Inflammation as a cause of acute myocardial infarction in patients with myeloproliferative neoplasm
2024-05-08AmedeoTirandiElisaSchiavettaEliaMaioliFabrizioMontecuccoLucaLiberale
Amedeo Tirandi,Elisa Schiavetta,Elia Maioli,Fabrizio Montecucco,Luca Liberale
Abstract Myeloproliferative neoplasms (MPN) are a group of diseases characterized by the clonal proliferation of hematopoietic progenitor or stem cells.They are clinically classifiable into four main diseases: chronic myeloid leukemia,essential thrombocythemia,polycythemia vera,and primary myelofibrosis.These pathologies are closely related to cardio-and cerebrovascular diseases due to the increased risk of arterial thrombosis,the most common underlying cause of acute myocardial infarction.Recent evidence shows that the classical Virchow triad (hypercoagulability,blood stasis,endothelial injury) might offer an explanation for such association.Indeed,patients with MPN might have a higher number and more reactive circulating platelets and leukocytes,a tendency toward blood stasis because of a high number of circulating red blood cells,endothelial injury or overactivation as a consequence of sustained inflammation caused by the neoplastic clonal cell.These abnormal cancer cells,especially when associated with the JAK2V617F mutation,tend to proliferate and secrete several inflammatory cytokines.This sustains a pro-inflammatory state throughout the body.The direct consequence is the induction of a pro-thrombotic state that acts as a determinant in favoring both venous and arterial thrombus formation.Clinically,MPN patients need to be carefully evaluated to be treated not only with cytoreductive treatments but also with cardiovascular protective strategies.
Key Words: Inflammation;Myeloproliferative neoplasm;Acute coronary syndrome;Myocardial infarction;Thrombosis;Cancer
INTRODUCTION
Myeloproliferative neoplasms (MPNs) are a group of diseases characterized by the clonal proliferation of hematopoietic progenitor or stem cells.MPNs are subdivided into four main diseases: Chronic myeloid leukemia,essential thrombocythemia (ET),polycythemia vera (PV),and primary myelofibrosis.Thrombosis is one of the most common complications of MPNs,which can occur in both arterial and venous vessels.As such,patients with MPN are at high risk of cardio-and cerebrovascular diseases such as myocardial infarction,deep venous thrombosis,and stroke[1,2].Epidemiologically,up to 75% of patients with MPNs experience a major adverse cardiovascular event (MACE) as a complication of their clinical condition,and about a third after a first acute coronary syndrome (ACS) have another MACE[3].Of interest,ACS might precede the development of a clinically overt MPNs[4].Such higher cardiovascular risk is probably related to the hyperviscosity and thrombocytosis that are found in these neoplastic conditions.The main key elements that contribute to this pro-thrombotic state are the augmented number of circulating platelets and their hyperactivation,the marked leukocytosis,the Janus kinase 2 (JAK2) mutation,and the inflammatory state that especially concern the endothelium.In addition,the concomitant presence of classic cardiovascular risk factors (such as smoking,dyslipidemia,hypertension,etc.) further contributes to the higher risk of possible cardiovascular acute diseases in these patients.In this editorial,we comment on a recent article by Mananet al[5] published in theWorld Journal of Cardiologyentitled “Acute myocardial infarction in myeloproliferative neoplasms”.We provide the key insights of the paper,re-discussing the main topics focusing on the major mechanism underlying the relation of MPNs and ACS.
HOW INFLAMMATION IN MYELOPROLIFERATIVE NEOPLASMS CAN PREDISPOSE TO ACUTE CORONARY SYNDROMES
Inflammation plays a central role in the pathogenesis of cardiac diseases,particularly in the development of atherosclerotic disease[6].In cases of MPN,the whole body undergoes a persistent inflammatory state,and patients typically suffer from inflammation-mediated symptoms such as fever,night sweats,weight loss,and fatigue[2].Accordingly,in patients with ET and PV,the presence of high levels of C-reactive protein is associated with a higher risk of thrombosis[7].Although more information is available concerning the role of inflammation in causing thrombosis,the underlying mechanisms through which MPNs contribute to the development of ACS are not completely understood.The concept is that thrombosis can affect both the arterial and venous vessels in MPN patients,and ACS are mainly caused by an arterial thrombosis of the coronary vessels[8].The basic principle for the development of a thrombus remains the notorious Virchow triad (hypercoagulability,blood stasis,endothelial injury)[9] (Figure 1).
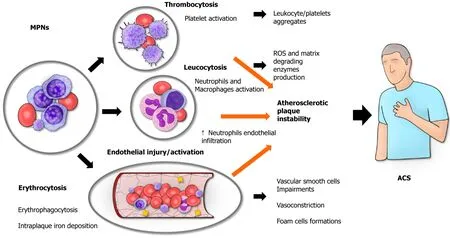
Figure 1 Myeloproliferative diseases in predisposing to acute coronary syndrome: The Virchow triad. Myeloproliferative neoplasms (MPNs) are a group of diseases characterized by the clonal proliferation of hematopoietic progenitor or stem cells.Patients with MPN are at high risk of cardiovascular events,especially those sustained by arterial thrombosis.Different causal links have been recently shown to account for increased acute myocardial infarction risk in patients with MPNs.ACS: Acute coronary syndrome;MPNs: Myeloproliferative neoplasms.
Hypercoagulability
MPNs,especially ET,are associated with increased platelet count as well as their functionality impairment.ET is characterized by an overproduction of platelets from megakaryocytes as these cells become excessively sensitive to thrombopoietin[10].As such,the risk of thrombosis is particularly higher in these patients.Such cells tend to be larger and more reactive[11].Further to their increased pro-thrombotic activity,dysfunctional platelets are less sensitive to the inhibitory effect mediated by aspirin or clopidogrel[12].Recently,the greater reactivity of platelets in MPNs has been related to the higher number of mitochondria within their membrane[13].
Leukocytosis is known to be a non-specific marker of acute myocardial infarction (AMI)[14],where it is thought to reflect the inflammatory response toward myocardial necrosis in AMI patients.In MPNs patients,it can also be an expression of a more aggressive disease or an exaggerated inflammatory response[3].As such,patients with AMI and marked leukocytosis are associated with a worse prognosis[15,16].On the other hand,leukocytosis itself is a possible cause of AMI.For instance,acute leukemia patients with marked leukocytosis are known to possibly have acute myocardial infarction as a complication of their clinical condition[16].In these patients,the presence of a pro-thrombophilic state and higher expression of adhesion molecules (e.g.,CD56) are thought to favor the onset of ACS[17].Similarly,patients with MPNs patients tend to have a pro-thrombotic state,and the presence of more circulating leukocytes can also reflect the presence of more reactive leukocytes with a tendency toward a dysregulated inflammatory response toward the onset of AMI.As such,leukocytosis in patients with PV can be considered as a possible hallmark of a higher cardiovascular risk[18].Indeed,pro-inflammatory states are known to increase the expression of procoagulants such as tissue factor,fibrinogen and adhesion molecules.
JAK2 is a non-receptor tyrosine kinase in the Janus kinase family.JAK2 mutations are implicated in MPNs,including PV,ET,and myelofibrosis[19].Furthermore,JAK2 mutation is also associated with a higher risk of ACS[3].The most prevalent JAK2 mutation in MPNs is called JAK2V617F.This mutation consists of a substitution of a valine with phenylalanine in position 617.The resulting neoplastic clones favor the development of inflammationviathe secretion of several inflammatory cytokines (e.g.,interleukin-1,interleukin-6,tumor necrosis factor-α,interferon-γ),resulting in mesenchymal and endothelial cell activation,bone marrow fibrosis,and eventually ending in acute myeloid leukemia[20].Furthermore,JAK2V617F is associated with a higher expression of adhesion molecules,especially integrins,resulting in the favoring of thrombus formation[2].Also,neutrophils harboring such mutations showed a higher tendency to form neutrophil extracellular traps[21-23].Again,NETosis is known to facilitate thrombus formation working as a scaffold for fibrin and cells as well as carrying different molecules with pro-coagulant activity[24].The result is a higher risk of thrombotic complications[25].Other MPN-associated mutations involve genes encoding for reticulum-associated protein calreticulin and thrombopoietin receptor[26].Of interest such mutations again result in the activation of JAK/STAT signaling and to date inhibitors of JAK2-driven signal have been approved for patients with myelofibrosis and PV[27].
Blood stasis
Blood stasis is typically found in MPNs patients.As all MPN relates with the expansion of a clone,and results in increased number of circulating cells a certain degree of blood stasis is expected in all patients with the disease[28].The higher hematocrit found in PV patients is secondary to the higher number of circulating erythrocytes found in these patients.Such a higher number of circulating red blood cells is associated with blood stasis,blood flow disturbances,and hyper-viscosity[29],therefore favoring the development of thrombosis[30].Similarly,in patients with ET cell count can hit high at over 1 million abnormal (see above) platelets/mL.Blood stasis together with the presence of over-reactive platelets can probably favor the development of thrombi because of platelet activation,as reported in studies on animal models that showed that platelet adhesion to the endothelium is directly related to the hematocrit levels[31].Clinically,PV patients are more prone to have vascular complications,such ACS[18].
Endothelial damage
The endothelium is a pivotal player in the pathophysiology of arterial thrombosis.Indeed,under physiological conditions endothelial cells exert anti-thrombotic roles by producing several mediators including nitric oxide.The endothelium probably participates in the formation of thrombi as a consequence of the hyper-coagulability state rather than being the primary origin of thrombus formation[32].However,under the pro-inflammatory pressure lead by the neoplastic clone,endothelial cells get dysfunctionally activated and secrete further pro-inflammatory cytokines propagating inflammation.Activated endothelial cells increase the expression of adhesion molecules including E-selectin on their surfaces in MPN patients[33].Although E-selectin is not considered a marker of unstable coronary plaque[34],the endothelial overexpression of E-selectin can trigger an excessive leukocyte response in MPN patients.As such,even the smallest plaque tear might favor an exaggerated intracoronary activation of platelets,causing the clinical manifestation of ACS.
Furthermore,endothelial cells with mutated JAK2 have been found in patients carrying the JAK2 V617F mutation[35].Of interest,such endothelial cells express a proadhesive phenotype with increased P-selectin expression that may be a further link with the increased thrombosis risk[36].Indeed,therapeutic approaches aiming at P-selectin blockade have shown preclinical potential to reduce thrombosis.Increased atherosclerosis may not be the only link between AMI and MPNs,as almost 20% of AMI in MPNs occurs in patients without significant atherosclerotic occlusive disease[37].Here,coronary vasoconstriction may play a role.Indeed,JAK2 V617F mice have shown increased arterial vasoconstriction due to their lower levels of nitric oxide,increased oxidative and inflammatory stress[38].Specifically,erythrocytes-derived microvescicles have been deemed responsible for such phenotype and proteomic analysis of particles derived from JAK2V617F erythrocytes suggested MPO as the potential mediator[38].
CONCLUSION
MPNs are associated with cardiovascular diseases,especially those sustained by a thrombotic event.MPNs arise from clonal hematopoiesis of indeterminate potential (CHIP),whose investigation in the last years provided fundamental insight into the causal link between thrombosis and MPNs.CHIP is defined as the presence of a clonal mutation in a driver gene,occurring with a variant burden of ≥ 2% but without any clinical evidence of a hematologic neoplasm.Patients with CHIP show a 10-fold increased risk of developing any hematologic malignancy,including MPNs[39].Of interest,the magnitude of risk enrichment due to CHIP is even higher than that of classical cardiovascular risk factors[39].Experimental and clinical observations further point at inflammation as the culprit link between CHIP and cardiovascular disease[40,41].Indeed,CHIP is nowadays seen as another characteristic of human aging,and it accompanies with another typical features of aging which is the appearance of a chronic low-grade pro-inflammatory state(inflamm-ageing).With recent trails showing the potential for anti-inflammatory therapies in cardiology[42],targeting specific inflammatory mediators may be a way to blunt prothrombotic state of patients with MPN.The role of CHIP and inflamm-ageing in cardiovascular disease development have been recently reviewed[41,43,44].
Mananet al[5] reviewed the recent literature and provided insight into the pathogenesis and clinical consequences of the association between hematological and cardiovascular diseases.Further research is needed to establish cardiovascular preventive strategies for MPN patients.
FOOTNOTES
Author contributions:Tirandi A wrote the paper and drew the image;Schiavetta E,and Maioli E critically revised the paper;Liberale L and Montecucco F supervised the entire work.All the authors read the final version of the manuscript and approve it for the submission and publication.
Conflict-of-interest statement:All the authors declare that they have no conflict of interest.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Italy
ORCID number:Amedeo Tirandi 0000-0003-1875-0160;Elisa Schiavetta 0009-0003-6281-996X;Elia Maioli 0009-0005-4125-9240;Fabrizio
Montecucco 0000-0003-0823-8729;Luca Liberale 0000-0003-1472-7975.
S-Editor:Liu JH
L-Editor:A
P-Editor:Zhang YL
杂志排行
World Journal of Cardiology的其它文章
- Facing ethical concerns in the age of precise gene therapy: Outlook on inherited arrhythmias
- Spontaneous coronary artery rupture after lung cancer surgery: A case report and review of literature
- Development and validation of a nomogram model for predicting the risk of pre-hospital delay in patients with acute myocardial infarction
- Seeing beneath the surface: Harnessing point-of-care ultrasound for internal jugular vein evaluation
- Risk of permanent pacemaker implantation following transcatheter aortic valve replacement: Which factors are most relevant?
- Cardiac rehabilitation after cardiac surgery: An important underutilized treatment strategy
