Endoscopic ultrasound-guided lauromacrogol injection for treatment of colorectal cavernous hemangioma: Two case reports
2024-05-07HuaTuoZhuWenGuoChenJingJieWangJiaNanGuoFenMingZhangGuoQiangXuHongTanChen
Hua-Tuo Zhu,Wen-Guo Chen,Jing-Jie Wang,Jia-Nan Guo,Fen-Ming Zhang,Guo-Qiang Xu,Hong-Tan Chen
Abstract BACKGROUND Colorectal cavernous hemangioma is a rare vascular malformation resulting in recurrent lower gastrointestinal hemorrhage,and can be misinterpreted as colitis.Surgical resection is currently the mainstay of treatment,with an emphasis on sphincter preservation.CASE SUMMARY We present details of two young patients with a history of persistent hematochezia diagnosed with colorectal cavernous hemangioma by endoscopic ultrasound (EUS).Cavernous hemangioma was relieved by several EUS-guided lauromacrogol injections and the patients achieved favorable clinical prognosis.CONCLUSION Multiple sequential EUS-guided injections of lauromacrogol is a safe,effective,cost-efficient,and minimally invasive alternative for colorectal cavernous hemangioma.
Key Words: Endoscopic ultrasound;Lauromacrogol injection;Colorectal cavernous hemangioma;Case report
lNTRODUCTlON
Colorectal cavernous hemangioma is an uncommon benign vascular neoplasm that usually present at birth.Anemia and painless lower gastrointestinal (GI) bleeding are the most common presenting symptoms.Some patients present with abdominal pain,diarrhea and constipation,or remain asymptomatic[1].Routine evaluation includes endoscopic examination,computed tomography (CT),and magnetic resonance imaging (MRI).The appearance at colonoscopy may present with chronic inflammatory changes,mucosal edema and ulceration because the dilated vessels may become obstructed by multiple thrombi,causing congestion and swelling of the colorectal mucosa.Misdiagnosis as inflammatory bowel diseases is common,resulting in delayed treatment[2,3].Endoscopic ultrasound (EUS) is effective for diagnosis of this submucosal lesion,and revealed a thickened colorectal wall with high echoic content but with obscure layer structures[4,5].
To date,management is still dominated by surgery,especially for patients with a wide range of lesions,and complete resection is an effective method to cure and pathologically confirm the disease[6].However,for patients with a single lesion or in cases an extensive resection is not feasible or in patients who refuse surgical intervention,nonoperative endoscopic treatment can be considered,such as sclerotherapy,electrocoagulation,or metal clipping for hemostasis[7].Only a few data are available on sclerotherapy of colorectal cavernous hemangioma.EUS-guided lauromacrogol injection to cure the disease has not been reported previously.
We report two cases of colorectal cavernous hemangioma successfully diagnosed by EUS,and treated by multiple sequential EUS-guided lauromacrogol injections.Clinical features and pathophysiology are discussed and a brief review of the current literature regarding colorectal cavernous hemangioma is included.
CASE PRESENTATlON
Chief complaints
Case 1:A 20-year-old man was admitted to our hospital for recurrent hematochezia for > 7 years,presenting with worsening painless fresh rectal bleeding for 1 month.
Case 2:A 26-year-old man with a history of increased stool frequency and intermittent hematochezia for > 1 year.
History of present illness
Case 1:Seven years previously,the patient began to have bloody stools without fever,abdominal distending pain,nausea and vomiting,or anal heaviness,and had been misdiagnosed with internal hemorrhoids,ulcerative proctitis,ischemic bowel disease and angiodysplasia in local hospitals.He had 1 month history of worsening painless fresh rectal bleeding before hospitalization.
Case 2:In the year before hospital admission,the patient developed increased stool frequency and intermittent bloody stools without abdominal pain,diarrhea,mucus,pus,or other symptoms.He attended our hospital and underwent colonoscopy that showed multiple bluish-purple nodules in the sigmoid colon.
History of past illness
Case 1:The patient underwent endoscopic ligation of hemorrhoids 5 years previously.
Case 2:There was no significant personal or family history.
Personal and family history
Case 1:The patient had no history of systemic syndromes such as Osler Weber Rendu and blue rubber bleb nevus syndromes.
Case 2:There was no significant personal or family history.
Physical examination
Case 1:On physical examination,the patient had an anemic appearance but the rest of the examination was unremarkable.
Case 2:No obvious abnormalities were observed.
Laboratory examinations
Case 1:Laboratory findings revealed microcytic hypochromic anemia with hemoglobin level of 6.4 g/dL (normal range 11.3 g/dL-15.1 g/dL).Other laboratory data showed no abnormalities.
Case 2:Stool occult blood examination was weakly positive.Other laboratory data showed no abnormalities.
Imaging examinations
Case 1:CT revealed diffuse thickening of the rectum with multiple calcification foci representing phleboliths,indicating vascular anomalies.And the enhanced rectal wall was not obvious in CT arterial phase (Figure 1).Endoscopic examination showed diffuse blue-red nodular lesions with vascular congestion of the distal rectal mucosa up to 10 cm in range (Figure 2A and B).Ultrasonographic (US) (12 MHz) was able to demonstrate a honeycomb appearance,and an anechoic zone indicated vascular signal in the rectal submucosa (Figure 2C).Linear EUS showed a submucosal vascular lesion in the rectal wall.EUS with color Doppler confirmed the venous nature of the outflowing vessel (Figure 2D).
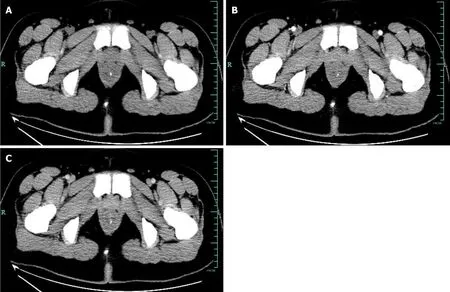
Figure 1 Enhanced total abdominal computed tomography showing that part of the rectum was thickened with edema and numerous tiny calcification foci indicative of phleboliths. A: Plain scan;B: Enhancement scan;C: Venous scan.

Figure 2 Colonoscopic and endoscopic ultrasound findings. A and B: Colonoscopy showed blue-red elevated nodular lesions;C: Endoscopic ultrasound (EUS) of rectum showed a vascular signal in the submucosa;D: EUS with color Doppler confirmed the venous nature of the outflowing vessel.
Case 2:Abdominal contrast-enhanced CT showed thickening of the proximal sigmoid colon wall with phleboliths.Colonoscopy showed multiple bluish-purple nodules limited to the proximal sigmoid,with a maximum size of 3.0 cm × 4.0 cm,and several isolated vascular masses were seen nearby (Figure 3A).Radial US scanning revealed nonechoic vascular signals in the submucosa of the anterior wall of the sigmoid colon (Figure 3B),and internal partitions were visible,with high echo spots in the partitions and sound shadows in the rear.The cross-section of the mass was about 14 mm × 16 mm.Doppler US showed small internal strips of blood flow (Figure 3C).And contrast-enhanced EUS using SonoVue (a contrast-enhanced ultrasound agent) distinctively revealed a septum-like structure and small internal enhancement in the anechoic areas (Figure 3D).

Figure 3 Endoscopic ultrasonography findings. A: Multiple bluish-purple nodules limited to proximal sigmoid;B: Nonechoic vascular signal in the submucosa of the anterior wall of the sigmoid,and a minute septum-like structure was visible,with high echo spots in the partitions;C: Doppler ultrasound detected small internal strips of blood flow;D: SonoVue imaging showed small internal enhancement in the anechoic areas.
FlNAL DlAGNOSlS
Case 1
Taking into consideration the clinical history,CT scan,endoscopic visualization of a blood-filled hemangioma,EUS demonstrated the sponge-like nature of the malformation,and a diagnosis of rectal cavernous hemangioma was made.
Case 2
Combination of clinical history,EUS findings,and CT scan led to a diagnosis of colorectal cavernous hemangioma.
TREATMENT
Case 1
Although surgical excision of the tumor is the only curative approach and the main determinant of prognosis,the patient declined any further surgical intervention because the lesion was close to the anus.With EUS guidance,a standard 22-gauge fine aspiration needle was inserted into the dilated vessels and 7 mL lauromacrogol was injected into the vessel,which generated hyperechoic clots in the lesion area (Video).EUS showed hyperechoic filling in the vascular lumen after injection (Figure 4A),and color Doppler US showed that the blood flow signal disappeared (Figure 4B).We chose another two points by intravascular injection of 9.5 mL and 10 mL lauromacrogol and achieved a similar effect to the previous endoscopic treatment.Colonoscopy showed that the elevated vascular mass was relieved and no obvious bleeding at the injection site (Figure 4C).He received antibiotics for 24 h and started to eat 12 h later and was discharged successfully.
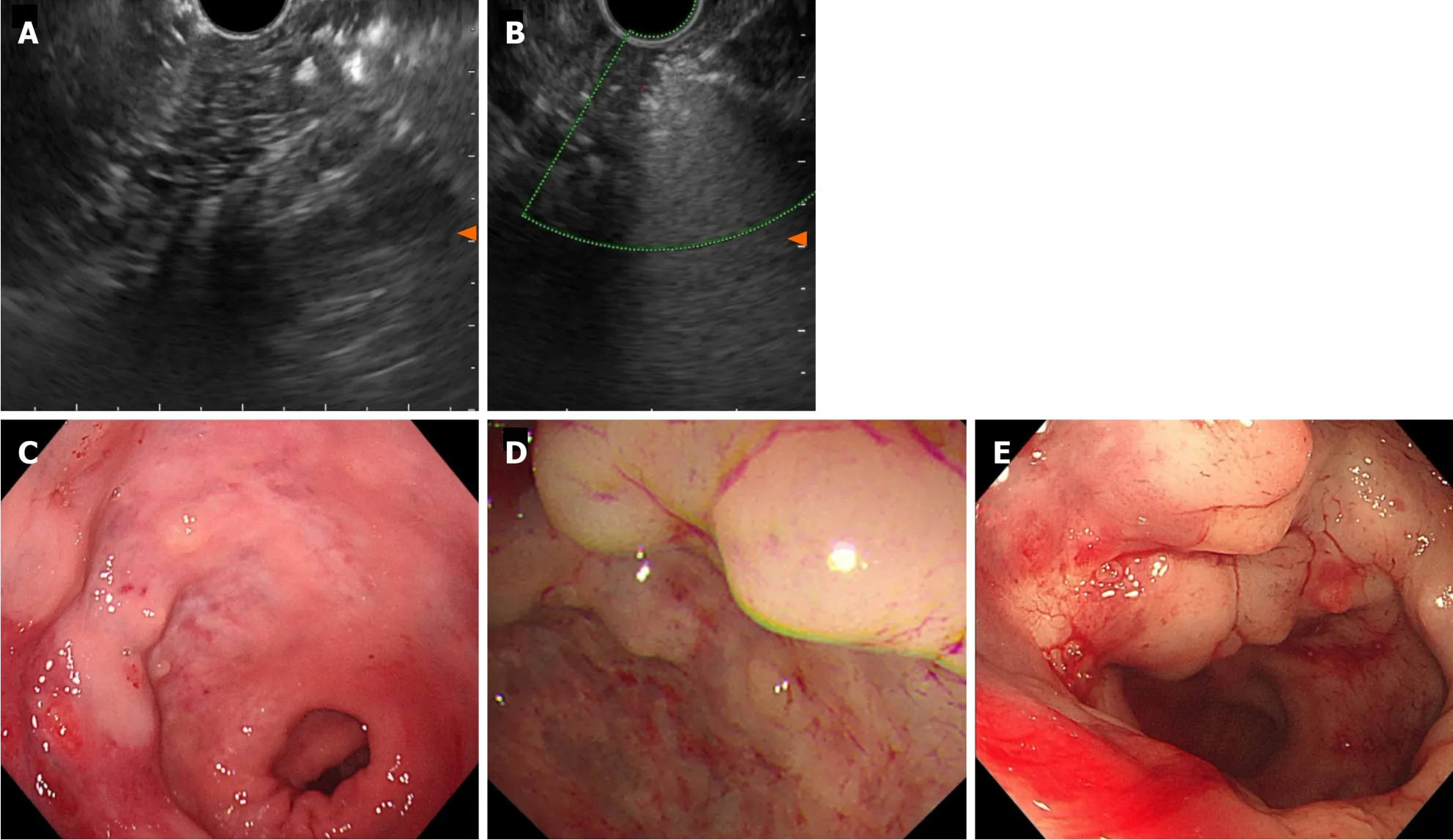
Figure 4 Endoscopic ultrasound after lauromacrogol treatment. A: Endoscopic ultrasound (EUS) showed hyperechoic filling in the vascular lumen after injection.Elevated vascular mass was relieved after EUS-guided lauromacrogol injection;B: EUS with color Doppler US showed that the blood flow signal disappeared;C: First scan;D: Second scan;E: Third scan.
Case 2
Under EUS guidance,a 22-gauge fine aspiration needle was inserted into the dilated vessels,and 30 mL lauromacrogol was injected into the vessel generating hyperechoic clots in the lesion area,and the lesion became pale and collapsed.There was no fever,abdominal distension,abdominal pain,hematemesis,black stool or other symptoms after the operation,and the patient was discharged on day 2 after the operation.
OUTCOME AND FOLLOW-UP
Case 1
Lauromacrogol injection significantly improved hematochezia symptoms,but the patient experienced refractory constipation and abdominal distension,which were resolved by administration of laxative and probiotics.The patient was rehospitalized for follow-up evaluation after 8 months and 22 months.Investigations revealed elevated hemoglobin of 13.3 g/dL-17.4 g/dL.Colonoscopy showed red-blue elevated nodular lesions and mucosal swelling,which were ameliorated by vascular congestion (Figure 4D and E).Lauromacrogol injection treatment was initiated through EUS guidance and postoperative EUS showed high echogenicity in the vascular tumor cavity,with Doppler US indicating reduced blood flow signals in the lesion.At present,the patient is generally in good condition during regular outpatient follow-up.
Case 2
In the following 2 months,the patient received endoscopic re-examination and EUS-guided sclerotherapy injection once a month,and the size and scope of the hemangiomas were significantly reduced (Figure 5A and B).At the fourth re-examination,only two small cavernous hemangiomas of 0.5 cm were visible in the sigmoid colon 38 cm from the anus.Small ulcers and scars could be seen around the hemangioma (Figure 5C).During follow-up of nearly 2 years,the patient reported no increase in stool frequency and blood.
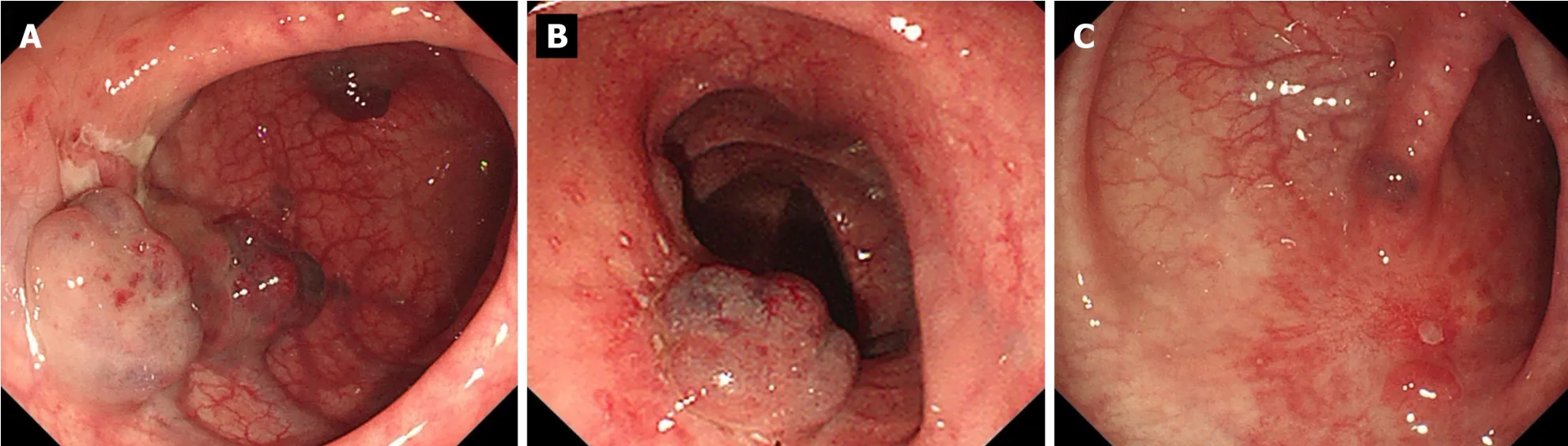
Figure 5 Endoscopic examination after lauromacrogol treatment. A and B: The size and scope of hemangiomas in each case were significantly reduced;C: There were only two small cavernous hemangiomas.Ulcers and scars were seen in the hemangioma areas after three sclerotherapy injections.
DlSCUSSlON
Colorectal hemangioma is an exceedingly rare congenital benign vascular disorder.The primary pathological subtypes encompass cavernous,capillary,and mixed forms,with cavernous hemangioma being the most prevalent.Cavernous hemangiomas can be further classified as localized or diffuse types,characterized by enlarged blood-filled spaces lined with abnormally thin vessel walls[8].Additionally,certain systemic syndromes such as Osler Weber Rendu and blue rubber bleb nevus syndromes may present histologically as telangiectatic,mixed or cavernous hemangiomas in the intestinal vasculature,of which colorectal hemangioma represents only a partial manifestation[9].
Colorectal cavernous hemangioma was first elucidated in 1839 but its pathogenesis is still not fully understood.This infrequent benign GI vascular lesion is considered to be a type of venous malformation that might be caused by the abnormal development of the mesoderm tissue during morphogenesis[10,11].Generally,colonic hemangiomas affect young people,with an equal ratio of males to females.Usually located in the rectosigmoid area,the size of the tumor varies from a few to dozens of centimeters.Mucosal edema,nodularity and vascular congestion erosion and indistinguishable edematous ulceration can lead to incorrect diagnosis as inflammatory bowel disease[12].Most patients have recurrent painless bleeding episodes,worsening with time,and half have chronic iron-deficient anemia.Abdominal or pelvic pain,obstruction,intussusception,or constipation may also be possible,although infrequent[13].Because of its risk of massive hemorrhage it should also be considered in patients with a history of proctitis to avoid unnecessary treatment.Early definitive diagnosis and timely therapy of the lesions are the main methods for curing colorectal cavernous hemangioma.
The preoperative diagnosis rate of this disease is low,and the lesion is usually found during enteroscopy,but the final diagnosis still needs to rely on postoperative pathological examination.Histologically,there are characteristic features but endoscopic biopsy should be avoided because of the high risk of severe hemorrhage.Clinical diagnosis of colorectal cavernous hemangioma should be made from a combination of clinical history,endoscopic findings,and cross-sectional imaging,preferably MRI or CT.Diffusely bowel-wall thickening with or without phleboliths can be accurately evaluated by CT[14].CT findings are nonspecific,making it difficult to differentiate colorectal cavernous hemangioma from the surrounding soft tissue and to determine the exact extent of the lesions[15].MRI can help evaluate the extent of the lesion and display the possible involvement of adjacent organs.High signal intensity can be seen in the perirectal fat with serpiginous structures correlating with the small vessels supplying the hemangioma on T2-weighted MRI[16].EUS can clearly display the intestinal wall hierarchy,determine the location and origin of the tumor,show echo of the lesion,and detect blood flow signals under Doppler US,providing a reliable diagnostic basis for colonic hemangioma.In the two cases reported in this paper,EUS showed that blue-purple lesions originated from the colorectal submucosa and appeared as honeycomb hypoechoic appearance,and Doppler US detected a small amount of blood flow signals.Contrastenhanced endoscopic ultrasonography can enhance the Doppler blood flow signal through injection of ultrasound contrast agent.Clearly showing whether there is abnormal perfusion in the blood flow of the lesion,which is helpful for the diagnosis of the disease and the judgment of blood supply.Therefore,EUS may be the best means of evaluation before treatment for colorectal cavernous hemangioma.
Complete surgical resection is currently considered the only effective way to cure the disease.Conservative options like banding and sclerotherapy have been reported,and they can decrease the amount and frequency of colorectal hematochezia[17].However,it has been reported that colorectal bleeding eventually recurs in most patients who receive nonoperative treatment[18].
Here,we report two cases of diffuse cavernous hemangioma of the rectosigmoid that was successfully treated by novel EUS-guided lauromacrogol injection,which achieved good clinical prognosis.Under the EUS guidance,the needle is accurately inserted into the cavernous hemangioma for the injection of lauromacrogol.The filling of lauromacrogol inside the hemangioma can be observed in real time during the EUS scan.During the operation,the injection can be stopped when the lesion is full of high echo of lauromacrogol.It can not only effectively reduce the risk of extravascular injection caused by "blind" injection under the direct vision of ordinary endoscope,but also monitor the changes of the echo in the hemangioma cavity during the injection process,and accurately control the injection amount of lauromacrogol.However,the local lesions may not penetrate completely due to the dilution of lauromacrogol with the blood flow after a single injection of lauromacrogol.Therefore,sequential treatment should be performed as appropriate.If the lesions are still residual in the follow-up,supplementary injection of sclerosing agent can be performed again.Compared with other nonsurgical conservative treatment methods,EUS-guided injection of lauromacrogol in the treatment of colorectal cavernous hemangioma is more targeted and thorough,with fewer intraoperative and postoperative complications.
These cases present a novel method of sclerotherapy under EUS guidance for colorectal cavernous hemangioma,suggesting that EUS could play a greater role in the interventional therapy of digestive cavernous hemangioma.The EUSguided injection of lauromacrogol for treatment of colorectal cavernous hemangioma is a safe,effective,cost-efficient,and minimally invasive technique,especially in instances where an extensive resection is not feasible or not tolerant for the patients.Multiple sequential EUS-guided injections of lauromacrogol can provide benefits to patients and reduce the likelihood of recurrence.However,long-term therapeutic effects and recurrence rates still require further follow-up and observation.Moreover,well-designed prospective studies are needed to evaluate the safety and feasibility of EUS-guided lauromacrogol injection in the treatment of GI hemangiomas,with particular consideration given to long-term follow-up.In the future,more cases should enable exploration of the potential of endoscopic therapy in curing GI hemangiomas and improving postoperative quality of life.
CONCLUSlON
Our cases show the safety and feasibility of EUS-guided lauromacrogol injection as an appropriate therapeutic option for colorectal cavernous hemangioma.It avoids surgery in some patients,but surveillance and repeated treatment are deemed necessary because of the likelihood of further lesions later in life.
ACKNOWLEDGEMENTS
We sincerely appreciate the patients and their families for their cooperation in information acquisition,treatment,and follow-up.
FOOTNOTES
Co-corresponding authors:Guo-Qiang Xu and Hong-Tan Chen.
Author contributions:Zhu HT and Chen WG contributed to manuscript writing and editing,and data collection;Wang JJ,Guo JN and Zhang FM contributed to data analysis;Xu GQ and Chen HT guided the treatment and contributed to conceptualization and supervision;All authors have read and approved the final manuscript.
Supported byNatural Science Foundation of Zhejiang Province,No.LY20H 030010;and Medical Health Science and Technology Project of Zhejiang Provincial Health Commission,No.2019-KY1-001-181.
lnformed consent statement:Informed written consent was obtained from the patient for publication of this report and any accompanying images.
Conflict-of-interest statement:The authors declare that they have no conflict of interest to disclose.
CARE Checklist (2016) statement:The authors have read the CARE Checklist (2016),and the manuscript was prepared and revised according to the CARE Checklist (2016).
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Hua-Tuo Zhu 0000-0002-4328-634X;Wen-Guo Chen 0000-0001-6286-1808;Jing-Jie Wang 0000-0002-8491-8649;Fen-Ming Zhang 0000-0002-1460-5196;Guo-Qiang Xu 0000-0003-1337-9120;Hong-Tan Chen 0000-0002-6686-8756.
S-Editor:Luo ML
L-Editor:A
P-Editor:Xu ZH
杂志排行
World Journal of Gastrointestinal Surgery的其它文章
- lndocyanine green: The guide to safer and more effective surgery
- Abdominal cocoon syndrome-a rare culprit behind small bowel ischemia and obstruction: Three case reports
- Link between mutations in ACVRL1 and PLA2G4A genes and chronic intestinal ulcers: A case report and review of literature
- Clinical efficacy and safety of erlotinib combined with chemotherapy in the treatment of advanced pancreatic cancer: A meta-analysis
- Endoscopic-ultrasound-guided biliary drainage with placement of electrocautery-enhanced lumen-apposing metal stent for palliation of malignant biliary obstruction: Updated meta-analysis
- lmpact of frailty on short-term postoperative outcomes in patients undergoing colorectal cancer surgery: A systematic review and meta-analysis
