从肠道菌群的角度分析衰弱在冠心病中的作用
2024-08-18冯荣鲁佳慧李赞李朝洋李亚芹崔红根
摘要:冠心病是老年人最常见的死亡原因。衰弱在老年患者中的发病率明显增加。衰弱评估能够很好预测老年冠心病患者预后。但是,衰弱导致冠心病不良预后的机制目前并不十分明确。近年来,由于基因测序技术的进步,肠道菌群与老年衰弱的相关性也更加明确,肠道菌群可能在衰弱合并冠心病的患者中发挥重要作用。现通过分析肠道菌群与衰弱的关系,说明肠道菌群对衰弱合并冠心病患者发生、发展的重要作用。通过早期调节衰弱患者肠道菌群,进而达到预防不良心血管事件发生,从而为临床对冠心病患者的二级预防提供理论依据。
关键词:冠心病;衰弱;肠道微生物组;衰弱评估
DOI:10.3969/j.issn.1674490X.2024.03.003
中图分类号:R54"""" 文献标志码:A"""" 文章编号:1674490X(2024)03001807
The role of frailty in coronary heart disease from the perspective of intestinal flora
FENG Rong, LU Jiahui, LI Zan, LI Zhaoyang, LI Yaqin, CUI Honggen
(Department of Cardiology, Affiliated Hospital of Hebei University, Baoding 071000, China)
Abstract: "Coronary heart disease is the most common cause of death in the elderly. The incidence of frailty in elderly patients is significantly increased. Frailty assessment can well predict the prognosis of elderly patients with coronary heart disease. However, the mechanism of frailty leading to poor prognosis of coronary heart disease is not very clear. Studies have shown that intestinal flora may play an important role in patients with frailty and coronary heart disease. In recent years, due to the advancement of gene sequencing technology, the correlation between intestinal flora and frailty in the elderly has become clearer. The main purposes of this review are to explain the important role of intestinal flora in the occurrence and development of patients with frailty and coronary heart disease by analyzing the relationship between intestinal flora and frailty through early intervention of intestinal flora in patients with frailty to achieve the purpose of preventing adverse cardiovascular events and in order to provide a theoretical basis for clinical secondary prevention of patients with coronary heart disease.
Key words: coronary heart disease;frailty; intestinal microbiome; frailty assessment"
冠状动脉粥样硬化性心脏病简称冠心病,目前已成为全球发病率和病死率最高的疾病,给各个国家医疗卫生带来了严重的经济负担。随着人口老龄化程度加深,衰弱在老年患者中更加常见,并且研究显示,衰弱与冠心病的不良预后密切相关[1]。肠道是人体重要的内分泌及免疫器官,其中肠道菌群及其衍生的代谢产物在维持心血管功能中起重要作用[2]。基因测序技术使肠道菌群与衰弱的相关性更明确[3],衰弱将导致患者肠道菌群失调,从而影响冠心病的发生发展[2]。
1 衰弱的概念及特征
衰弱是一种随着年龄增长,机体逐渐出现的生理储备功能下降,抗应激能力减退,肌肉力量及耐力的衰减等[4]。衰弱除表现为躯体功能的减弱外,还表现在内环境功能的紊乱,进而导致跌倒、残疾、甚至死亡等不良事件。衰弱在老年患者中越来越常见。一项关于60岁以上10万名非衰弱的全球社区老年人衰弱和衰弱前的发生率的荟萃分析表明,在中位随访时间3年后就有13.6%发生衰弱[5]。
2 衰弱的评估
衰弱评估已成为评估冠心病预后的一种重要方法。由于衰弱是多种功能障碍共同导致的老年综合征。因此,对其评估需要全面考量。目前衰弱评估主要包括两个方面[6]:(1)通过体外表现,利用衰弱评估工具评估患者精神状态及躯体功能;(2)通过内在生化指标反映机体内环境的稳态状况,间接反映衰弱。
2.1 衰弱评估工具在冠心病中的应用
衰弱评估工具种类很多,包括Fried衰弱表型 (Fried Frailty Phenotype,FFP)、衰弱筛查量表(the Frail Scale,FRAIL)、临床衰弱评估量表(Clinical Frailty Scale,CFS)、埃德蒙衰弱量表(Edmonton Frail Scale,EFS)及衰弱指数等[7]。不同量表对不同类型冠心病评估效能有差别。
2.1.1 CFS在稳定性冠心病(stable coronary heart disease,SCAD)中的应用
SCAD的主要特征是病情的长期稳定。然而,医护人员须考虑斑块破裂后发生不良事件的可能性。与非衰弱患者相比,衰弱合并SCAD患者的住院率及病死率明显增高[8]。Shimono等[9]应用CFS评估65岁或以上有SCAD并拟行择期经皮冠状动脉介入术(percutaneous coronary intervention,PCI)患者的衰弱情况,观察衰弱与长期临床结局之间相关性。在中位随访时间3年后显示,PCI术前的衰弱与主要不良心血管事件(major adverse cardiovascular events,MACE)独立相关(HR 4.27,95% CI 1.86~9.80,Plt;0.001)。并且,衰弱组主要出血事件的累积发生率高于非衰弱组(P=0.001)。
2.1.2 CFS及衰弱指数在急性冠状动脉综合征(acute coronary syndrome,ACS)中的应用
ACS是冠心病中最严重类型,可导致心律失常、心力衰竭,甚至猝死。然而,急性冠状动脉事件全球登记库(global registry of acute coronary events,GRACE) 和心肌梗死溶栓治疗(thrombolysis in myocardial infarction, TIMI)对老年ACS患者不良心血管事件的预测效能略显不足。因为老龄化使当代人们“生物学”年龄和“实际”年龄不完全匹配。Campo等[10]应用FFP、握力、简易体能状况量表(Short Physical Performance Battery,SPPB)、CFS、哥伦比亚衰弱指数、多维预后指数(multidimensional prognostic index,MPI)和EFS分别评估ACS患者衰弱情况,并观察这些量表和GRACE、TIMI风险评分在预测不良心血管事件和全因死亡率的效能,结果显示,这些量表和GRACE、TIMI风险评分均能预测ACS患者MACE。并且,SPPB可明显改善GRACE和TIMI风险评分效能,提高约15%(Plt;0.001)。此外,Anand等[11]同样表明,CFS能够改善GRACE评分在衰弱合并急性心肌梗死患者12个月死亡风险中的预测效能(AUC 0.86
vs 0.80,P=0.04)。然而,Pavasini等[12]却表明,SPPB、哥伦比亚衰弱指数、EFS及CFS并不能提高衰弱合并ACS患者PCI术后出血风险评分的预测效能。
2.2 常见的生化指标与衰弱的关系
生化指标能够间接反映机体的衰弱状态。Dai等[13]通过分析白细胞介素-6(interleukin-6,IL-6)、白蛋白和25-羟维生素D水平与衰弱合并慢性冠状动脉综合征(chronic coronary syndromes, CCS)患者预后的相关性后表明,IL-6是预后的危险因素,而白蛋白和25-羟维生素D是其保护因素。IL-6可能成为预测和干预衰弱合并CCS患者的潜在靶点。此外,Xu等[14]研究显示,C反应蛋白、D-二聚体和纤维蛋白原是衰弱独立的危险因素,而血红蛋白可能是衰弱的保护因素。
3 衰弱患者的肠道菌群对冠心病的影响
衰弱能够破坏肠道菌群平衡,使肠内有益菌数量大量减少,有害菌数量大量增长,导致机体出现各种心血管疾病[15]。
3.1 肠道菌群的重要性
肠道微生物是机体重要组成部分。人类肠道微生物中,细菌的种类超过15 000种,数量超过10万亿,其中最常见的包含4大细菌门,如厚壁菌门、拟杆菌门、放线菌科及变形菌门[16]。根据他们在人体内的作用,可大致分为有益菌、有害菌和中性菌。各菌群互相依存、相互制约,在调节机体新陈代谢、免疫功能和神经功能等方面起重要作用[17]。
3.2 肠道菌群与衰弱的关系
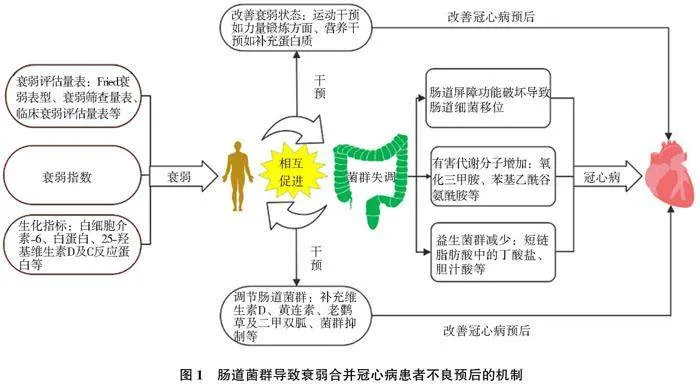
衰弱与肠道菌群关系密切,衰弱将导致菌群失调,引起机体患病。肠道菌群紊乱进一步加重衰弱,两者间相互作用,共同促进疾病的进展。Rashidah等[18]通过分析衰弱老人和健康老人肠道微生物群组成和肠道通透性指标的差异,得出衰弱老人的肠道菌群主要以厚壁菌门减少为主,其中以小杆菌属、乳杆菌属和瘤胃球菌属等有益菌减少最为明显。此外,衰弱组血清连蛋白,促炎细胞因子,如肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)、高迁移率族蛋白B1 (high mobility group box-1 protein HMGB1), IL-6、巨噬细胞炎症蛋白1和氨基酸等增加,导致肠壁通透性升高,菌群移位,从而引起机体患病。
3.3 衰弱患者肠道菌群对冠心病的影响
衰弱患者肠道菌群的改变与冠心病的严重程度密切相关。肠道菌群影响冠心病的途径主要包括[19]:(1)衰弱导致肠道屏障功能紊乱,使有害菌群透过肠道屏障进入体循环,促进冠心病的发生发展;(2)衰弱使肠道菌群失调,导致致病菌群明显增加,其释放的有害代谢分子,诱发动脉粥样硬化;(3)衰弱导致益生菌群减少,造成人体代谢功能紊乱,促使粥样斑块形成。
3.3.1 衰弱导致肠道菌群移位,促进冠心病的发生发展
肠道屏障可以限制肠道细菌从肠道流出,并维持机体健康。然而,衰弱使肠道屏障破裂,导致肠道通透性增加,细菌转移到各脏器,从而诱发心血管疾病。Khan等[20]分析29例心肌梗死患者血液中的微生物群组成,得出与健康对照组相比,心肌梗死组α多样性显著降低。α多样性常用来估算细菌多样性,值越大,说明群落多样性越高,菌群稳定性越好,抵抗外界破坏和环境变迁的能力越强。此外,心肌梗死患者放线菌和双歧杆菌丰富。虽然该研究并未明确说明心肌梗死组是否合并衰弱,但通过对一般资料的分析显示,该组患者的平均年龄为(59.24±12.90)岁,接近老年水平。并且,该研究只采急性心肌梗死之后的血液样本,因此无法区分心肌梗死之前血液微生物组的成分是否对结果有影响。Khan等[21]同样提出,变形菌门和酸杆菌门在ACS发生发展中的重要作用,并且,厚壁菌门、乳杆菌属可能是导致CCS急性加重的重要原因。
3.3.2 致病菌群释放的有害代谢分子诱发动脉粥样硬化
健康人体内致病菌或者机会致病菌数目很少,他们产生的有毒代谢物不足以对机体健康产生危害。但是,衰弱将使有害菌数量增加,诱发动脉粥样硬化。氧化三甲胺(trimetlylamine oxide, TMAO)是有害代谢物中最具代表性的一种。TMAO促进动脉粥样硬化的分子机制主要包括[22]:(1)TMAO可激活血管平滑肌细胞和内皮细胞丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)信号通路、核因子-κB (nuclear factor kappa-B,NF-κB)信号通路、NOD样受体热蛋白结构域相关蛋白3(NOD-like receptor thermal protein domain associated protein 3, NLRP3)及热休克蛋白60(heat shock protein 60,HSP60),导致炎症因子表达增加;(2)TMAO可导致清道夫受体(如CD36、SR-A1等),表达增加,导致巨噬细胞摄取更多氧化修饰的低密度脂蛋白(oxidized low density lipoprotein,ox-LDL)形成泡沫细胞;(3)TMAO通过抑制肝脏中合成胆汁酸所必需的关键酶的表达,从而减少胆汁酸含量,导致逆向胆固醇转运减少;(4)TMAO增加血小板细胞内质网中钙的释放,导致血小板聚集和血栓形成。此外,苯乙酰谷氨酰胺(phenylacetyl glutamine,PAGln ) [23] 、尿毒症毒素[24]等在动脉粥样硬化的发生发展中同样起重要作用。
3.3.3 益生菌群减少,导致机体代谢紊乱,促使斑块形成
益生菌在维持肠道正常屏障功能、减轻炎症反应及调节机体代谢等方面发挥重要作用[25]。其中产短链脂肪酸(short chain fatty acid,SCFA)的细菌在维持心血管健康方面尤为重要。SCFA主要包括丁酸、乙酸、丙酸,其中以丁酸盐最重要。SCFA调节心功能的机制主要包括[26]:(1)诱导肠上皮细胞紧密连接,维持正常的肠道屏障功能;(2)抑制组蛋白脱乙酰基酶3(histone deacetylase 3,HDAC3)/SP1-miR-27a通路,促进ATP结合盒转运体A1的表达,增加胆固醇逆转运;(3)增加膜联蛋白A1的表达,诱导巨噬细胞的胞葬作用。胞葬作用指吞噬细胞(如巨噬细胞、树突细胞等)清除程序性死亡细胞,从而减轻炎症反应的过程;(4)增强过氧化物酶体增殖物激活受体γ,进而降低血脂、血糖和炎症细胞因子水平。此外,胆汁酸在肠肝循环过程中,可通过促进胆固醇代谢,增加胰岛素敏感性及胰岛素分泌等,改善心血管功能[27]。
3.4 干预衰弱及肠道菌群,改善心血管功能
3.4.1 改善衰弱状态,调节心功能
Beigiene·等[28]通过对衰弱合并ACS患者进行为期(18.9±1.7)d的不同运动干预后表明,与传统物理治疗手段相比,机械装置辅助运动的患者更有效地将缓慢步态速度逆转到正常。传统治疗手段包括呼吸训练和使用测力计的有氧训练。Han等[29]同样表明,锻炼-营养干预能有效改善患者衰弱状态,其中运动干预主要表现在力量锻炼方面,营养干预以补充蛋白质为主。
3.4.2 调节肠道菌群,改善心功能
Wang等[30]研究表明,补充维生素D可以调节肠道微生物群,降低小鼠血浆TMAO水平。其机制可能和维生素D调节肠道菌群组成有关,使厚壁菌门显著减少,拟杆菌门增加。小檗碱[31]、老鹳草素[32]及二甲双胍[33]等在调节肠道菌群、改善心功能方面也发挥重要作用。此外,Liang等[34]研究益生菌对小鼠TMAO及相关脂质代谢的影响显示,动物双歧杆菌亚种和乳酸菌可在盲肠内定植,并降解三甲胺及改变肠道菌群结构。该研究为通过菌群移植治疗衰弱合并冠心病提供新的方向。
4 小结与展望
本综述从衰弱与冠心病的关系、衰弱与肠道菌群的关系及肠道菌群与冠心病的关系出发(图1),说明了肠道菌群在衰弱合并冠心病中的重要作用。这提示医护人员,早期评估患者的衰弱状态、肠道菌群的变化,能有效改善冠心病预后,从而达到早干预、早治疗。并且,在以后的临床工作中,更应该注重微生物群-肠-心轴在心血管疾病发生发展中的重要作用。
参考文献:
[1]ILIE A C, TARANU S M, STEFANIU R, et al. Chronic coronary syndrome in frail old population[J]. Life, 2022, 12(8): 1133. DOI: 10.3390/life12081133.
[2]WANG M, WANG Z N, LEE Y J, et al. Dietary meat, trimethylamine N-oxide-related metabolites, and incident cardiovascular disease among older adults: the cardiovascular health study[J]. Arterioscler Thromb Vasc Biol, 2022, 42(9): e273-e288. DOI: 10.1161/ATVBAHA.121.316533.
[3]XU Y S, WANG Y H, LI H W, et al. Altered fecal microbiota composition in older adults with frailty[J]. Front Cell Infect Microbiol, 2021, 11: 696186. DOI: 10.3389/fcimb.2021.696186.
[4]IJAZ N, BUTA B, XUE Q L, et al. Interventions for frailty among older adults with cardiovascular disease: JACC state-of-the-art review[J]. J Am Coll Cardiol, 2022, 79(5): 482-503. DOI: 10.1016/j.jacc.2021.11.029.
[5]OFORI-ASENSO R, CHIN K L, MAZIDI M, et al. Global incidence of frailty and prefrailty among community-dwelling older adults: a systematic review and meta-analysis[J]. JAMA Netw Open, 2019, 2(8): e198398. DOI: 10.1001/jamanetworkopen.2019.8398.
[6]SEPU""" ′LVEDA M, ARAUNA D, GARCA F, et al. Frailty in aging and the search for the optimal biomarker:a review[J]. Biomedicines, 2022, 10(6): 1426. DOI: 10.3390/biomedicines10061426.
[7]GIALLAURIA F, LORENZO A D, VENTURINI E, et al. Frailty in acute and chronic coronary syndrome patients entering cardiac rehabilitation[J]. J Clin Med, 2021, 10(8): 1696. DOI: 10.3390/jcm10081696.
[8]BARBALHO S M, TOFANO R J, CHAGAS E F B, et al. Benchside to the bedside of frailty and cardiovascular aging: main shared cellular and molecular mechanisms[J]. Exp Gerontol, 2021, 148: 111302. DOI: 10.1016/j.exger.2021.111302.
[9]SHIMONO H, TOKUSHIGE A, KANDA D, et al. Association of preoperative clinical frailty and clinical outcomes in elderly patients with stable coronary artery disease after percutaneous coronary intervention[J]. Heart Vessels, 2023, 38(10): 1205-1217. DOI: 10.1007/s00380-023-02276-3.
[10]CAMPO G, MAIETTI E, TONET E, et al. The assessment of scales of frailty and physical performance improves prediction of major adverse cardiac events in older adults with acute coronary syndrome[J]. J Gerontol A Biol Sci Med Sci, 2020, 75(6): 1113-1119. DOI: 10.1093/gerona/glz123.
[11]ANAND A, CUDMORE S, ROBERTSON S, et al. Frailty assessment and risk prediction by GRACE score in older patients with acute myocardial infarction[J]. BMC Geriatr, 2020, 20(1): 102. DOI: 10.1186/s12877-020-1500-9.
[12]PAVASINI R, MAIETTI E, TONET E, et al. Bleeding risk scores and scales of frailty for the prediction of haemorrhagic events in older adults with acute coronary syndrome: insights from the FRASER study[J].Cardiovasc Drugs Ther, 2019, 33(5): 523-532. DOI: 10.1007/s10557-019-06911-y.
[13]DAI J R, LI J, HE X, et al. A relationship among the blood serum levels of interleukin-6, albumin, and 25-hydroxyvitamin D and frailty in elderly patients with chronic coronary syndrome[J]. Aging Med, 2022, 5(1): 17-29. DOI: 10.1002/agm2.12201.
[14]XU L, ZHANG J, SHEN S, et al. Clinical frailty scale and biomarkers for assessing frailty in elder inpatients in China[J]. J Nutr Health Aging, 2021, 25(1): 77-83. DOI: 10.1007/s12603-020-1455-8.
[15]DAMICO F, BARONE M, BRIGIDI P, et al. Gut microbiota in relation to frailty and clinical outcomes[J].Curr Opin Clin Nutr Metab Care, 2023, 26(3): 219-225. DOI: 10.1097/MCO.0000000000000926.
[16]ILLIANO P, BRAMBILLA R, PAROLINI C. The mutual interplay of gut microbiota, diet and human disease[J]. FEBS J, 2020, 287(5): 833-855. DOI: 10.1111/febs.15217.
[17]KOMODROMOU I, ANDREOU E, VLAHOYIANNIS A, et al. Exploring the dynamic relationship between the gut microbiome and body composition across the human lifespan: a systematic review[J]. Nutrients, 2024, 16(5): 660. DOI: 10.3390/nu16050660.
[18]RASHIDAH N H, LIM S M, NEOH C F, et al. Differential gut microbiota and intestinal permeability between frail and healthy older adults: a systematic review[J]. Ageing Res Rev, 2022, 82: 101744. DOI: 10.1016/j.arr.2022.101744.
[19]RAEVSKY K P, POPOV S P, KONYAEV V V, et al. Features of the intestinal microbiota in the elderly in the development of coronary heart disease[J]. Adv Gerontol, 2023, 36(2): 247-250.
[20]KHAN I, KHAN I, KAKAKHEL M A, et al. Comparison of microbial populations in the blood of patients with myocardial infarction and healthy individuals[J]. Front Microbiol, 2022, 13: 845038. DOI: 10.3389/fmicb.2022.845038.
[21]KHAN I, KHAN I, USMAN M, et al. Analysis of the blood bacterial composition of patients with acute coronary syndrome and chronic coronary syndrome[J]. Front Cell Infect Microbiol, 2022, 12: 943808. DOI: 10.3389/fcimb.2022.943808.
[22]SHEN X Y, LI L H, SUN Z, et al. Gut microbiota and atherosclerosis-focusing on the plaque stability[J]. Front Cardiovasc Med, 2021, 8: 668532. DOI: 10.3389/fcvm.2021.668532.
[23]KRISHNAMOORTHY N K, KALYAN M, HEDIYAL T A, et al. Role of the gut bacteria-derived metabolite phenylacetylglutamine in Health and Diseases[J]. ACS Omega, 2024, 9(3): 3164-3172. DOI: 10.1021/acsomega.3c08184.
[24]CUNHA R S D, SANTOS A F, BARRETO F C, et al. How do uremic toxins affect the endothelium? [J]. Toxins (Basel), 2020, 12(6). DOI: 10.3390/toxins12060412.
[25]OMORAIN V L, RAMJI D P. The potential of probiotics in the prevention and treatment of atherosclerosis[J]. Mol Nutr Food Res, 2020, 64(4): e1900797. DOI: 10.1002/mnfr.201900797.
[26]CHEN W J, ZHANG S, WU J F, et al. Butyrate-producing bacteria and the gut-heart axis in atherosclerosis[J]. Clin Chim Acta, 2020, 507: 236-241. DOI: 10.1016/j.cca.2020.04.037.
[27]VOURAKIS M, MAYER G, ROUSSEAU G. The role of gut microbiota on cholesterol metabolism in atherosclerosis[J]. Int J Mol Sci, 2021, 22(15): 8074. DOI: 10.3390/ijms22158074.
[28]BEIGIENE"""""" · A, PETRUEVICˇIENE" · D, BARASAITE" · V, et al. Frailty and different exercise interventions to improve gait speed in older adults after acute coronary syndrome[J]. Medicina, 2021, 57(12): 1344. DOI: 10.3390/medicina57121344.
[29]HAN C Y, MILLER M, YAXLEY A, et al. Effectiveness of combined exercise and nutrition interventions in prefrail or frail older hospitalised patients: a systematic review and meta-analysis[J]. BMJ Open, 2020, 10(12): e040146. DOI: 10.1136/bmjopen-2020-040146.
[30]WANG X, LI X Q, DONG Y M. Vitamin D decreases plasma trimethylamine-N-oxide level in mice by regulating gut microbiota[J].Biomed Res Int, 2020, 2020: 9896743. DOI: 10.1155/2020/9896743.
[31]LI X X, SU C Y, JIANG Z B, et al. Berberine attenuates choline-induced atherosclerosis by inhibiting trimethylamine and trimethylamine-N-oxide production via manipulating the gut microbiome[J]. NPJ Biofilms Microbiomes, 2021, 7(1): 36. DOI: 10.1038/s41522-021-00205-8.
[32]LIN K Y, WANG X D, LI J, et al. Anti-atherosclerotic effects of geraniin through the gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway in mice[J]. Phytomedicine, 2022, 101: 154104. DOI: 10.1016/j.phymed.2022.154104.
[33]ZHANG H X, LAI J, ZHANG L H, et al. The co-regulation of the gut microbiome and host genes might play essential roles in metformin gastrointestinal intolerance[J]. Toxicol Appl Pharmacol, 2023, 481: 116732. DOI: 10.1016/j.taap.2023.116732.
[34]LIANG X, ZHANG Z, LV Y Y, et al. Reduction of intestinal trimethylamine by probiotics ameliorated lipid metabolic disorders associated with atherosclerosis[J]. Nutrition, 2020, 79/80: 110941. DOI: 10.1016/j.nut.2020.110941.
(责任编辑:高艳华)
本文引用:冯荣,鲁佳慧,李赞,等.从肠道菌群的角度分析衰弱在冠心病中的作用[J].医学研究与教育,2024,41(3):1824.DOI:10.3969/j.issn.1674490X.2024.03.003.
基金项目:2020 年度河北省医学科学研究项目(20200203)
第一作者:冯荣 (1995—),男,四川南充人,在读硕士,主要从事冠心病研究。E-mail: 2485585221@qq.com
通信作者:李亚芹 (1972—),女,河北保定人, 副主任医师,硕士,硕士生导师,主要从事冠心病诊断与治疗。E-mail: liyaqin2014@163.com