Tf2O活化苯基次磷酸直接合成双硫代磷酸酯
2024-05-15刘海超徐文博权正军
刘海超 徐文博 权正军
DOI:10.16783/j.cnki.nwnuz.2024.03.003
收稿日期:2024-03-08;修改稿收到日期:2024-04-08
基金项目:国家自然科学基金资助项目(22061038,22067018,21562036)
作者简介:刘海超(1997—),男,甘肃临泽人,硕士研究生.主要研究方向为含磷有机化合物的合成和绿色有机合成.
E-mail:1435510317@qq.com
*通信联系人,男,教授,博士,博士研究生导师.主要研究方向为杂环有机化合物合成、催化有机合成、含磷有机化合物的合成和绿色有机合成.
E-mail:quanzj@nwnu.edu.cn
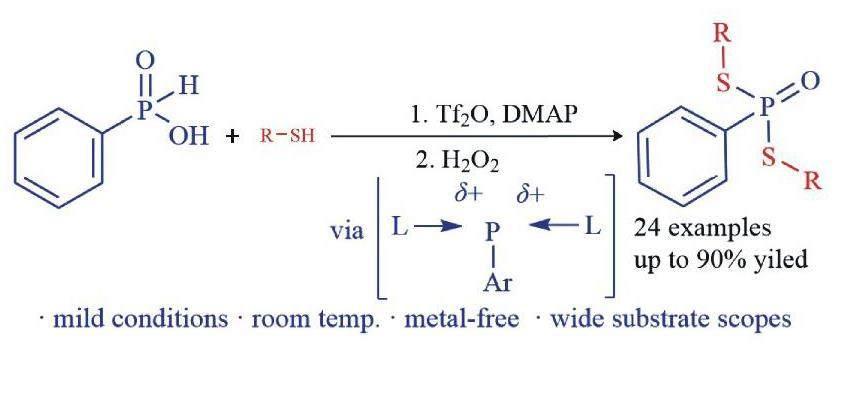
摘要:使用Tf2O為活化剂,DMAP为稳定剂,活化易得的苯基次磷酸产生磷双阳离子等价物,原位生成的阳离子等价物与各种含SH化合物进行偶联反应,合成相应的双硫代磷酸酯.该方法操作步骤简单,无需金属催化剂,底物适用性广泛,为合成磷硫化合物提供了一种新颖且有效的方法.
关键词:苯基次磷酸;磷双阳离子等价物;双硫代磷酸酯;活化
中图分类号:O 627.51 文献标志码:A 文章编号:1001-988Ⅹ(2024)03-0025-09
Direct synthesis of phenylphosphonodithioates via Tf2O activatingphenylphosphinic acid
LIU Hai-chao,XU Wen-bo,QUAN Zheng-jun
(College of Chemistry and Chemical Engineering,Northwest Normal University,Lanzhou 730070,Gansu,China)
Abstract:By using Tf2O as the activator and DMAP as the stabilizer,readily available phenylphosphinic acid can be activated to generate phosphonium dication equivalents.These in situ generated cation equivalents can directly couple with thiol-containing compounds to form the corresponding dithiophosphates.This one-pot method provides a convenient and practical approach to access a variety of dithiophosphates.The reaction system is simple,does not require metal catalysts and has a wide substrate scope.
Key words:phenylphosphinic acid;phosphenium dication equivalents;dithiophosphates;activation
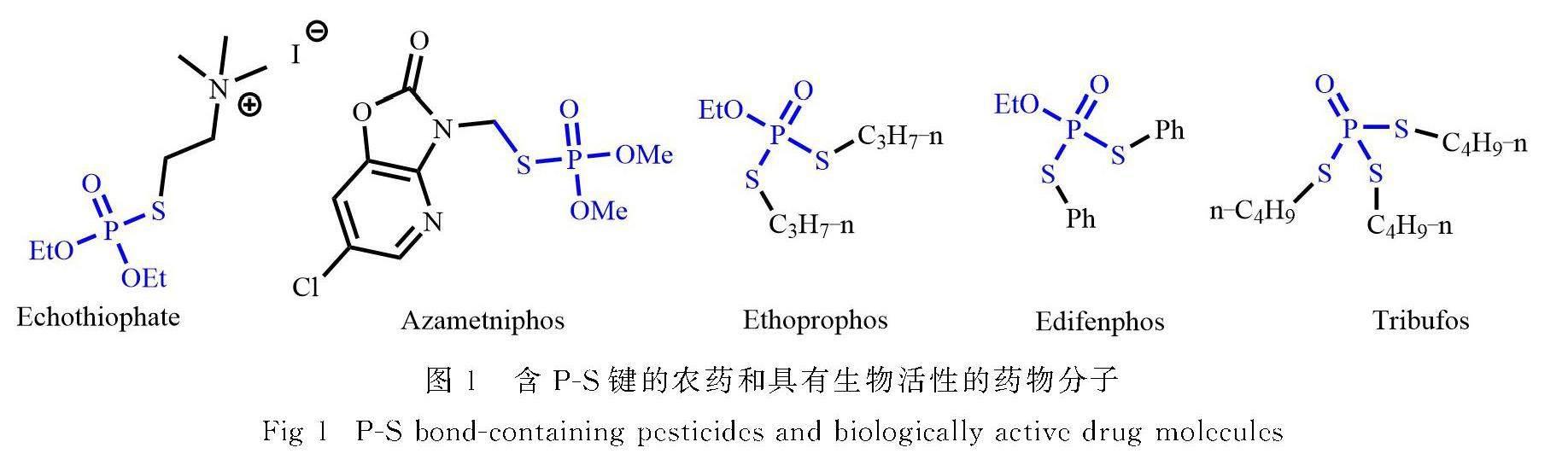
地球上的生命以磷元素(P)为主导,磷是生命的基本元素,磷酸及其衍生物几乎支撑了所有生物功能;同时,有机磷化合物因其独特的生物学和物理特性,在有机合成、催化和药物等领域具有广泛应用[1-6].其中,硫代磷酸酯类化合物由于其同时具有硫原子和磷原子,在有机化学中被广泛用于合成中间体,也在医药和农用化学品等领域得到了广泛应用[7].例如,含有P-S键的分子被用于抗癌剂[8]、抗病毒剂[9]、心脏保护治疗剂[10]和乙酰胆碱酯酶抑制剂[11]等方面.含硫有机磷化合物在广谱放射防护剂、抗癌药物氨磷汀[12]以及抗青光眼药物[13]中也展现治疗潜力(图1).因此,硫代磷酸酯及其衍生物由于其独特的性质在过去几十年中受到了广泛关注.
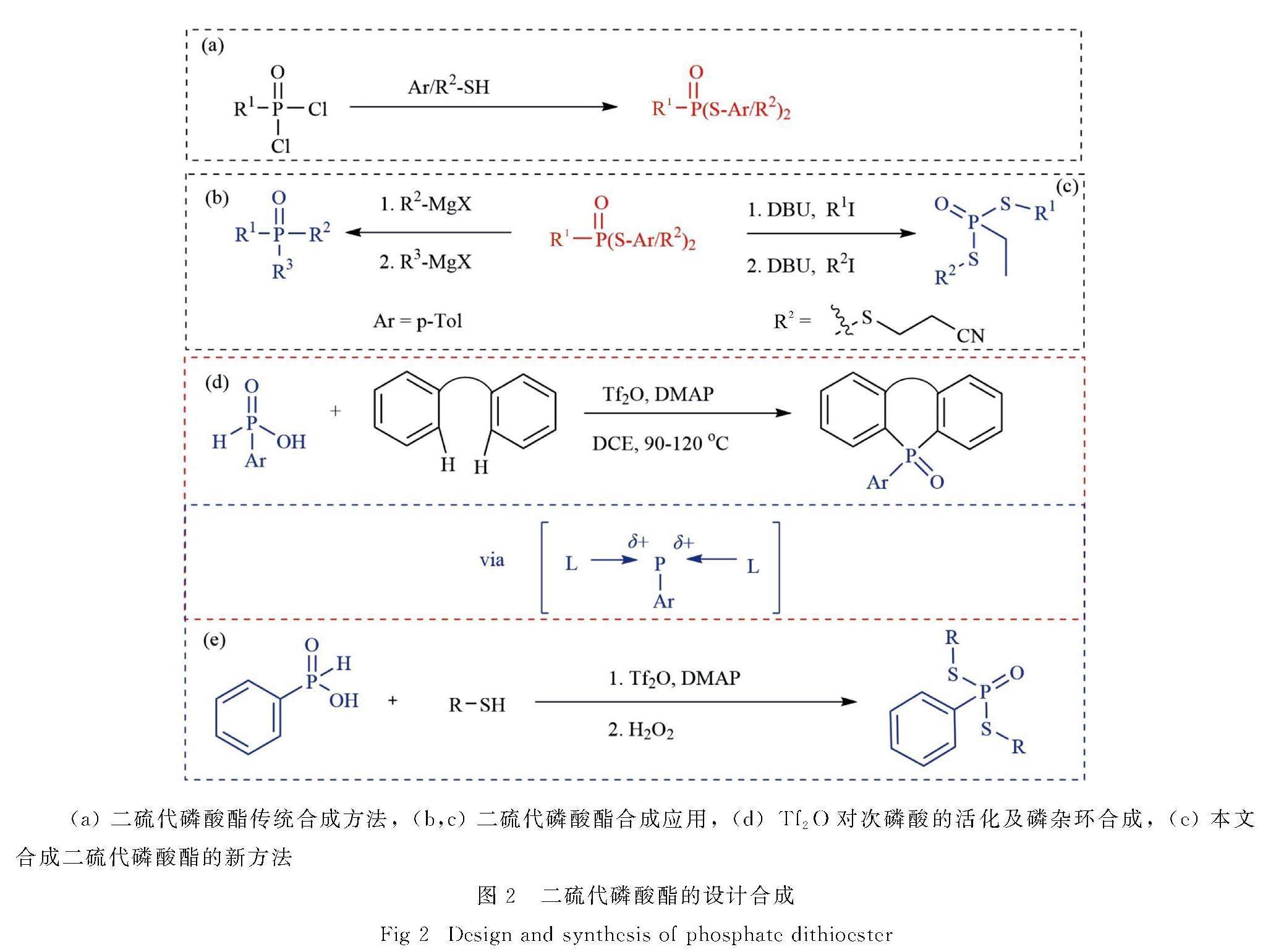
通常,硫代磷酸酯是通过硫醇或硫酚对卤化磷的亲核取代反应而合成[14-16].然而,这类方法通常需要使用有毒的氯、溴以及难闻硫醇或硫酚等试剂.为了克服这些缺点,已经发展出了许多替代方法合成硫代磷酸酯.这些方法可以分为两大类:一种是对磷酰化试剂的改进,利用磷酸二烷基酯、亚磷酸三烷基酯、二苯基磷和白磷为磷源,与含硫试剂反应合成硫代磷酸酯[17-19];另一种是对硫试剂的改进,使用二硫化物、磺酰氯和磺酰肼等多种含硫试剂用作硫源合成硫代磷酸酯[20-22].近期,人们还发展了合成硫代磷酸酯的新方法,即利用硫粉(S8)为硫源,与二芳基碘鎓盐或重氮盐、卤代烃、芳基硼酸等和P-H化合物的三组分反应,合成硫代磷酸酯[23-27].这些方法无需事先制备硫酚或硫醇试剂,减少了操作步骤,并且以高产率获得各种S-芳基硫代磷酸酯.
相对于单硫代磷酸酯的合成,双硫代磷酸酯的合成方法较为单一,目前主要通过二氯氧磷与硫醇的亲核反应来实现(图2a).例如,2017年,OSullivan等[28]报道了一种二氯化磷与氰基乙基硫醇进行取代反应,从而合成S,S-二烷基磷酰二硫代酸酯的方法.他们还采用顺序烷基化-去保护-烷基化策略,成功制备了不对称的二取代二硫代磷酸酯.同年,Hosoya等[29]利用硫醇和苯基磷酰二氯反应合成了双硫代磷酸酯.随后,通过两种格氏试剂与二硫代磷酸酯反应,获得了广泛的不对称有机磷化合物(图2b,c).然而,由于卤试剂的使用会产生大量卤化物废弃物,而且卤化过程具有较高的毒性和环境污染问题,因此采用简便的方法制备双硫代磷酸酯是有机磷合成领域的前沿和热点.
2020年,Miura等[30]报道了一种利用苯基次磷酸替代氯化磷和三氟甲基磺酸酐(Tf2O)反应生成磷双阳离子中间体的方法.随后,该中间体与二芳基化合物发生C-H活化偶联反应,从而制得含磷杂环化合物(图2d).受Miura等工作的启发,文中设计了一种利用廉价易得的苯基次磷酸作为反应底物,替代氯化磷合成双硫代磷酸酯的方法(图2e).通过Tf2O活化苯基次磷酸产生磷双阳离子,同时使用4-二甲氨基吡啶(DMAP)为稳定剂,原位与硫醇、硫酚反应,高效合成了双硫代磷酸酯.这一方法避免了氯化磷的使用,为此类化合物的合成提供了一条新的途径.
1 材料与方法
以合成化合物3的典型实验为例,将苯基次磷酸(1a)(0.2 mmol)、间甲基苯硫酚(2a)(0.4 mmol)和DMAP(0.48 mmol)在氩气保护下加入Shlenk反應管,随后加入甲苯溶剂(2 mL),接着将Tf2O(0.48 mmol)滴加到混合体系中.在室温下搅拌6 h,通过TLC分析监测反应进度.反应结束后,向反应体系中滴加3滴H2O2(30%),室温继续搅拌反应15 min,用水和乙酸乙酯(3×15 mL)进行萃取.合并有机相,通过无水MgSO4干燥,过滤,旋蒸去除溶剂.最后通过硅胶柱色谱法(石油醚∶乙酸乙酯=20∶1~5∶1 )纯化得到相应的产物3a.
2 结果与讨论
选择苯基次磷酸(1a)和间甲苯硫酚(2a)作为模板底物,以确定最佳的反应条件,如表1所示.在在反应结束后,向反应体系中滴加3滴H2O2(30%),室温继续搅拌反应15 min,得到稳定的五价磷目标化合物.文中主要从碱、溶剂、温度和反应时间等方面进行优化.首先,研究了不同的碱对反应的影响(表1,编号1-7).结果表明,在不加碱的条件下,只能获得微量产物(表1,编号1),这证明碱对反应的重要性.进一步分析发现,相对于其他有机碱或无机碱,DMAP表现最佳,产率明显提高,以67%产率得到产物3a.随后,考察了不同溶剂对反应的影响(表1,编号8-11),结果发现甲苯是最佳的反应溶剂,在其他溶剂中都可以发生反应,但产率都会有不同程度的下降.接着,对反应温度进行了考察(表1,编号12-13),发现改变温度对反应影响不大.随后,对反应时间进行了优化(表1,编号13-15),结果显示最佳反应时间为6 h,3a的产率最高,为78%.延长时间会导致目标产物产率下降,主要是生成的中间产物不稳定所致.
2.1 底物适用范围
在最优的反应条件下,对反应的适用性进行了研究(图3).结果表明,无论底物上存在吸电子基团或给电子基团,都能够以较高的产率得到目标产物,最高收率可达90%(3b).值得注意的是,当苯环上间位连有取代基时,目标产物的收率会有所降低(3a,3k,3n,3r),然而,邻位带有取代基的目标产物的产率却不受影响(3c,3l,3o,3q).因此,笔者认为空间位阻对目标产物的产率影响不大,主要是电子效应起作用.含有氟、氯、溴等取代基的底物也能够与苯基次磷酸发生偶联反应,得到中等产率的目标产物.当苯环上存在两个取代基时,目标化合物3d和3e的产率分别为60%和63%.当使用脂肪族的硫醇作为底物时,也能以22%~60%的产率得到目标产物(3u-3x).然而,利用叔丁基硫醇或对硝基苯硫酚时,没有分离到目标产物(3aa,3ab).
2.2 可能的反应机理
参考文献[1],提出了一种可能的反应机理(图4).首先,苯基次磷酸发生互变异构生成相应的二羟基膦,在2倍量的Tf2O作用下脱去2个
羟基,形成相应的中间体(Ⅰ).然后,在路易斯碱
(L)的作用下,脱去2个三氟甲磺酸根离子(TfO-),生成高度亲电、不饱和的P(Ⅲ)双阳离子中间体(Ⅱ).接着,该中间体与2分子的R-SH发生亲核反应,生成不稳定的次磷酸酯Ⅲ,最后经H2O2氧化,形成目标产物3.
2.3 化合物的结构与表征
通过使用Varian Mercury plus-400和Agilent 600 MHz DD2 仪器,以CDCl3为溶剂,Me4Si为内标测定所有化合物结构的核磁共振氢谱、碳谱、磷谱;用 Bruker Daltonics APEX II 47e and Orbitrap Elite 质谱仪测定质谱数据;用XT-4显微熔点仪测定熔点,所用试剂均为分析纯,使用前经重蒸、干燥处理.
S,S-di-m-tolyl phenylphosphonodithioate(3a).White oil.Yield: 78%.1H NMR(400 MHz,CDCl3)δ: 7.82(dd,J=7.3,14.2 Hz,2H),7.46(dd,J=3.7,7.3 Hz,1H),7.37(dd,J=4.4,7.5 Hz,2H),7.26(t,J=3.1 Hz,4H),7.14(d,J=7.6 Hz,1H),2.24(s,6H).13C NMR(101 MHz,CDCl3)δ: 139.35(d,J=2.3 Hz),136.51(d,J=4.2 Hz),134.15,133.07,132.91(d,J=4.4 Hz),131.97,131.86,130.50(d,J=2.8 Hz),129.27(d,J=2.3 Hz),128.66,128.52,126.09(d,J=6.2 Hz),21.49.31P NMR(202 MHz,CDCl3)δ: 49.62.
S,S-di-p-tolyl phenylphosphonodithioate(3b).White oil.Yield: 90%.1H NMR(600 MHz,CDCl3)δ: 7.79(dd,J=6.9,13.9 Hz,2H),7.51~7.45(m,1H),7.38(dd,J=4.4,7.7 Hz,2H),7.35~7.30(m,4H),7.06(d,J=7.9 Hz,4H),2.29(d,J=2.0 Hz,6H).13C NMR(126 MHz,CDCl3)δ: 139.65(d,J=3.1 Hz),135.66(d,J=4.1 Hz),133.46(d,J=10.7 Hz),132.58(d,
J=3.4 Hz),131.72(d,J=10.7 Hz),130.08(d,J=2.3 Hz),128.41,128.29,122.58(d,J=6.0 Hz).31P NMR(162 MHz,CDCl3)δ: 50.87.HRMS(ESI)m/z:Calcd for C20H19OPS2[M+Na]+:393.0501,Found:393.0507.
a Reaction conditions: 1(0.2 mmol), 2(0.4 mmol), DMAP(0.48 mmol) and Tf2O(0.48 mmol), Toluene(2 mL),at 25 ℃ for 6 h.
Then,added H2O2(30%) 3 drops,r.t., 15 min.
b Isolated yield.
S,S-di-o-tolyl phenylphosphonodithioate(3c).White oil.Yield: 85%.1H NMR(600 MHz,CDCl3)δ: 7.85~7.79(m,2H),7.48(dd,J=1.8,7.5 Hz,3H),7.39(dd,J=4.5,7.6 Hz,2H),7.21(dd,J=1.6,7.4 Hz,2H),7.17(d,J=7.5 Hz,2H),7.07(dd,J=2.0,7.5 Hz,2H),2.34(s,6H).13C NMR(126 MHz,CDCl3)δ: 143.17(d,J=4.2 Hz),137.21(d,J=4.0 Hz),134.74,133.88,132.61(d,J=3.3 Hz),131.47(d,J=10.9 Hz),130.86(d,J=2.4 Hz),129.77(d,J=3.0 Hz),128.44,128.33,126.57(d,J=2.4 Hz),125.87(d,J=6.3 Hz),21.53.31P NMR(202 MHz,CDCl3)δ: 49.02.
S,S-bis(3,5-dimethylphenyl)phenylphosphonodithioate(3d).White oil.Yield: 60%.1H NMR(600 MHz,CDCl3)δ: 7.81(dd,J=8.2,13.9 Hz,2H),7.48(dd,J=1.7,7.3 Hz,1H),7.39(dd,J=4.4,7.6 Hz,2H),7.35(dd,J=2.0,8.0 Hz,2H),6.99(d,J=2.2 Hz,2H),6.88(dd,J=2.1,8.0 Hz,2H),2.29(s,6H),2.26(d,J=2.1 Hz,6H).13C NMR(126 MHz,CDCl3)δ: 142.90(d,J=4.1 Hz),139.89(d,J=3.2 Hz),137.11(d,J=3.8 Hz),134.96,134.11,133.03~130.72(m),128.28(d,J=14.1 Hz),127.40(d,J=2.5 Hz),122.27(d,J=6.4 Hz),21.28(d,J=32.1 Hz).31P NMR(202 MHz,CDCl3)δ: 49.02.
S,S-bis(2,4-dimethylphenyl)phenylphosphonodithioate(3e).White oil.Yield: 63%.1H NMR(600 MHz,CDCl3)δ: 7.81(dd,J=8.2,13.9 Hz,2H),7.48(dd,J=1.7,7.3 Hz,1H),7.39(dd,J=4.4,7.6 Hz,2H),7.35(dd,J=2.0,8.0 Hz,2H),6.99(d,J=2.2 Hz,2H),6.88(dd,J=2.1,8.0 Hz,2H),2.29(s,6H),2.26(d,J=2.1 Hz,6H).13C NMR(126 MHz,CDCl3)δ: 142.91(d,J=4.1 Hz),139.90(d,J=3.0 Hz),137.12(d,J=3.9 Hz),134.96,134.11,132.45(d,J=3.4 Hz),132.13~130.91(m),128.30(d,J=14.0 Hz),127.41(d,J=2.6 Hz),122.27(d,J=6.3 Hz),21.30(d,J=32.3 Hz).31P NMR(202 MHz,CDCl3)δ: 49.04.
S,S-bis(4-methoxyphenyl)phenylphosphonodithioate(3f).White oil.Yield: 58%.1H NMR(500 MHz,CDCl3)δ: 7.81~7.73(m,2H),7.49(dd,J=1.8,7.4 Hz,1H),7.37(dd,J=3.2,8.2 Hz,6H),6.81~6.75(m,4H),3.76(s,6H).13C NMR(126 MHz,CDCl3)δ: 160.76(d,J=2.7 Hz),137.34(d,J=3.8 Hz),136.08~130.28(m),128.35(d,J=14.2 Hz),116.36(d,J=6.2 Hz),114.89(d,J=2.3 Hz),55.33.31P NMR(202 MHz,CDCl3)δ: 50.03.
S,S-bis(4-ethylphenyl)phenylphosphonodithioate(3g).White oil.Yield: 60%.1H NMR(400 MHz,CDCl3)δ: 7.85~7.74(m,2H),7.49(dd,J=4.6,7.1 Hz,1H),7.43~7.35(m,4H),7.26(dd,J=3.1,5.9 Hz,1H),7.22~7.05(m,4H),2.83~2.45(m,4H),1.33~1.00(m,6H).13C NMR(126 MHz,CDCl3)δ: 148.66(d,J=4.1 Hz),145.84(d,J=3.0 Hz),137.22(d,J=3.8 Hz),135.84~135.67(m),132.80~130.86(m),130.38~127.75(m),126.54(d,J=2.3 Hz),122.81(d,J=6.3 Hz),28.05(d,J=128.7 Hz),17.63~11.75(m).31P NMR(202 MHz,CDCl3)δ: 49.86.
S,S-bis(4-isopropylphenyl)phenylphosphonodithioate(3h).White oil.Yield: 77%.1H NMR(600 MHz,CDCl3)δ: 7.78(dd,J=7.0,14.0 Hz,2H),7.50~7.43(m,1H),7.41~7.33(m,6H),7.16~7.09(m,4H),2.84(q,J=7.0 Hz,2H),1.19(dd,J=1.0,6.9 Hz,12H).13C NMR(126 MHz,CDCl3)δ: 150.40(d,J=3.1 Hz),135.75(d,J=4.1 Hz),133.96,133.11,132.56(d,J=3.4 Hz),131.61,128.32(d,J=14.3 Hz),127.50(d,J=2.3 Hz),122.93(d,J=6.2 Hz),33.86,23.81.31P NMR(202 MHz,CDCl3)δ: 50.05.
S,S-bis(4-chlorophenyl)phenylphosphonodithioate(3i).White oil.Yield: 45%.1H NMR(400 MHz,CDCl3)δ: 8.07(dd,J=8.5,17.4 Hz,2H),7.81(t,J=8.3 Hz,1H),7.72(q,J=7.9 Hz,2H),7.69~7.60(m,4H),7.56~7.50(m,4H).13C NMR(101 MHz,CDCl3)δ: 137.33(d,J=4.1 Hz),136.68(d,J=3.4 Hz),133.72~133.50(m),132.07(d,J=11.1 Hz),130.04(d,J=2.3 Hz),129.17(d,J=14.4 Hz),124.90(d,J=6.2 Hz).31P NMR(202 MHz,CDCl3)δ: 48.51.
S,S-bis(4-bromophenyl)phenylphosphonodithioate(3j).White oil.Yield: 41%.1H NMR(500 MHz,CDCl3)δ: 7.80(dd,J=7.0,14.2 Hz,1H),7.54(dd,J=5.3,7.4 Hz,0H),7.44(dd,J=4.5,7.6 Hz,1H),7.40~7.34(m,2H),7.27~7.22(m,2H).13C NMR(126 MHz,CDCl3)δ: 136.84(d,J=4.2 Hz),136.20(d,J=3.4 Hz),133.13(t,J=3.1 Hz),131.63,131.55,129.56(d,J=2.3 Hz),128.67(d,J=14.6 Hz),124.43(d,J=6.2 Hz).31P NMR(202 MHz,CDCl3)δ: 48.92.
S,S-bis(3-chlorophenyl)phenylphosphonodithioate(3k).White oil.Yield: 54%.1H NMR(500 MHz,CDCl3)δ: 7.84~7.77(m,2H),7.55(dd,J=1.9,7.3 Hz,1H),7.49~7.40(m,4H),7.37(q,J=7.8 Hz,2H),7.31(q,J=8.3 Hz,2H),7.21(t,J=7.9 Hz,2H).13C NMR(126 MHz,CDCl3)δ: 135.13(d,J=4.2 Hz),134.77(d,J=2.5 Hz),133.72(d,J=4.2 Hz),133.25(d,J=3.5 Hz),133.10,132.24,131.58,131.49,130.23(d,J=2.3 Hz),129.83(d,J=2.7 Hz),128.71(d,J=14.6 Hz),127.78(d,J=6.0 Hz).31P NMR(202 MHz,CDCl3)δ: 48.96.
S,S-bis(2-chlorophenyl)phenylphosphonodithioate(3l).White oil.Yield: 34%.1H NMR(500 MHz,CDCl3)δ: 7.93~7.85(m,2H),7.72(dd,J=1.9,7.8 Hz,2H),7.52(dd,J=1.8,7.3 Hz,1H),7.42(dd,J=4.7,7.5 Hz,2H),7.37(dd,J=1.5,7.9 Hz,2H),7.25(dd,J=1.7,7.8 Hz,2H),7.19(dd,J=1.5,7.6 Hz,2H).13C NMR(126 MHz,CDCl3)δ: 138.77(d,J=5.1 Hz),137.78(d,J=4.1 Hz),133.69,133.11(d,J=3.5 Hz),132.82,131.70,131.60,130.72(d,J=2.7 Hz),130.27(d,J=2.2 Hz),128.55,128.44,127.33(d,J=2.2 Hz),126.15(d,J=5.9 Hz).31P NMR(202 MHz,CDCl3)δ: 49.55.
S,S-bis(4-fluorophenyl)phenylphosphonodithioate(3m).White oil.Yield: 56%.1H NMR(500 MHz,CDCl3)δ: 7.78(dd,J=7.5,14.1 Hz,2H),7.52(dd,J=2.0,7.4 Hz,1H),7.42(dd,J=6.4,7.3 Hz,6H),6.96(t,J=8.6 Hz,4H).13C NMR(126 MHz,CDCl3)δ: 164.71(d,J=3.1 Hz),162.72(d,J=3.1 Hz),137.79(d,J=4.0 Hz),133.23,132.97(d,J=3.4 Hz),132.38,131.64,128.53,121.13(d,J=3.4 Hz).19F NMR(376 MHz,CDCl3)δ: -111.16(dd,J=4.8,8.8 Hz).31P NMR(202 MHz,CDCl3)δ: 49.40.
S,S-bis(3-fluorophenyl)phenylphosphonodithioate(3n).White oil.Yield: 33%.1H NMR(400 MHz,CDCl3)δ: 7.81(dd,J=7.5,14.4 Hz,2H),7.57~7.49(m,1H),7.44(dd,J=4.5,7.5 Hz,2H),7.24(dd,J=4.3,7.1 Hz,4H),7.22~7.15(m,2H),7.03(dd,J=2.6,7.3 Hz,2H).13C NMR(101 MHz,CDCl3)δ: 163.83,161.34,133.84~131.29(m),130.69(dd,J=2.3,8.2 Hz),128.92(d,J=14.5 Hz),128.00(d,J=8.1 Hz),122.62(dd,J=4.3,22.7 Hz),117.04(dd,J=2.7,21.1 Hz).19F NMR(376 MHz,CDCl3)δ: -100.52~-118.72(m).31P NMR(202 MHz,CDCl3)δ: 48.86.
S,S-bis(2-fluorophenyl)phenylphosphonodithioate(3o).White oil.Yield: 36%.1H NMR(400 MHz,CDCl3)δ: 7.86(dd,J=7.6,14.4 Hz,2H),7.58(t,J=7.3 Hz,2H),7.54~7.46(m,1H),
7.42(t,J=6.4 Hz,2H),7.33(q,J=7.3 Hz,2H),7.12~6.98(m,4H).
13C NMR(101 MHz,CDCl3)δ: 164.30(d,J=4.7 Hz),161.81(d,J=4.6 Hz),138.33(d,J=3.9 Hz),133.83,133.32(d,J=3.4 Hz),132.75,132.19(dd,J=2.8,8.0 Hz),131.82,131.71,128.77,128.62,125.03(dd,J=2.3,3.9 Hz),116.47(dd,J=2.4,22.7 Hz),114.13~112.99(m).19F NMR(376 MHz,CDCl3)δ: -105.72(d,J=7.6 Hz).31P NMR(202 MHz,CDCl3)δ: 49.54.
S,S-di(naphthalen-1-yl)phenylphosphonodithioate(3p).White oil.Yield: 66%.1H NMR(500 MHz,CDCl3)δ: 8.28~8.22(m,2H),7.82(d,J=8.3 Hz,2H),7.80~7.72(m,5H),7.47~7.42(m,3H),7.35(t,J=7.7 Hz,2H),7.28~7.22(m,2H).13C NMR(126 MHz,CDCl3)δ: 136.13(d,J=5.3 Hz),135.10(d,J=3.0 Hz),133.88,133.03,132.54(d,J=3.5 Hz),131.57,131.49,130.52(d,J=3.2 Hz),128.25,128.14,126.96,126.33,126.11,125.58(d,J=3.0 Hz),123.83(d,J=6.7 Hz).31P NMR(202 MHz,CDCl3)δ: 49.51.
S,S-bis(2-bromophenyl)phenylphosphonodithioate(3q).White oil.Yield: 43%.1H NMR(400 MHz,CDCl3)δ: 7.91(dd,J=7.6,14.6 Hz,2H),7.76(d,J=7.8 Hz,2H),7.55(dd,J=7.7,15.5 Hz,3H),7.44(dd,J=4.7,7.6 Hz,2H),7.25(t,J=7.4 Hz,2H),7.17(t,J=7.7 Hz,2H).13C NMR(101 MHz,CDCl3)δ: 137.81(d,J=4.1 Hz),133.85(d,J=1.9 Hz),133.38(d,J=3.5 Hz),131.95(d,J=11.4 Hz),130.95,129.73(d,J=5.6 Hz),128.84,128.65(d,J=8.4 Hz),128.21.31P NMR(162 MHz,CDCl3)δ: 50.73.
S,S-bis(3-bromophenyl)phenylphosphonodithioate(3r).White oil.Yield: 45%.1H NMR(400 MHz,CDCl3)δ: 7.81(dd,J=7.6,14.3 Hz,2H),7.57(s,3H),7.45(dd,J=7.8,16.2 Hz,6H),7.16(t,J=7.9 Hz,2H).13C NMR(101 MHz,CDCl3)δ: 138.12(d,J=4.4 Hz),134.41(d,J=4.3 Hz),133.51(d,J=3.4 Hz),133.35,132.94(d,J=2.9 Hz),132.26,131.91,131.80,131.69,130.76(d,J=2.2 Hz),130.60,129.02,128.89(d,J=2.0 Hz),128.21(d,J=6.1 Hz),127.97,122.95(d,J=2.7 Hz).31P NMR(162 MHz,CDCl3)δ: 50.24.
S,S-bis(4-(tert-butyl)phenyl)phenylphosphonodithioate(3s).White oil.Yield: 61%.1H NMR(400 MHz,CDCl3)δ: 7.80(dd,J=7.1,13.8 Hz,2H),7.49(dd,J=5.9,7.7 Hz,1H),7.38(dd,J=2.3,8.7 Hz,6H),7.31~7.26(m,4H),1.28(s,18H).13C NMR(101 MHz,CDCl3)δ: 152.88(d,J=3.0 Hz),135.66(d,J=4.1 Hz),133.23,132.79(d,J=3.4 Hz),131.87(d,J=10.8 Hz),128.62,128.48,126.65(d,J=2.4 Hz),126.39,122.93(d,J=6.2 Hz),34.93,31.43.31P NMR(162 MHz,CDCl3)δ: 51.20.
S,S-diphenyl phenylphosphonodithioate(3t).
White oil.Yield: 58%.1H NMR(400 MHz,CDCl3)δ: 7.79(dd,J=7.6,14.1 Hz,3H),7.46(d,J=7.5 Hz,6H),7.25(t,J=7.4 Hz,6H).13C NMR(101 MHz,CDCl3)δ: 135.94(d,J=4.3 Hz),132.97(d,J=3.3 Hz),131.94,129.64(d,J=2.8 Hz),128.59,126.44(d,J=6.1 Hz).31P NMR(162 MHz,CDCl3)δ: 51.35.
S,S-dicyclohexyl phenylphosphonodithioate(3u).
White oil.Yield: 67%.1H NMR(600 MHz,CDCl3)δ: 7.94~7.87(m,2H),7.54~7.42(m,3H),3.44~3.34(m,2H),2.12~2.06(m,2H),1.87(dd,J=7.3,12.6 Hz,2H),1.74~1.67(m,2H),1.53(dd,J=4.0,12.9 Hz,4H),1.47~1.16(m,8H).13C NMR(126 MHz,CDCl3)δ: 136.94,136.07,132.26(d,J=3.3 Hz),131.10,131.01,128.53,128.42,46.17(d,J=2.9 Hz),35.43(dd,J=4.4,13.8 Hz),25.84(d,J=17.0 Hz),25.31.31P NMR(202 MHz,CDCl3)δ: 51.59.
S,S-dicyclopentyl phenylphosphonodithioate(3v).
White oil.Yield: 22%.1H NMR(400 MHz,CDCl3)δ: 7.92(dd,J=7.4,14.1 Hz,2H),7.54~7.46(m,3H),3.57(dd,J=6.5,10.3 Hz,2H),2.13(q,J=8.4 Hz,2H),1.93(q,J=7.3 Hz,2H),1.68(d,J=15.5 Hz,6H),1.53(dd,J=6.8,14.0 Hz,6H).13C NMR(101 MHz,CDCl3)δ: 136.69,133.66~130.34(m),128.75(d,J=14.1 Hz),45.39(d,J=3.1 Hz),35.60(t,J=5.2 Hz),24.50(d,J=10.3 Hz).31P NMR(162 MHz,CDCl3)δ: 52.47.
S,S-dibenzyl phenylphosphonodithioate(3w).
White oil.Yield: 50%.1H NMR(600 MHz,CDCl3)δ: 7.86(dd,J=7.0,14.4 Hz,2H),7.54~7.48(m,1H),7.42(dd,J=6.9,8.8 Hz,2H),7.30~7.17(m,10H),4.20~4.09(m,4H).13C NMR(151 MHz,CDCl3)δ: 136.55(d,J=5.7 Hz),134.80,134.07,132.67(d,J=3.3 Hz),131.10,131.03,129.10,128.63,128.55,127.58,77.96~74.41(m),35.16(d,J=2.9 Hz).31P NMR(202 MHz,CDCl3)δ: 53.24.
S,S-diphenethyl phenylphosphonodithioate(3x).
White oil.Yield: 23%.1H NMR(400 MHz,CDCl3)δ: 7.97~7.87(m,2H),7.56(dd,J=3.8,7.4 Hz,1H),7.50(dd,J=4.2,7.5 Hz,2H),7.28(t,J=7.3 Hz,4H),7.22(d,J=7.0 Hz,2H),7.18~7.11(m,4H),3.16(dd,J=7.7,13.0 Hz,4H),2.97(t,J=7.7 Hz,4H).13C NMR(101 MHz,CDCl3)δ: 139.57,132.98,131.40,129.06,126.91,37.29(d,J=4.4 Hz),32.55.31P NMR(162 MHz,CDCl3)δ: 55.73.
3 結束语
简而言之,利用简单易得的硫醇或者硫酚作为硫源,苯基次磷酸作为磷源,在三氟甲磺酸酐与N,N-二甲氨基吡啶的作用下,在温和条件下通过磷双阳离子与SH化合物的偶联反应实现了双硫代磷酸酯的合成.该反应提供了一种简便的合成双硫代磷酸酯的途径,具有条件温和、操作简便和范围广泛等特点.
参考文献:
[1] DEMKOWICZ S,RACHON J,DAS′KO M,et al.Selected organophosphorus compounds with biological activity:Applications in medicine[J].RSC Adv,2016,6(9):7101.
[2] LI W,ZHANG J.Recent developments in the synthesis and utilization of chiral β-aminophosphine derivatives as catalysts or ligands[J].Chem Soc Rev,2016,45(6):1657.
[3] XU J,LIU R Y,YEUNG C S,et al.Monophosphine ligands promote Pd-catalyzed C-S cross-coupling reactions at room temperature with soluble bases[J].ACS Catal,2019,9(7):6461.
[4] MANGHI M C,MASIOL M,CALZAVARA R,et al.The use of phosphonates in agriculture.chemical,biological properties and legislative issues[J].Chemosphere,2021:283131187.
[5] GUTERMAN R,RABIEE K A,GILROY J B,et al.Polymer network formation using the phosphane-ene reaction:A thiol-ene analogue with diverse postpolymerization chemistry[J].Chem Mater,2015,27(4):1412.
[6] WU K,LI Q,SU W,et al.Experimental and theoretical study of phosphine-catalyzed reaction modes in the reaction of α-substituted allenes with aryl Imines[J].Angew Chem Int Ed,2023,62(51):e202314191.
[7] ZHANG A,SUN J,LIN C,et al.Enantioselective interaction of acid α-naphthyl acetate esterase with chiral organophosphorus insecticides[J].J Agric Food Chem,2014,62(7):1477.
[8] DURGAM G G,VIRAG T,WALKER M D,et al.Synthesis,structure-activity relationships,and biological evaluation of fatty alcohol phosphates as lysophosphatidic acid receptor ligands,activators of PPARγ,and inhibitors of autotaxin[J].J Med Chem,2005,48(15):4919.
[9] ROUX L,PRIET S,PAYROT N,et al.Ester prodrugs of acyclic nucleoside thiophosphonates compared to phosphonates:Synthesis,antiviral activity and decomposition study[J].Eur J Med Chem,2013:63869.
[10] KUMAR T S,YANG T,MISHRA S,et al.5′-Phosphate and 5′-phosphonate ester derivatives of (N)-methanocarba adenosine with in vivo cardioprotective activity[J].J Med Chem,2013,56(3):902.
[11] KABOUDIN B,EMADI S,HADIZADEH A.Synthesis of novel phosphorothioates and phosphorodithioates and their differential inhibition of cholinesterases[J].Bioorg Chem,2009,37(4):101.
[12] KOUKOURAKIS M.Amifostine in clinical oncology:Current use and future applications[J].Anti-Cancer Drugs,2002,13(3):181.
[13] PRATT-JOHNSON J A,DRANCE S M,INNES R.Comparison between pilocarpine and echothiophate for chronic simple glaucoma:A comparison between 4% pilocarpine and 0.06% echothiophate iodide in the diurnal management of chronic simple glaucoma[J].Arch Ophthalmol,1964,72(4):485.
[14] TIMPERLEY C M,SAUNDERS S A,SZPALEK J,et al.Fluorinated phosphorus compounds:Part 8.The reactions of bis(fluoroalkyl) phosphorochloridates with sulfur nucleophiles[J].J Fluorine Chem,2003,119(2):161.
[15] LIU Y C,LEE C F.N-Chlorosuccinimide-promoted synthesis of thiophosphates from thiols and phosphonates under mild conditions[J].Green Chem,2014,16(1):357.
[16] PANMAND D S,TIWARI A D,PANDA S S,et al.New benzotriazole-based reagents for the phosphonylation of various N-,O-,and S-nucleophiles[J].Tetrahedron Lett,2014,55(43):5898.
[17] LU G,CHEN J,HUANGFU X,et al.Visible-light-mediated direct synthesis of phosphorotrithioates as potent anti-inflammatory agents from white phosphorus[J].Org Chem Front,2019,6(2):190.
[18] HUANGFU X,WANG Y,LU G,et al.Direct synthesis of phosphorotrithioites and phosphorotrithioates from white phosphorus and thiols[J].Green Chem,2020,22(16):5303.
[19] ZHANG Y,CAO Y,CHI Y,et al.Formation of N-P(O)-S bonds from white phosphorus via a four-component reaction[J].Adv Synth Catal,2022,364(13):2221.
[20] LIU T,ZHANG Y,YU R,et al.An alternative metal-free aerobic oxidative cross-dehydrogenative coupling of sulfonyl hydrazides with secondary phosphine oxides[J].Synthesis,2020,52(2):253.
[21] ZHANG P,LI W,ZHU X,et al.Photoredox and copper-catalyzed sulfonylphosphorothiolation of alkenes toward β-sulfonyl phosphorothioates[J].Adv Synth Catal,2022,364(18):3316.
[22] CHEN Z W,PRATHEEPKUMAR A,BAI R,et al.Cesium carbonate-catalyzed synthesis of phosphorothioates via S-phosphination of thioketones[J].Chem Commun,2022,58(78):11001.
[23] LIU X,JIANG W,HUANG C,et al.Electrochemical phosphorothiolation and 1,4-S→C phospho-Fries rearrangement:Controlled access to phosphorothiolated and mercapto-phosphono substituted indolizines[J].Org Chem Front,2023,10(20):5198.
[24] SHI S,CHEN J,ZHUO S,et al.Iodide-catalyzed phosphorothiolation of heteroarenes using P(O)H compounds and elemental sulfur[J].Adv Synth Catal,2019,361(13):3210.
[25] XU J,ZHANG L,LI X,et al.Phosphorothiolation of aryl boronic acids using P(O)H compounds and elemental sulfur[J].Org Lett,2016,18(6):1266.
[26] SHI S,ZHANG P,LUO C,et al.Copper-catalyzed remote C(sp3)-H phosphorothiolation of sulfonamides and carboxamides in a multicomponent reaction[J].Org Lett,2020,22(5):1760.
[27] ZHANG P,YU G,LI W,et al.Copper-catalyzed multicomponent trifluoromethylphosphorothiolation of alkenes:access to CF3-containing alkyl phosphorothioates[J].Org Lett,2021,23(15):5848.
[28] JONES D J,OLEARY E M,OSULLIVAN T P Synthesis of symmetrically and unsymmetrically substituted S,S-dialkyl phosphonodithioates[J].Tetrahedron Lett,2017,58(44):4212.
[29] NISHIYAMA Y,HAZAMA Y,YOSHIDA S,et al.Synthesis of unsymmetrical tertiary phosphine oxides via sequential substitution reaction of phosphonic acid dithioesters with grignard reagents[J].Org Lett,2017,19(14):3899.
[30] NISHIMURA K,HIRANO K,MIURA M.Direct synthesis of dibenzophospholes from biaryls by double C—P bond formation via phosphenium dication equivalents[J].Org Lett,2020,22(8):3185.
(責任编辑 陆泉芳)
