lnflammatory responses in esophageal mucosa before and after laparoscopic antireflux surgery
2024-05-07PelinErgunSezgiKipcakNurSelviGunelEserYildirimSozmenSerhatBor
Pelin Ergun,Sezgi Kipcak,Nur Selvi Gunel,Eser Yildirim Sozmen,Serhat Bor
Abstract BACKGROUND Currently,the primary treatment for gastroesophageal reflux is acid suppression with proton pump inhibitors,but they are not a cure,and some patients don’t respond well or refuse long-term use.Therefore,alternative therapies are needed to understand the disease and develop better treatments.Laparoscopic anti-reflux surgery (LARS) can resolve symptoms of these patients and plays a significant role in evaluating esophageal healing after preventing harmful effects.Successful LARS improves typical gastroesophageal reflux symptoms in most patients,mainly by reducing the exposure time to gastric contents in the esophagus.Amelioration of the inflammatory response and a recovery response in the esophageal epithelium is expected following the cessation of the noxious attack.AIM To explore the role of inflammatory biomolecules in LARS and assess the time required for esophageal epithelial recovery.METHODS Of 22 patients with LARS (pre-and post/5.8 ± 3.8 months after LARS) and 25 healthy controls (HCs) were included.All subjects underwent 24-h multichannel intraluminal impedance-pH monitoring and upper gastrointestinal endoscopy,during which esophageal biopsy samples were collected using endoscopic techniques.Inflammatory molecules in esophageal biopsies were investigated by reverse transcription-polymerase chain reaction and multiplex-enzyme-linked immunosorbent assay.RESULTS Post-LARS samples showed significant increases in proinflammatory cytokines [interleukin (IL)-1β,interferon-γ,CX-C chemokine ligand 2 (CXCL2)],anti-inflammatory cytokines [CC chemokine ligand (CCL) 11,CCL13,CCL17,CCL26,CCL1,CCL7,CCL8,CCL24,IL-4,IL-10],and homeostatic cytokines (CCL27,CCL20,CCL19,CCL23,CCL25,CXCL12,migration inhibitory factor) compared to both HCs and pre-LARS samples.CCL17 and CCL21 levels were higher in pre-LARS than in HCs (P < 0.05).The mRNA expression levels of AKT1,fibroblast growth factor 2,HRAS,and mitogen-activated protein kinase 4 were significantly decreased post-LARS vs pre-LARS.CCL2 and epidermal growth factor gene levels were significantly increased in the pre-LARS compared to the HCs (P < 0.05).CONCLUSION The presence of proinflammatory proteins post-LARS suggests ongoing inflammation in the epithelium.Elevated homeostatic cytokine levels indicate cell balance is maintained for about 6 months after LARS.The anti-inflammatory response post-LARS shows suppression of inflammatory damage and ongoing postoperative recovery.
Key Words: Anti-reflux surgery;Gastroesophageal reflux disease;Cytokine;Ⅰnflammatory response;Esophagus
lNTRODUCTlON
Gastroesophageal reflux disease (GERD) is a chronic public health problem characterized by typical symptoms of heartburn and/or regurgitation.It is a common worldwide condition and ranks among the most prevalent diseases in adults[1].Although GERD is widespread,the factors initiating the pathogenesis of the disease are not fully understood.Two theories exist on the disease’s pathogenesis.The first of these is the direct effect of gastric contents on the surface epithelium,where the inflammatory process begins in the lumen and advances with the assistance of dilated intercellular spaces[2].However,this theory is insufficient to explain patients without erosion.Except for erosive esophagitis,no difference has been shown in other phenotypes of GERD according to the level of dilated intercellular spaces[1].The other theory is cytokine-mediated damage.According to this theory,proinflammatory cytokines recruit immune cells,and tissue damage occurs as a result of the inflammatory response mediated by incoming immune cells starting from the basolateral cell layers[3].Substances like acid and pepsin in the reflux content contribute to this damage[4].
The primary treatment modality currently is inhibiting gastric acid secretion with proton pump inhibitors (PPIs).However,some patients resist long-term drug usage.While erosive reflux patients generally respond well to PPIs,others require continuous medical treatment due to the absence of a cure through drug use[5].In addition,some phenotypes of the disease do not totally or even partially respond to PPIs.Drug therapy provides temporary relief but is not a definitive treatment method.For treatment of the disease,exploring alternative therapeutic approaches becomes imperative.Understanding the disease pathogenesis is crucial to identifying target molecules for the development of preventive or therapeutic medications.
Since symptom resolution can be achieved in up to 93.1% of patients following laparoscopic anti-reflux surgery (LARS)[6],this modality is crucial for assessing the healing process of the esophageal epithelium after preventing the effects of noxious agents.The aim of this study was to investigate the role of inflammatory and recovery biomolecules after LARS by exploring the inflammatory pathways that may contribute to the pathogenesis of the disease.Additionally,we aimed to determine healing time frame to ascertain whether a meaningful period for healing allows the esophageal epithelium to fully recover.
MATERlALS AND METHODS
Subjects
In total,35 patients with GERD who had been approved for LARS by the Ege University GERD Study Group,and 25 healthy controls (HCs) were included in the study.However,the follow-up upper gastrointestinal (GI) endoscopy continued with 23 patients,as 12 patients did not attend their post-LARS upper GI endoscopy appointments.The interval between the two upper GI endoscopies ranged from 2 to 18 months (mean 5.8 ± 3.8 months).All patients had pyrosis and/or regurgitation at least once a week or more frequently and completed the GERDQ (Validated Mayo Clinic) and QoLRAD (quality of life) questionnaires.Patients were ceased proton-pump inhibitors,H2 blockers,and antacids at least 10 d pre-procedure.
Esophageal motility tests were done before placing the multichannel intraluminal impedance-pH (MII-pH) catheter at the upper lower esophageal sphincter (LES) boundary.Data were analyzed using MMS software version 8.1 (MMS -Laborie,the Netherlands).An eight-channel motility catheter with four radial and four circumferential openings was used for motility measurements.After an 8-h fast,the catheter was placed 50-55 cm deepviathe nasal passage.LES location was identified using intragastric pressure.For 24-h MII-pH monitoring,a calibrated MII-pH catheter (MMS -Laborie,the Netherlands) was positioned 5 cm above the LES,connected to a recording device (MMS -Laborie,the Netherlands).All HCs had normal intraesophageal 24-h MII-pH and high-resolution manometry and a negative history of upper GI disease or surgery.The patients with GERD who were treated with LARS already had a pathological reflux burden according to MII-pH monitoring and/or endoscopically observed esophageal erosions.Surgical indications were determined by the entire GERD team,which included specialists in gastroenterology,surgery,ENT,pulmonary medicine,and psychiatry.
The exclusion criteria for both patients and HCs included primary esophageal motility disorders,Barrett’s esophagus,previous upper GI surgery,chronic renal failure,severe coronary artery disease,severe chronic obstructive pulmonary disease,uncontrolled diabetes mellitus,pregnancy,lactation,and other disorders that may affect the study,with the exception of cancer (except non-melanoma skin cancer).
Biopsy specimens
Upper GI endoscopy was conducted by one gastroenterologist (Bor S),and the biopsy samples were taken by one technician.Esophageal biopsy specimens (n=4) were endoscopically taken from normal mucosa 3-5 cm above the Z-line without erosion using biopsy forceps (Radial Jaw 4,opening diameter 2.8 mm,Boston Scientific,United States).Two biopsies were preserved in RNAzol®(GeneCopoeia,Rockville,MD) for subsequent mRNA studies at -80 °C,while the remaining samples were immediately frozen at -80 °C for later protein measurements (Figure 1).

Figure 1 Study design. Pre-LARS: Pre-laparoscopic anti-reflux surgery;Post-LARS: Post-laparoscopic anti-reflux surgery;HRM: High-resolution manometry;MIIpH: Multichannel intraluminal impedance-pH;PPI: Proton pump inhibitor;RT-PCR: Real-time polymerase chain reaction;ELISA: Enzyme-linked immunosorbent assay;GI: Gastrointestinal.
Gene expression
The biopsy samples were homogenized using a Bioprep-6 Homogenizer (Hangzhou Allsheng Instruments Inc.,Zhejiang,China),and total RNA was isolated with an Aurum™ Total RNA Mini Kit (Bio-Rad Laboratories,Inc.,Hercules,CA) following to the manufacturer’s instructions.The absorbance,indicating the concentration and purity of the total RNA,was measured at 260/280 nm with a NanoDrop spectrophotometer (Thermo Scientific,Wilmington,DE) using 2 μL of each homogenized and isolated sample.
cDNA was synthesized from total RNA in each sample using qPCR and an iScript cDNA Synthesis Kit with a reverse transcriptase enzyme (Bio-Rad Laboratories,Inc.,Hercules,CA) following to the manufacturer’s instructions.Real-time polymerase chain reaction was conducted using a LightCycler®480 (Roche Diagnostics Inc.,Basel,CH).iTaq Universal SYBR Green Supermix (Bio-Rad Laboratories,Inc.,Hercules,CA) and two different primer libraries -(Human JAK/STAT Signaling Primer Library and Human NFκB Primer Library) Real Time Primers (LLC) -were employed according to the manufacturer’s specifications.The housekeeping genes selected were actin-beta,beta-2-microglobulin,and ribosomal protein L13a.
Multiplex protein measurements
The biopsy samples were homogenized using a Bioprep-6 Homogenizer (Hangzhou Allsheng Instruments Inc.,Zhejiang,China),and total protein was extracted with a Bio-Plex TM Cell Lysis Kit (Bio-Rad Laboratories,Inc.,Hercules,CA) according to the manufacturer’s instructions.After centrifugation (4500 rpm for 10 min)[7],the isolated proteins were divided into aliquots,and protein amounts were determined using the Lowry method[7].The protein levels of chemokines and phospho-cell signaling proteins were measured using Bio-Plex Multiplex Immunoassays (Human Chemokine 40-Plex panel,Pro Cell Signaling Phospho 7-plex panel,Pro Cell Signaling Phospho NFκB p65 ve Pro Cell Signaling Phospho p38 MAPK,Bio-Rad Laboratories,Inc.,Hercules,CA) according to the manufacturer’s instructions.
Statistical analysis
The 2-ΔΔCtmethod was used for the quantitation analysis of gene expression.The corresponding gene expression levels in each group were compared.Gene expression levels in each group were compared,and genes with a fold change ≥ 1.5 were included in the evaluation.Statistical analyses were performed using ANOVA,Student’sttest (for parametric data) and the Mann-WhitneyUtest (for nonparametric data) with IBM®SPSS®Statistics 25.0.APvalue of < 0.05 was considered statistically significant in all comparisons.A paired samplest-test was applied for pre-LARS and post-LARS comparisons.Parametric values were presented as mean ± SD,while nonparametric tests used median and variance values.
RESULTS
Study group
One patient with erosive reflux disease (ERD) C/D out of 23 patients and 5 out of 25 HCs were excluded for various reasons: The presence of multiple polyps observed during upper GI endoscopy,excessive bleeding during biopsies,desaturation,and other related issues with the sedation procedure,as well as the relapse of erosions and/or symptoms after LARS.Ultimately,a total of 22 patients [10 ERD A/B,6 ERD C/D,6 non-ERD (NERD)] and 20 HCs were included in the study (Table 1).

Table 1 Demographics
Gene expression
CC chemokine ligand (CCL)2 (-2.3-fold) and epidermal growth factor (EGF) (-2.2-fold) gene expression levels were lower in the pre-LARS group compared to the HC group (Supplementary Table 1).On the other hand,mRNA expression levels of JUN (1.7-fold) and RAF1 (1.6-fold) were increased,while those of fibroblast growth factor 2 (FGF2) (-5.2-fold),mitogenactivated protein kinase 4 (MAP2K4) (-3.1-fold),EP300 (-2.8-fold),MCM5 (-2.8-fold),AKT1 (-2.4-fold),IRF9 (-2.3-fold),PIK3R2 (-2.3-fold),MYC (-2.2-fold),B-cell CLL/lymphoma 3 (BCL3) (-2.0-fold),PIAS4 (-2.0-fold),interferon (IFN) (alpha,beta and omega) receptor 1 (-2.0-fold),HRAS (-1.8-fold),IKBKE (-1.8-fold),RELA (-1.8-fold),TICAM1 (-1.5-fold),and PTPN11 (-1.5-fold) were decreased in the post-LARS group compared to levels in HCs (Figure 2A and Supplementary Table 2).

Figure 2 Significant gene expression. A: Significant gene expression in the post-laparoscopic anti-reflux surgery group compared to healthy controls;B: Significant gene expression in the post-laparoscopic anti-reflux surgery group compared to pre-laparoscopic anti-reflux surgery group.All comparisons are given as fold changes.BCL3: B-cell CLL/lymphoma 3;FGF2: Fibroblast growth factor 2;IFNAR1: Interferon (alpha,beta and omega) receptor 1;IKBKE: Inhibitor of kappa light polypeptide gene enhancer in B-cells,kinase epsilon;JUN: Jun proto-oncogene;MAP2K4: Mitogen-activated protein kinase 4;EGF: Epidermal growth factor;TNFRSF1A: Tumor necrosis factor receptor superfamily,member 1A.

Table 2 Cell signaling proteins
The fold changes in the post-LARS group compared to the pre-LARS group depicted in Figure 2B and Supplementary Table 3.While EGF (1.5-fold) and BCL3 (2.7-fold) expression increased after LARS,FGF2 (-5.4-fold),RIPK1 (-4.6-fold),tumor necrosis factor receptor superfamily,member 1 (-4.0-fold),MAP2K4 (-2.4-fold),HRAS (-2.2-fold) and AKT1 (-1.7 fold) decreased compared to pre-LARS measurements.

Table 3 Cytokine results indicating the homeostatic response
Protein levels
In the pre-LARS group,c-Jun levels were significantly lower compared to those in HCs (P< 0.05).Nuclear factor kappa-beta (NFκB) (P< 0.01),MEK1,and p38-MAPK levels were significantly higher than those in HCs (P< 0.05) (Table 2).The levels of the proinflammatory cytokines interleukin (IL)-1β,IFNγ and C-X-C chemokine ligand 2 (CXCL2) were significantly higher in the post-LARS compared to both the pre-LARS and HC groups (P< 0.05) (Figure 3A,Supplementary Table 4).
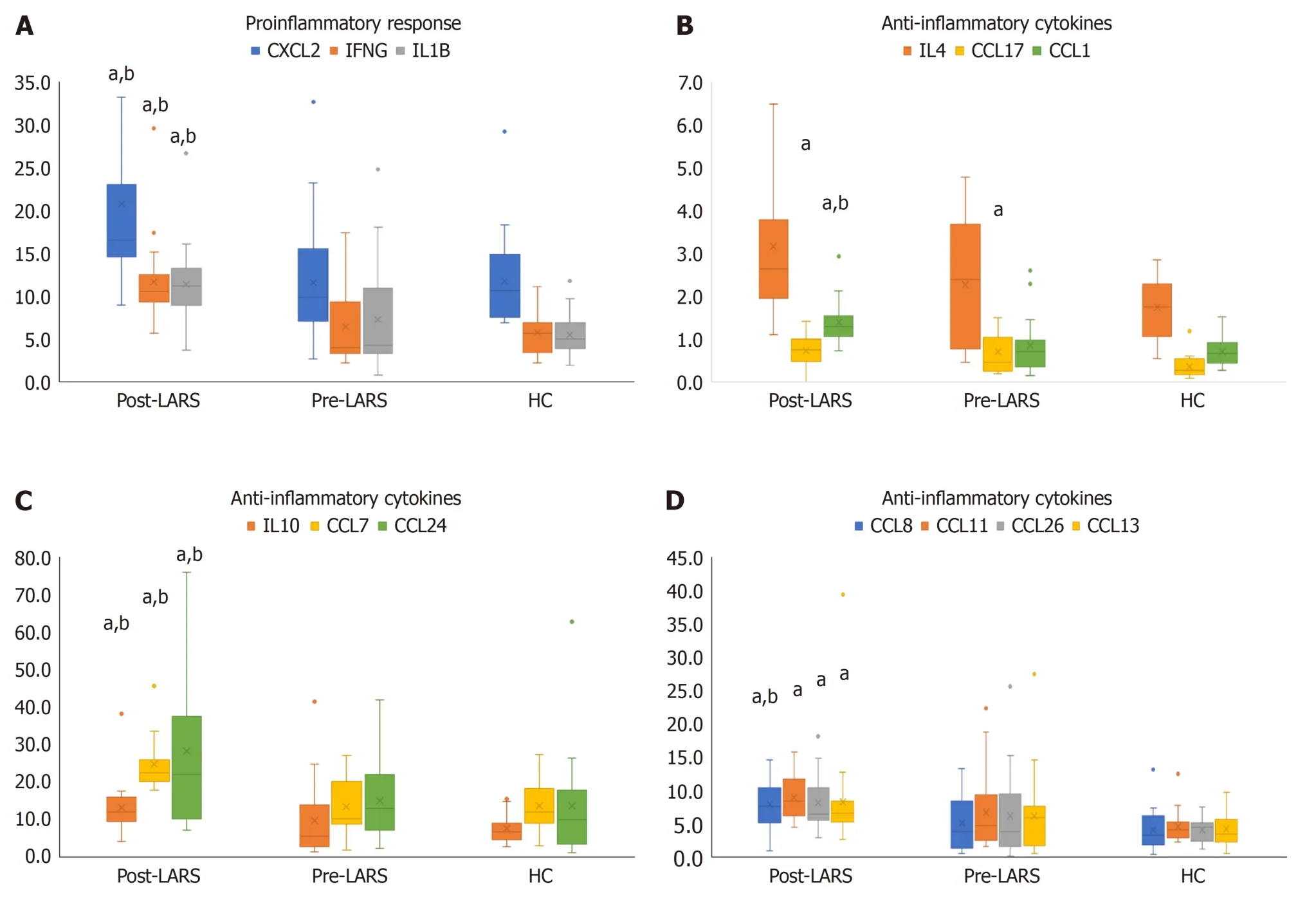
Figure 3 Cytokine results. A: Cytokine results indicating the proinflammatory response;B-D: Cytokine results indicating the anti-inflammatory response.aP < 0.05 vs healthy control;bP < 0.05 vs pre-laparoscopic anti-reflux surgery.CXCL: CXC motif chemokine ligand;IFN: Interferon gamma;IL: Interleukin;LARS: Laparoscopic anti-reflux surgery;HC: Healthy control.
Anti-inflammatory cytokines,including CCL11,CCL13,CCL17,CCL26,CCL1,CCL7,CCL8,CCL24,IL-4 and IL-10,showed a significant increase compared to levels in the HC and/or pre-LARS groups (P< 0.05) (Figure 3B-D,Supplementary Table 4).Specifically,CCL17 levels were higher in the pre-LARS group than in the HC group (P< 0.05).
The levels of homeostatic cytokines,including CCL27,CCL20,CCL19,CCL23,CCL25,CXCL12 and migration inhibitory factor (MIF),were higher in the post-LARS group than in the HC and/or pre-LARS groups (Table 3,Supplementary Table 4).Specifically,CCL21 levels were higher in the pre-LARS group than in the HC group (P< 0.05).
DlSCUSSlON
GERD is typically treated with PPIs,aimed at suppressing gastric acid secretion.However,long-term drug use can pose challenges,and some patients may exhibit a low response to PPI treatment,necessitating a more permanent solution.Laparoscopic antireflux surgery is offered as an alternative method to alleviate reflux symptoms,with a success rate of approximately 90%[8,9] in experienced centers.Following LARS,the contact of the esophagus with gastric contents and noxious agents is significantly reduced,leading to a drastic alleviation of symptoms and observable healing of the epithelium during endoscopy,as seen in our patients.
In this study,CCL21 levels were found to be higher in pre-LARS patients compared to controls.These intriguing findings may be explained by the role of the CCL21/CCR7 axis in the regulation of T-cell immunity.Unsoeldet al[10] observed that transgenic mice with high expression of CCL21 failed in the CD4 T-cell response against local skin infections.They suggested that a high concentration of CCL21 downregulated CCR-7,which is responsible for mediating the T-cell adaptive immune response and peripheral tolerance[10,11].It could be speculated that reflux disease is associated with an imbalance between CCL21 and CCR7 expression,characterized by an increase in favor of CCL21.
Chemotactic response of antireflux surgery
We investigated cellular-level changes before and after surgical treatment to comprehend the pathophysiological mechanism underlying GERD.Upon comparing data from patients’ post-LARS to those from HCs,we observed an increase in IL1β,MEK-1,p38 MAP kinase,and certain chemokine levels (CCL1,CCL19,CCL20,CCL21,CCL23,and CCL24) in the post-LARS group.These findings suggest an elevation in the inflammatory process in reflux disease through the toll-like receptors (TLR) signaling pathway and MEK/ERK pathway.While the MEK/ERK pathway is primarily activated by growth factors,osmotic stress,and cytokines[12],p38-MAPK is predominantly triggered by oxidative stress,UV radiation,hypoxia,ischemia,and specific proinflammatory cytokines like IL-1 and tumor necrosis factor-alpha (TNFα)[13].
Additionally,the expression level of RAF1,an activator of the MEK/ERK pathway that transmits chemical signals outside the cell to the cell nucleus,was significantly increased in post-LARS patients.Overactivity of these pathways results in NFκB activation and subsequently increased levels of pro-inflammatory cytokines,especially IL1β and IFNγ.In our study,the elevated IL1β levels may have been regulated by these two pathways and NFκB[14,15].
When we evaluated our data concerning the type of reflux,we observed varied responses in protein levels after surgery among different reflux phenotypes (Supplementary Table 5).Interestingly,there was no significant change in notable chemokine and protein levels in reflux patients with ERD C/D after surgery.This might be explained by the limited number of patients (n=6) in this group or the time of control endoscopy after surgery (approximately 5.8 months after LARS).
While MAPK4,TNF receptor,HRAs and AKT1 gene expression decreased,the expression of proinflammatory molecules (IL1β,IFNγ),chemotactic molecules (MIF,CCL1,CCL7,CCL11,CCL25,CCL27,CXCL2),and macrophage activation-related proteins (IL10,CCL19) increased after surgery in patients with ERD A/B and NERD compared to presurgical levels.
IL1β,a potent proinflammatory regulator,is secreted from many immune cells and triggers the production of acute phase proteins,proinflammatory cytokines,and adhesion molecules.It also activates T and B lymphocytes[16].Together,IFNγ and TNFα are precursors of the inflammatory response.IFNγ,predominantly secreted from activated T lymphocytes,is a crucial cytokine with pleiotropic immunological functions.Although elevated mostly in pathogenic infections,it has many functions,including promoting macrophage growth,antigen production,activation the innate immune system,fostering lymphocyte-endothelial interaction,regulating type 1 T helper (Th1)/Th2 balance,and controlling cellular proliferation and apoptosis[17].IFNγ can also trigger IL1β synthesis[18].
The elevation of CXCL2 levels in post-LARS provides evidence of the presence of neutrophils in the tissue[19].IL1β also induces the production of macrophage MIF,a regulator of innate immunity.MIF mostly causes macrophage accumulation in hypersensitivity regions[20].MIF contributes to the activation of NFκB by inhibiting the MEK/ERK signaling pathway and IKBA,an inhibitor of NFκB[21].It might be suggested that the TLR signaling pathway through MAP kinase and the MEK/ERK pathway was suppressed after surgery,likely due to depletion of stimulants in the lumen.On the other hand,the proinflammatory status,demonstrated by increases in IL1β,NFκB,and IFNγ levels,remained active in tissues after surgery.
This could be explained by two theories: Oxidative stress that might be elevated due to ischemia-reperfusion after surgery stimulates NFκB activation by increasing nuclear factor E2-related factor 2 and heme oxygenase levels.The second explanation involves chloride sensing regulation of NACHT,LRR,and PYD domains-containing protein 3 (NLRP3) inflammasome activation[22,23].Recent studies have shown that the chloride concentration in cells is a critical control point for NLRP3 inflammasome activation.Mayes-Hopfingeret al[22] revealed that decreased intracellular Cl activates the NLRP3 inflammasome,promoting an immune response by switching the proinflammatory status of a phagocyte.Although their study was conducted in macrophages,we can speculate that depletion of intracellular chloride concentration due to a decrease in extracellular chloride concentration,achieved by blocking acid flux to the esophagus with surgical intervention,might activate the NLRP3 inflammasome in the esophageal epithelium.These theories warrant further study.
TECK/CCL25 and CTACK/CCL27 levels were also increased in the post-LARS group compared to both the pre-LARS and HC groups.T memory and effector lymphocytes activated by IL1β rapidly migrate to the inflammatory epitheliumviaCCL25 and CCL27.However,it is known that these two chemokines primarily actviamemory T cells[19].CCL25 and CCL27 have more homeostatic effects on memory cells[19,24].
Additionally,I309/CCL1 and monocyte chemoattractant protein (MCP)2/CCL8,which have a homeostatic effect on memory T cells and have anti-inflammatory effects on Th2 and regulatory T cells during inflammation,were significantly increased after surgery.Moreover,there was an increase in MCP3/CCL7[25] and IL-10 levels,which can block the Th1 response that mediates monocyte motility,supported the anti-inflammatory activation post-LARS.
Our study showed that EOTAXIN-2/CCL24,MIP1d/CCL15,and MIP3b/CCL19 levels increased in NERD patients after surgery.EOTAXIN2/CCL24,responsible for the recruitment of basophils and eosinophils,promotes cell migration and regulates inflammatory and fibrotic activities.It is secreted from various cells,especially activated fibroblasts,leading to fibroblast proliferation and collagen synthesis[26].The increase in CCL24 levels indicates that the collagen deposition and reorganization process was active,with the effect of anti-inflammatory regulation in post-LARS tissues.Increased EGF expression in this group also supports the healing process and proliferation[27] and provides information about the presence of eosinophils or basophils in tissues.
CC chemokines are well-known chemoattractants for monocytes (RANTES,MCP 1-5),eosinophils (eotaxins 1-3),basophils (MCP 4-5),and lymphocytes [macrophage inflammatory protein (MIP)-1α and β].Our study demonstrated an increase in CC chemokines and many chemoattractants (MIP1,MIP3,EOTAXIN-2) after surgery in NERD patients.An increase in TNFα and IL1β might suggest that surgical treatment in NERD patients induced inflammatory processes,likely in response to surgical intervention,even after approximately 6 months.
Anti-inflammatory process following antireflux surgery
On the other hand,elevated levels of CCL1,CCL11 and CCL24 indicate that the Th2 response is activated,and that the resolution of the inflammatory response is increased post-LARS.CCL11 mediates the Th2 response as well as eosinophil and basophil migration[19].In addition to its anti-inflammatory properties,CCL17 also helps maintain homeostatic balance by mediating the transition of effector memory T cells to the inflammatory region.
MPIF1/CCL23 levels increased after surgery in patients with ERD A/B.CCL23 secreted by neutrophilsviaCXCL2,supports the inflammatory response by activating lymphocytes,monocytes and macrophages[28].However,homeostatic chemokines,such as MIP3-b/CCL19 and SDF1-a+b/CXCL12 were also increased[24] along with CCL25 and CCL27.CCL19 helps stabilize the inflammatory response by inducing naive T cells and central memory T cells to return to lymph nodes[25].Similarly,neutrophils,monocytes and B cells return to the bone marrow and mediate the suppression of the inflammatory response[19].
IL-4 levels increased after surgery in NERD patients compared to HCs.IL-4 has potent cytoprotective properties[29].We thought that it may have a major role in preserving the mucosal integrity after surgery.IL-4 also exerts anti-inflammatory effects by inducing the production of CCL7 and CCL11 from peripheral cells in the inflammatory region[25].These two elevated chemokines may be secretedviaIL-4.IL-4 can also suppress important cytokines in the proinflammatory process,such as IL1β and TNFα[30,31].The significant increase in important anti-inflammatory cytokines such as IL-4 and IL-10 in the NERD and ERD A/B groups may have caused the suppression of important proinflammatory markers in the postoperative group[32].
These findings suggest that the postoperative recovery process is ongoing after successful surgery.In addition,the proinflammatory effect is still ongoing,and it is possible that the anti-inflammatory response overwhelms the ongoing proinflammatory process.After the operation,patients were rescored for symptoms,improvements noted in the control endoscopy,and relapsed patients were excluded.But there may be patients whose mucosal damage had healed but who still had insensible acid attacks.Therefore,reflux symptoms that may occur after LARS may not always indicate failure of the surgery.A limitation of our study is the inability to perform a 24-h pH-impedance test in the post-LARS group,preventing the collection of rational data on acid attacks.In addition,only three patients (11,14,and 18 months) visited our clinic for control endoscopy after LARS (the other 19 patients were observed for < 6 months).Although no significant change was observed in pro-inflammatory and chemotactic cytokines when these three patients were excluded,it was noted that levels returned to the preoperative level (data not shown) according to the inflammatory cytokine levels in these three patients.However,a statistical calculation could not be made because we only had three patients in the long term.More patients are needed to examine the long-term effects.
CONCLUSlON
In conclusion,inflammatory processes,especially involving the TLR signaling pathway,may play a significant role in the pathophysiology of reflux disease.Surgical treatment of reflux disease yields varied responses in cells: The MEK/ERK pathway is suppressed,while inflammatory molecule levels,particularly NFκB and IL1β,increase through different mechanisms,including TLR signaling.Surgical treatment induces chemotactic cytokines and inflammatory responses in NERD patients.Elevated levels of macrophage activation markers after surgery in patients with NERD and erosive A/B promote macrophage differentiation into the M2a and M2b phenotypes,crucial for the tissue healing process.
The post-LARS group was included in the study approximately 6 months (2-18 months) after the operations.Proinflammatory proteins like IL1β,IFNγ,and CXCL2 persist,indicating the status of the ongoing inflammatory response.Additionally,high levels of CCL25,CCL27,CXCL12,CCL17,CCL1,CCL20,and MIF homeostatic cytokines/chemokines are present about 6 months post-operation,aiming to preserve cell homeostasis.Anti-inflammatory response proteins,including IL-10,CCL1,CCL7,CCL8,MCP4/CCL13,EOTAXIN3/CCL26,EOTAXIN/CCL11,TARC/CCL17,and IL-4,are also observed,working to suppress inflammatory damage responses in patients with GERD in the post-LARS group.These persistent cytokine levels suggest that,even at 6 months post-operation,complete recovery has not been achieved.Therefore,it is advisable to continue avoiding refluxogenic foods to prevent symptom recurrence.
ARTlCLE HlGHLlGHTS
Research background
Laparoscopic anti-reflux surgery (LARS) is the preferred therapeutic approach for gastroesophageal reflux disease (GERD),as it effectively prevents the reflux of gastric contents into the esophagus.While there is existing knowledge about the recovery period of LARS (typically reported as 8-10 wk in the literature),limited data is available regarding the healing process within the esophageal mucosa following this procedure.This study aims to illuminate the recovery process of patients with GERD who have undergone LARS,with a specific focus on the inflammatory pathways within the esophageal mucosa.
Research motivation
Patients who have undergone LARS often report the eventual healing of symptoms such as heartburn and regurgitation after the surgery.However,a small percentage continues to experience GERD symptoms even post-LARS.The available data on LARS is primarily derived from patients’ responses.
Research objectives
We aim to focus on the inflammatory and recovery processes within the esophageal mucosa before and after the surgery.
Research methods
Twenty-two patients with GERD (the same patients before and after LARS) and 25 healthy controls (HCs) were enrolled in the study.Esophageal biopsies were homogenized,and the expressions of inflammatory and cell signaling genes were measured using real-time polymerase chain reaction.Protein levels were assessed using the multiplex enzyme-linked immunosorbent assay method.
Research results
The approximate period between pre-and post-LARS was 6 months (5.8 ± 3.8 months).We demonstrated that proinflammatory cytokines remained activated in post-LARS patients.However,we also observed a significant increase in homeostatic and anti-inflammatory cytokines in the post-LARS group compared to both pre-LARS and HCs.
Research conclusions
We conclude that the toll-like receptor signal is involved in the activation of inflammatory cytokines,while the MEK/ERK pathway is suppressed after LARS.Despite the higher levels of inflammatory cytokines,regulatory and anti-inflammatory markers were also activated in these patients.The persistence of cytokine levels suggests that recovery may not be complete even at 6 months.Patients who have undergone LARS should avoid refluxogenic foods to prevent short-term GERD symptoms.
Research perspectives
We plan a follow-up study with esophageal biopsies and 24-h multichannel intraluminal impedance-pH impedance monitoring in the long term for those patients.
ACKNOWLEDGEMENTS
The results of this study were presented and awarded with a travel grant at IUBMB-FEBS-PABMB 2022 Congress between 9 to 14 July 2022 at Lisbon,Portugal.
FOOTNOTES
Author contributions:Ergun P,Yildirim Sozmen E,and Bor S contributed to the conceptualization,writing-review and editing;Ergun P and Bor S were involved in the investigation;Ergun P,Kipcak S,Selvi Gunel N,Yildirim Sozmen E,and Bor S participated in the methodology and project administration;Ergun P,Kipcak S,Selvi Gunel N,and Yildirim Sozmen E contributed to the software;Ergun P was involved in the writing-original draft;and all authors have read and agreed to the published version of the manuscript.
Supported bythe Scientific and Technological Research Council of Turkiye/TUBİTAK,No.118S260;and Turkish Society of Gastroenterology,No.797-TGD-2021.
lnstitutional review board statement:This study was performed in line with the principles of the Declaration of Helsinki.Approval was granted by the Ethics Committee of Ege University,Izmir,Turkiye (18-2.1/36,20/02/2018).
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Data sharing statement:No additional data are available.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:United States
ORClD number:Pelin Ergun 0000-0002-3155-0633;Sezgi Kipcak 0000-0003-0615-3844;Nur Selvi Gunel 0000-0003-0612-2263;Eser Yildirim Sozmen 0000-0002-6383-6724;Serhat Bor 0000-0001-5766-9598.
Corresponding Author's Membership in Professional Societies:Turkish Society of Gastroenterology;Turkish Biochemical Society.
S-Editor:Wang JJ
L-Editor:A
P-Editor:Xu ZH
杂志排行
World Journal of Gastrointestinal Surgery的其它文章
- lndocyanine green: The guide to safer and more effective surgery
- Endoscopic ultrasound-guided lauromacrogol injection for treatment of colorectal cavernous hemangioma: Two case reports
- Abdominal cocoon syndrome-a rare culprit behind small bowel ischemia and obstruction: Three case reports
- Link between mutations in ACVRL1 and PLA2G4A genes and chronic intestinal ulcers: A case report and review of literature
- Clinical efficacy and safety of erlotinib combined with chemotherapy in the treatment of advanced pancreatic cancer: A meta-analysis
- Endoscopic-ultrasound-guided biliary drainage with placement of electrocautery-enhanced lumen-apposing metal stent for palliation of malignant biliary obstruction: Updated meta-analysis
