Clinical efficacy and safety of erlotinib combined with chemotherapy in the treatment of advanced pancreatic cancer: A meta-analysis
2024-05-07XiaoYanLiuHongNianPanYueYu
Xiao-Yan Liu,Hong-Nian Pan,Yue Yu
Abstract BACKGROUND Advanced pancreatic cancer is resistant to chemotherapeutic drugs,resulting in limited treatment efficacy and poor prognosis.Combined administration of the chemotherapeutic gemcitabine and erlotinib is considered a potential first-line treatment for advanced pancreatic cancer.However,their comparative benefits and potential risks remain unclear.AIM To assess the clinical efficacy and safety of erlotinib combined with other chemotherapy regimens for the treatment of advanced pancreatic cancer.METHODS Literature on the clinical efficacy and safety of erlotinib combined with chemotherapy for advanced pancreatic cancer was retrieved through an online search.The retrieved literature was subjected to a methodological qualitative assessment and was analyzed using the RevMan 5.3 software.Ten randomized controlled trials involving 2444 patients with advanced pancreatic cancer were included in the meta-analysis.RESULTS Compared with chemotherapeutic treatment,erlotinib combined with chemotherapy significantly prolonged the progression-free survival time of pancreatic cancer patients [hazard ratio (HR)=0.78,95%CI: 0.66-0.92,P=0.003].Meanwhile,the overall survival (HR=0.99,95%CI: 0.72-1.37,and P=0.95) and disease control rate (OR=0.93,95%CI: 0.45-0.91,P=0.84) were not significantly favorable.In terms of safety,the erlotinib and chemotherapy combination was associated with a significantly higher risk of diarrhea (OR=3.59,95%CI: 1.63-7.90,P < 0.05) and rash (OR=3.63,95%CI: 1.64-8.01,P < 0.05) compared with single-agent chemotherapy.Moreover,the risk of vomiting (OR=1.27,95%CI: 0.62-2.59,P=0.51),regurgitation/anorexia (OR=1.61,95%CI: 0.25-10.31,P=0.62),and infection (OR=0.72,95%CI: 0.28-1.87,P=0.50) were not significant in either group.CONCLUSION Compared with a single chemotherapeutic modality,erlotinib combined with gemcitabine can prolong progression-free survival in pancreatic cancer,but does not improve survival benefit or disease control rate,and can increase the risk of diarrhea and rash.
Key Words: Erlotinib;Chemotherapy;Advanced pancreatic cancer;Efficacy;Safety;Meta-analysis
lNTRODUCTlON
Pancreatic cancer is one of the most common malignant gastrointestinal tumors worldwide,and its incidence and mortality rates are increasing each year[1].The current mainstay of pancreatic cancer treatment is surgical resection,and early surgery has been shown to increase the likelihood of successful resection,with postoperative 5-year survival rates ranging from 70% to 85%[2].However,pancreatic tumors tend to infiltrate surrounding tissues,and early detection and metastasis prevention pose significant challenges.Consequently,most pancreatic cancers are diagnosed at advanced stages.Only 10% of advanced pancreatic cancers can be surgically resected,resulting in a 5-year survival rate of less than 4%[3].As such,systemic chemotherapy is the primary treatment for pancreatic cancer.However,despite the efficacy of cytotoxic drugs in inducing tumor cell apoptosis,the abnormal permeability of blood vessels surrounding the tumor tissue diminishes the influx of chemotherapeutic agents into the tumor.Furthermore,residual tumor cells can acquire essential growth-promoting substances from the surrounding blood supply,which enables continued proliferation,ultimately limiting the efficacy of chemotherapeutic drugs.This underscores the urgent need for novel and effective approaches.
The evolving fields of molecular biology and tumor immunology have paved the way for targeted drug therapy for pancreatic cancer at the molecular level.This approach allows the design of drugs that selectively act on oncogenic sites within the body,inducing tumor cell-specific death while sparing normal tissue cells surrounding the tumor[4,5].Erlotinib is a tyrosine kinase inhibitor (EGFR antagonist) used as a molecularly targeted therapeutic drug that competitively binds to the catalytic site of the intracellular region of the tyrosine kinase receptor with adenosine triphosphate,inhibiting the phosphorylation reaction.This,in turn,blocks downstream proliferative signaling and hinders liganddependent HER-1/EGFR activity in tumor cells,ultimately suppressing tumor cell proliferation[6,7].When used in conjunction with chemotherapeutic drugs for pancreatic cancer treatment,erlotinib has demonstrated favorable outcomes.Hence,this study aimed to evaluate the clinical efficacy and safety of erlotinib monotherapy combined with chemotherapy for the treatment of advanced pancreatic cancer,with the goal of offering valuable insights into the clinical management of this disease.
MATERlALS AND METHODS
Literature search
Literature searches were conducted in the PubMed,Embase,and Cochrane Library databases from inception to November 2023.The search terms used were “pancreatic cancer”,“pancreatic adenocarcinoma”,“erlotinib”,“gemcitabine”,“Erlotinib monotherapy combined with chemotherapy”,“advanced pancreatic cancer”,etc.
Inclusion and exclusion criteria
The inclusion criteria were: (1) Randomized controlled trials published in English;(2) studies conducted in patients with pathologically diagnosed advanced pancreatic cancer;(3) studies where patients in the control group were treated with chemotherapeutic drugs (gemcitabine and capecitabine) and those in the observation group were treated with erlotinib combined with chemotherapy;and (4) studies that assessed disease control rate (DCR),overall survival (OS),and progression-free survival (PFS) as efficacy indices.Articles that met the following criteria were excluded: (1) retrospective studies;(2) studies with incomplete data;and (3) case reports and news articles.
Extraction of the literature
The literature was screened by two professionals,and relevant data were extracted.The screened information was crosschecked and disagreements were resolved by discussion with a third author.The extracted information primarily included the (1) article title,literature source,authors,and publication date;(2) literature type and relevant elements for assessing the risk of bias;(3) interventions provided to the control and study groups,patients’ age,etc.;and (4) outcome indicators found in the literature,which included efficacy (DCR,PFS,and OS) and adverse effects (diarrhea,rash,vomiting,regurgitation/anorexia,and infection).
Evaluation of the quality of the literature
The Cochrane International Collaboration was used to evaluate the quality of the literature based on the sequence of randomization,allocation concealment,blinding implementation,presence of other biases,patient withdrawals,and loss to follow-up[8].
Statistical analysis
A meta-analysis of the efficacy and safety of erlotinib monotherapy in combination with chemotherapy for advanced pancreatic cancer was performed using RevMan 5.3 software.Dichotomous variables were evaluated as relative risks (RRs) or odds ratios (ORs) and 95%CIs for the effect analysis.For continuous variables,the mean,standard deviation,and 95%CI were used as effect statistics.Measurement data were compared using the chi-square test and combined withI2values for the size of heterogeneity,where aPvalue of > 0.10 and anI2value of < 50% indicated good statistical homogeneity.Meta-analysis was performed using a fixed-effects model,with aPvalue of ≤ 0.10 and anI2value of ≥ 50% indicating the presence of statistical heterogeneity.Finally,a meta-analysis was performed using a random-effects model.If the screened literature data were not subjected to meta-analysis,a descriptive analysis was performed.
RESULTS
Results of the literature search
The literature search and screening processes are depicted in Figure 1.Initially,806 papers were retrieved by searching the databases of related websites using relevant keywords.After Endnote processing,178 duplicates were removed,leaving 628 papers for further consideration.Subsequently,the titles and abstracts of conference papers,reviews,case reports,non-randomized controlled trials,and duplicate publications were screened,resulting in the exclusion of an additional 393 articles.Finally,235 articles were retained for further examination.After reading these articles,225 were excluded,resulting in the inclusion of 10 articles in the final analysis.
Baseline characteristics of studies included in the literature
Ten articles[9-18],all of which were randomized controlled trial studies,published between 2007 and 2020,with a total sample size of 2444 patients,were screened.The patients in the control group were treated with gemcitabine,capecitabine,or gemcitabine,whereas those in the study group were treated with gemcitabine or erlotinib.Characteristics of the included studies are listed in Table 1.

Table 1 Baseline characteristics of studies included in the meta-analysis
Literature quality assessment
The included studies exhibited a low risk of bias in terms of randomized sequence methods,blinding of literature results,and selective reporting.The overall quality of the studies was rated as B (Figure 2).

Figure 2 Literature bias evaluation chart.
Meta-analysis results of DCR
Five[9-11,13,16] of the 10 included articles reported DCR,including 509 patients in the control group and 585 patients in the study group.Statistical heterogeneity was observed among studies (P=0.0001,I2=83%).Therefore,using a randomeffects model,a meta-analysis showed that there was no significant difference in the DCR between the two groups of patients [OR=0.93 (0.45-1.91),P=0.84] (Figure 3A).
Meta-analysis results of OS
All 10 studies[9-18] reported the effect of erlotinib combination chemotherapy on OS in patients with pancreatic cancer,including 1258 patients in the control group and 1186 patients in the study group.Statistical heterogeneity was observed among the studies (P < 0.0001,I2=92%).Using a random-effects model,a meta-analysis showed that there was no significant difference in the comparison of OS between the two groups of patients [HR=0.99 (0.72-1.37),P=0.95](Figure 3B).
Results of the meta-analysis of PFS
All 10 studies[9-18] reported the effect of erlotinib combination chemotherapy on PFS in patients with pancreatic cancer,including 1258 patients in the control group and 1186 patients in the study group.Significant heterogeneity was observed among the studies (P< 0.0001,I2=73%).Using a random-effects model,the meta-analysis showed that the study group had significantly prolonged PFS compared with the control group (P< 0.001,Figure 3C).
Adverse reactions
Results of the meta-analysis of diarrhea incidence:Three studies[12,13,16] reported the incidence of diarrhea,and no statistical heterogeneity was found (P> 0.05,I2=0%).Hence,they were analyzed using a fixed-effects model.Results showed that the incidence of diarrhea in the study group was 3.59 times higher than that in the control group,and this difference was significant (P< 0.001;Figure 4A).

Figure 4 Forest plot of the meta-analysis comparing the incidence of adverse reactions in the two study groups. A: Incidence of diarrhea;B: Incidence of rashes;C: Incidence of vomiting;D: Regurgitation/anorexia;E: Infections.
Results of the meta-analysis of rash incidence: Three studies[13,16,17] reported the incidence of rash,but no statistical heterogeneity was found (P> 0.05,I2=0%).Hence,these studies were analyzed using a fixed-effects model.Results showed that the incidence of rash in the study group was 3.63 times higher than that in the control group,and this difference was significant (P< 0.001;Figure 4B).
Results of the meta-analysis of vomiting incidence: Four studies[12,13,16,17] reported on the incidence of vomiting,among which no statistical heterogeneity was found (P> 0.05,I2=0%);therefore,they were analyzed using a fixed-effects model.Results showed that the incidence of vomiting in the study group was 1.27 times higher than that in the control group.However,this difference was not statistically significant (P> 0.05;Figure 4C).
Meta-analysis results of regurgitation/anorexia:Three studies[12,16,17] reported the incidence of regurgitation/anorexia,among which a statistical heterogeneity was observed (P< 0.05,I2=85%);hence,these studies were analyzed using a random-effects model.Results showed that the incidence of regurgitation/anorexia was 1.60 times higher in the study group than that in the control group,but the difference was not significant (P> 0.05,Figure 4D).
Results of the meta-analysis of infection incidence: Two studies[13,16] reported the incidence of infections,and statistical heterogeneity was observed (P> 0.05,I2=0%).Therefore,they were analyzed using a fixed-effects model.The results showed that the incidence of infection in the study group was 0.72 times higher than that in the control group;however,the difference was not statistically significant (P> 0.05,Figure 4E).
Publication bias:Funnel plots were constructed based on the clinical efficacy and safety of erlotinib monotherapy combined with chemotherapy for advanced pancreatic cancer,and showed good symmetry on both sides of the funnel plots with less publication bias (Figure 5).
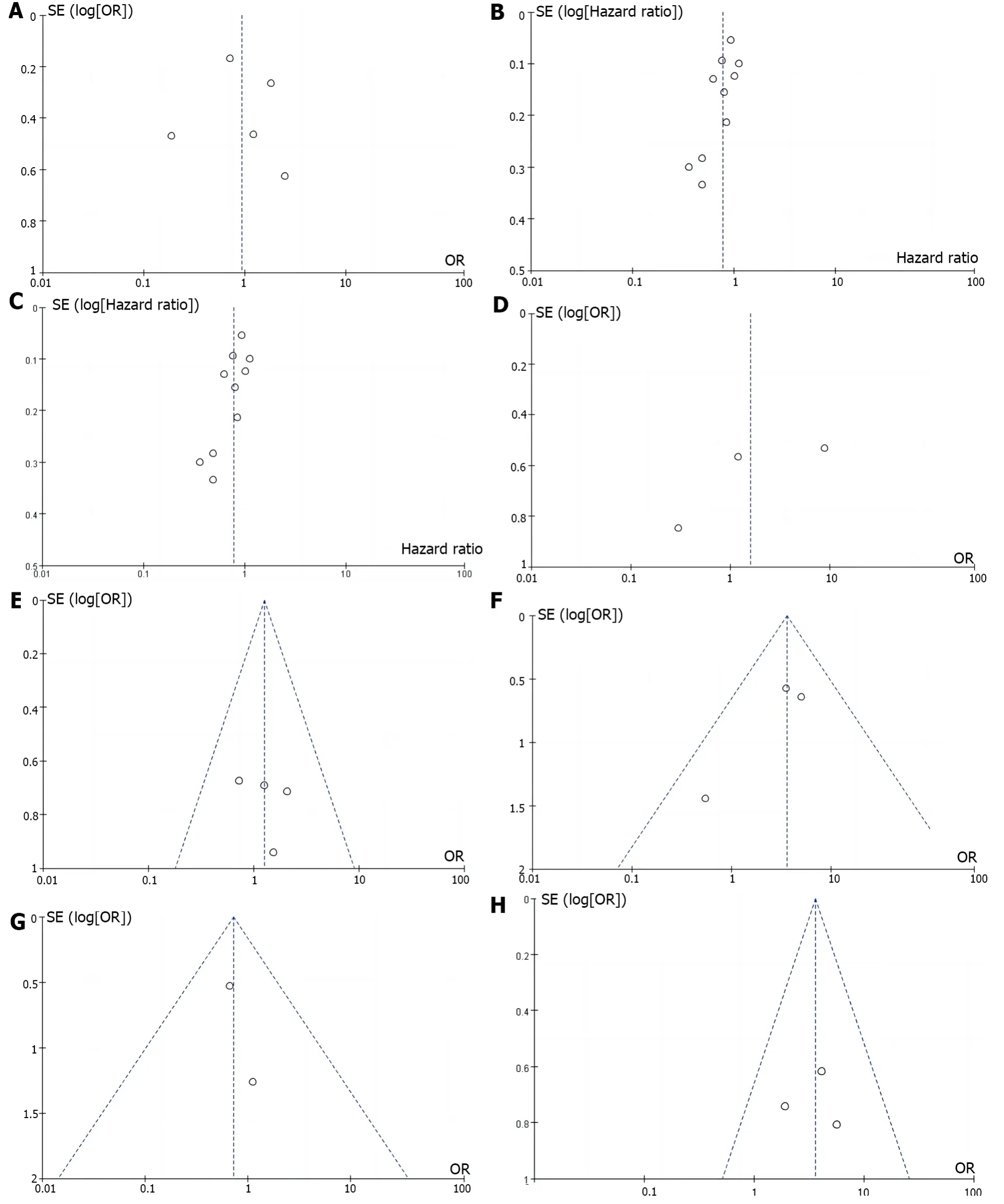
Figure 5 Plot of publication bias. A: Funnel plot depicting publication bias in the literature reporting disease control rate;B: Funnel plot depicting publication bias in the literature reporting overall survival;C: Funnel plot of publication bias in the literature reporting the incidence of progression-free survival;D: Funnel plot of publication bias in the literature reporting the incidence of regurgitation of anorexia nervosa;E: Inverted funnel plot of publication bias in the literature reporting the incidence of vomiting;F: Inverted funnel plot of publication bias in the literature reporting the incidence of diarrhea;G: Inverted funnel plot of publication bias in studies reporting the incidence of infections;H: Inverted funnel plot of publication bias in studies reporting the incidence of rashes.
DlSCUSSlON
Most patients with pancreatic cancer are diagnosed at an intermediate or advanced stage,with radical surgery considered feasible in only 15% to 20% of patients;however,the rates of recurrence and metastasis are high after surgery[19,20].Therefore,patients with intermediate or advanced pancreatic cancer,as well as those who undergo radical surgery require further intervention.Novel pharmacological mechanisms of action for molecularly targeted drugs have garnered the attention of the medical community in recent years,leading to their rapid development.Erlotinib is a novel epidermal growth factor receptor tyrosine kinase inhibitor that can be orally administered.It effectively inhibits EGFR and blocks intracellular tyrosine kinase phosphorylation.The overexpression or mutation of EGFR in many tumors can lead to uncontrolled cell growth and malignancy.Erlotinib inhibits tumor cell proliferation,invasion,and metastasis by blocking EGFR,promoting apoptosis,enhancing sensitivity to chemotherapy,and improving the therapeutic effects.Erlotinib inhibits EGFR;hinders tumor cell proliferation,invasion,and metastasis;promotes tumor cell apoptosis;enhances sensitivity to chemotherapy;improves therapeutic effects;and prolongs the survival of patients with tumors[21,22].However,whether erlotinib combined with chemotherapeutic drugs has significant advantages over chemotherapeutic drugs alone in the treatment of pancreatic cancer currently remains unclear.Hence,we conducted a meta-analysis of existing clinical studies to compare the efficacy and safety of erlotinib combined with chemotherapeutics and chemotherapeutic drugs alone for the treatment of pancreatic cancer.
Although molecularly targeted agents exhibit good clinical efficacy in the treatment of pancreatic cancer,the results of published clinical studies vary significantly.Mooreet al[11] conducted a phase III clinical randomized controlled trial (RCT) and randomized 569 patients with locally progressive or distant metastatic pancreatic cancer into two groups in a 1:1 ratio.One group received erlotinib in combination with gemcitabine,while the other received gemcitabine alone.Results showed median OS rates of 6.24 months and 5.91 months in these groups (P=0.038),respectively,with the combination therapy showing a better survival benefit than single-agent gemcitabine.Despite the positive results of this study,the survival benefit in the trial group was extremely limited and inconsistent with the conclusions of several subsequent prospective clinical RCTs.In a phase III clinical RCT,the LAP07 study,published in 2016,Hammelet al[12] enrolled 442 patients with locally progressive pancreatic cancer.Trial and control groups were treated with erlotinib in combination with gemcitabine or gemcitabine alone,respectively.Results showed that the median OS durations of the two groups were 11.9 and 13.6 months,respectively (P=0.09),and the difference was not significant.Nevertheless,the results of this study showed that erlotinib combined with chemotherapy significantly prolonged the PFS of pancreatic cancer patients compared with chemotherapy alone (HR=0.78,95%CI: 0.66-0.92,P=0.003).This finding indicates that erlotinib combined with chemotherapy can help patients stabilize their disease and prolong the progression-free time of pancreatic cancer.However,this combination treatment had no significant benefits on OS (HR=0.99,95%CI: 0.72-1.37,P=0.95) or DCR (OR=0.93,95%CI: 0.45-0.91,P=0.84).Second,in terms of safety,erlotinib combined with gemcitabine increased the risk of diarrhea (OR=3.59,95%CI: 1.63-7.90,P< 0.05) and rash (OR=3.63,95%CI: 1.64-8.01,P< 0.05) in patients with advanced pancreatic cancer compared with single-agent chemotherapy,which is not conducive to the stabilization of the disease and recovery.Although the risk of vomiting (OR=1.27,95%CI: 0.62-2.59,P=0.51),regurgitation/anorexia (OR=1.61,95%CI: 0.25-10.31,P=0.62),and infection (OR=0.72,95%CI: 0.28-1.87,P=0.50) occurred in the two groups of patients,the difference was not significant.This observation indicates that erlotinib combination chemotherapy did not increase the risk of vomiting,regurgitation/anorexia,or infection in patients with advanced pancreatic cancer compared with single-agent chemotherapy.Moreover,the included studies exhibited good baseline comparability,and the meta-analysis was well-standardized.The symmetry on both sides of the funnel plots of DCR,OS,PFS,and adverse effects in patients after treatment indicated that publication bias had minimal impact on the study results and that the outcomes maintained a high level of confidence.Therefore,the results of this meta-analysis are reliable and stable,providing an evidence-based reference for the clinical treatment of advanced pancreatic cancer,and guiding further research.
Systematic evaluation enables the analysis and assessment of existing clinical studies,provides guidance for clinical practice,and suggests directions for future research.Several suggestions can be made based on the limitations of this study.The process of reporting RCTs should be standardized,especially the description of methodological quality,the endpoints should be described in detail,the follow-up and recording of safety and long-term efficacy indicators should be strengthened,the economic indicators related to the report should be collected in order to make it is easier to evaluate the economic aspects of the interventions,and more high-quality,large-sample clinical studies should be conducted to validate the conclusions of this meta-analysis.Additional high-quality large-sample clinical studies are required to validate the findings of this meta-analysis.The use of erlotinib in combination with other chemotherapeutic agents to improve patient outcomes remains an important topic in clinical research.
CONCLUSlON
Overall,the results of the present meta-analysis show that,in contrast to single-agent chemotherapy,the combination of erlotinib and chemotherapy prolongs PFS in patients with pancreatic cancer.Furthermore,it does not elevate the risk of vomiting,regurgitation/anorexia,or infections in patients with advanced pancreatic cancer.However,this combination does not enhance survival benefits or DCR and may increase the risk of diarrhea and rash.
ARTlCLE HlGHLlGHTS
Research background
Pancreatic cancer is one of the most common malignant gastrointestinal tumors worldwide,and its incidence and mortality rates are increasing each year.The current mainstay of pancreatic cancer treatment is surgical resection,and early surgery has been shown to increase the likelihood of successful resection,with postoperative 5-year survival rates ranging from 70% to 85%.However,pancreatic tumors tend to infiltrate surrounding tissues,and early detection and metastasis prevention pose significant challenges.Erlotinib is a tyrosine kinase inhibitor (EGFR antagonist) used as a molecularly targeted therapeutic drug that competitively binds to the catalytic site of the intracellular region of the tyrosine kinase receptor with adenosine triphosphate,inhibiting the phosphorylation reaction.
Research motivation
This study evaluated the clinical efficacy and safety of erlotinib monotherapy combined with chemotherapy for the treatment of advanced pancreatic cancer,with the goal of offering valuable insights into the clinical management of this disease.
Research objectives
This study aimed to evaluate the clinical efficacy and safety of erlotinib monotherapy combined with chemotherapy for the treatment of advanced pancreatic cancer.
Research methods
Literature searches were conducted in the PubMed,Embase,and Cochrane Library databases from inception to November 2023.A meta-analysis of the efficacy and safety of erlotinib monotherapy in combination with chemotherapy for advanced pancreatic cancer was performed using RevMan 5.3 software.
Research results
Compared with chemotherapeutic treatment,erlotinib combined with chemotherapy significantly prolonged the progression-free survival time of pancreatic cancer patients.Meanwhile,the overall survival and disease control rate were not significantly favorable.In terms of safety,the erlotinib and chemotherapy combination was associated with a significantly higher risk of diarrhea and rash compared with single-agent chemotherapy.Moreover,the risk of vomiting,regurgitation/anorexia,and infection were not significant in either group.
Research conclusions
this study aimed to evaluate the clinical efficacy and safety of erlotinib monotherapy combined with chemotherapy for the treatment of advanced pancreatic cancer,with the goal of offering valuable insights into the clinical management of this disease.
I choose, answered he, the chest which stands at the foot of your bed; whatever lies on the top of the bed, and whatever is under the side of the cave
Research perspectives
Given the above clinical and methodological heterogeneity and the small sample size used in the stratified analysis,the conclusions of this study need to be verified further in the clinical setting.
FOOTNOTES
Co-corresponding authors:Hong-Nian Pan and Yue Yu
Author contributions:Liu XY and Pan HN acquired,analyzed,and interpreted the data;drafted,revised,and approved the manuscript;Yu Y conceived and designed the study,and critically revised and approved the final manuscript.Pan HN and Yu Y contributed efforts of equal substance throughout the research process;and the choice of these researchers as co-corresponding authors acknowledges and respects this equal contribution,while recognizing the spirit of teamwork and collaboration of this study.
Supported byNational Natural Science Foundation of China,No.31 870993;Fundamental Research Funds for the Central Universities,No.WK9110 000005;Anhui Provincial Health Research Project,No.AHWJ2022c020;Anhui Medical University Campus Level Research Fund,No.2020xkj229;Lu'an City Science and Technology Plan Project,No.2022Lakj009;and New Technology and Project of Lu'an People's Hospital,No.2021xjs10.
Conflict-of-interest statement:The authors declare no conflicts of interest.
PRlSMA 2009 Checklist statement:The authors have read the PRISMA 2009 Checklist,and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Xiao-Yan Liu 0009-0005-9226-5384;Hong-Nian Pan 0009-0008-2004-0209;Yue Yu 0000-0002-3617-2037.
S-Editor:Wang JL
L-Editor:A
P-Editor:Cai YX
杂志排行
World Journal of Gastrointestinal Surgery的其它文章
- lndocyanine green: The guide to safer and more effective surgery
- Endoscopic ultrasound-guided lauromacrogol injection for treatment of colorectal cavernous hemangioma: Two case reports
- Abdominal cocoon syndrome-a rare culprit behind small bowel ischemia and obstruction: Three case reports
- Link between mutations in ACVRL1 and PLA2G4A genes and chronic intestinal ulcers: A case report and review of literature
- Endoscopic-ultrasound-guided biliary drainage with placement of electrocautery-enhanced lumen-apposing metal stent for palliation of malignant biliary obstruction: Updated meta-analysis
- lmpact of frailty on short-term postoperative outcomes in patients undergoing colorectal cancer surgery: A systematic review and meta-analysis
