMPK,and Rho/ROCK[J].Molecular and Ce-llular Biochemistry,440(1-2):77-88.doi:10.1007/s 11010-017-3157-z.
van Andel M M,Groenink M,Zwinderman A H,Mulder B J M,de Waard V.2019.The potential beneficial effects ofresveratrol on cardiovascular complications in Marfan syn‐drome patients—Insights from rodent-based animal studies[J].International Journal of Molecular Sciences,20(5):1122.doi:10.3390/ijms20051122.
Wang G H,Ruan L,Wu R P,Jin Z.2023.Effects of aerobic exercise andresveratrol on adipocytokines in rats with non‐alcoholic fatty liver disease[J].Science&Sports,38(1):57-67.doi:10.1016/j.scispo.2021.12.008.
Wei Z Y,Chen G J,Hu T T,Mo X H,Hou X X,Cao K,Wang L Q,Pan Z Y,Wu Q,Li X,Ye F,Zouboulis C C,Ju Q.2021.Resveratrol ameliorates lipid accumulation andinflammation in human SZ95 sebocytes via the AMPK signaling pathways in vitro[J].Journal of Dermatological Science,103(3):156-166.doi:10.1016/j.jdermsci.2021.07.010.
Xiong L,Pei J,Bao P J,Wang X D,Guo S K,Cao M L,Kang Y D,Yan P,Guo X.2023.The effect of the feeding system on fat deposition in yak subcutaneous fat[J].International Journal of Molecular Sciences,24(8):7381.doi:10.3390/ijms24087381.
Zhang B Y,Sun Z Q,Yu Z,Li H H,Luo H L,Wang B.2022a.Transcriptome and targeted metabolome analysis provide insights into bile acids'new roles and mechanisms on fat deposition and meat quality in lamb[J].Food Research International,162:111941.doi:10.1016/j.foodres.2022.111941.
Zhang C,Meng S D,Shao Q,Wang X Y,Li C X,Chen W B,Li Y X,Huang S C,Ma Y B.2022b.Protective effects of monoammonium glycyrrhizinate on fatty deposit degenera‐tion induced in primary calf hepatocytes by sodium oleate administration in vitro[J].Research in Veterinary Science,150:213-223.doi:10.1016/j.rvsc.2022.05.011.
Zhang D K,Wei Y H,Huang Q N,Chen Y,Zeng K,Yang W Q,Chen J,Chen J W.2022c.Important hormones regula-ting lipid metabolism[J].Molecules,27(20):7052.doi:10.3390/molecules27207052.
Zhang L X,Li C X,Kakar M U,Khan M S,Wu P F,Amir R M,Dai D F,Naveed M,Li Q Y,Saeed M,Shen J Q,Rajput S A,Li J H.2021.Resveratrol(RV):A pharmaco‐logical review and call for further research[J]Biomedi‐cine&Pharmacotherapy,143:112164.doi:10.1016/j.bio‐pha.2021.112164.
Zhang S B,Cui YY,Gao X T,Wei C W,Wang Q,Yang B,Sun W Y,Luo Y Y,Jiang Q Y,Huang Y N.2023.Resveratrol inhibits the formation and accumulation of lipid droplets through AdipoQ signal pathway and lipid metabolism lncRNAs[J].The Journal of Nutritional Biochemistry,117:109351.doi:10.1016/j.jnutbio.2023.109351.
Zhao G,Shi X B,Sun Z B,Zhao P F,Lu Z M.2021.PAQR4 promotes the development of hepatocellular carcinoma by activatinoeG93PT6GUXPl0B7gWNq5kFUT7/iWmeZavN9IGgnFL4=g PI3K/AKT pathway[J].Acta Biochimica et Bio‐physicaSinica,53(12):1602-1613.doi:10.1093/abbs/gmab 143.
Zhou Z Q,Moore T M,Drew B G,Ribas V,Wanagat J,Civelek M,Segawa M,Wolf D M,Norheim F,Seldin M M,Strumwasser A R,Whitney K A,Lester E,Reddish B R,Vergnes L,Reue K,Rajbhandari P,Tontonoz P,Lee J,Mahata S K,Hewitt S C,Shirihai O,Gastonbury C,Small K S,Laakso M,Jensen J,Lee S,Drevon CA,Korach K S,Lusis A J,HevenerA L.2020.Estrogen receptorαcontrols metabolism in white and brown adipocytes by regulating Polg1 and mitochondrial remodeling[J].Science Transla‐tional Medicine,12(555):eaax8096.doi:10.1126/scitrans‐lmed.aax8096.
(责任编辑刘可丹)
