Endoscopic-ultrasound-guided biliary drainage with placement of electrocautery-enhanced lumen-apposing metal stent for palliation of malignant biliary obstruction: Updated meta-analysis
2024-05-07ZuXiangPengFangFangChenWenTangXuZengHongJuanDuRuXianPiHongMingLiuXiaoXiaoLu
Zu-Xiang Peng,Fang-Fang Chen,Wen Tang,Xu Zeng,Hong-Juan Du,Ru-Xian Pi,Hong-Ming Liu,Xiao-Xiao Lu
Abstract BACKGROUND Endoscopic ultrasound-guided biliary drainage using electrocautery-enhanced (ECE) delivery of lumen-apposing metal stent (LAMS) is gradually being recognized as a viable palliative technique for malignant biliary obstruction after endoscopic retrograde cholangiopancreatography (ERCP) failure.However,most of the studies that have assessed its efficacy and safety were small and heterogeneous.Prior meta-analyses of six or fewer studies that were published 2 years ago were therefore underpowered to yield convincing evidence.AIM To update the efficacy and safety of ECE-LAMS for treatment of biliary obstruction after ERCP failure.METHODS We searched PubMed,EMBASE,and Scopus databases from the inception of the ECE technique to May 13,2022.Primary outcome measure was pooled technical success rate,and secondary outcomes were pooled rates of clinical success,reintervention,and adverse events.Meta-analysis was performed using a randomeffects model following Freeman-Tukey double-arcsine transformation in R software (version 4.1.3).RESULTS Fourteen eligible studies involving 620 participants were ultimately included.The pooled rate of technical success was 96.7%,and clinical success was 91.0%.Adverse events were reported in 17.5% of patients.Overall reintervention rate was 7.3%.Subgroup analyses showed results were generally consistent.CONCLUSION ECE-LAMS has favorable success with acceptable adverse events in relieving biliary obstruction when ERCP is impossible.The consistency of results across most subgroups suggested that this is a generalizable approach.
Key Words: Biliary obstruction;Biliary drainage;Electrocautery-enhanced lumen-apposing metal stents;Endoscopic ultrasound;Endoscopic retrograde cholangiopancreatography failure
lNTRODUCTlON
Endoscopic retrograde cholangiopancreatography (ERCP)-guided biliary drainage is the first-line therapeutic technique for management of patients with distal malignant biliary obstruction[1].However,even though it is performed by experts,its failure rate is up to 15%,especially when patients have gastric outlet obstruction,surgically altered anatomy,tumoral involvement of the papilla,and ampulla obscured by prior stents[2-5].
Previously,surgical bypass or percutaneous transhepatic biliary drainage (PTBD) was considered as an alternative for palliative treatment of malignant biliary obstruction when ERCP failed[6].Although surgical biliary bypass has a high rate of technical success,this approach showed a high rate of major complications and mortality[7,8].PTBD has had a high rate of complication and leads to poor health-related quality of life,and its adverse event rate is up to 60%[9-11].
Endoscopic ultrasound (EUS)-BD has emerged over the past decade as a safer and more effective treatment in patients who failed ERCP.When compared with PTBD,EUS-BD showed a higher rate of clinical success,lower rate of adverse events,and lower reintervention rate[12,13].However,EUS-BD using conventional stent involves multiple procedural steps,which considerably increases the risk of adverse events[14].
A revolutionary stent delivery system,electrocautery-enhanced (ECE) technology,was launched in 2013 for the placement of lumen-apposing metal stents (LAMSs),which simplified the procedure and allowed one-step stent deployment[15].EUS-BD with dedicated ECE-LAMS is gradually being recognized as a viable technique when ERCP is impossible.Nevertheless,ambiguous evidence for its efficacy and safety still hampers adoption of this approach in clinical practice.In an effort to help overcome such ambiguity,we conducted a descriptive meta-analysis in 2021 focusing on studies published before April 2020,which investigated the EUS-guided choledochoduodenostomy using ECE-LAMS for biliary drainage.However,only six studies involving 270 patients were considered eligible for analysis[16].Intriguingly,EUS-BD using ECE-LAMS has been widely performed in several tertiary centers worldwide during the last 2 years.Hence,an updated meta-analysis was warranted to determine further the feasibility and safety of ECE-LAMS for palliation of biliary obstruction when ERCP is impossible.
MATERlALS AND METHODS
Search strategy and selection criteria
This systematic review and meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline[17].We searched PubMed,EMBASE,and Scopus databases from January 1,2012 to May 13,2022,since the introduction of ECE-LAMS in 2013[18].The following keywords were used: “endoscopic ultrasound”,“EUS”,“lumen-apposing metal stent”,“LAMS”,“electrocautery-enhanced”,“electrocautery-enabled”,“biliary drainage”,“transmural drainage”,“biliary obstruction”,“bile duct obstruction”,and “obstructive jaundice”.Searches were restricted to human studies and peer-reviewed studies published in English language journals.The Supplementary material show the details of the search strategy.
Two authors (Peng ZX and Chen FF) independently filtered titles and abstracts of potentially eligible articles following duplicate removal.Case reports,reviews,meta-analyses,letters to the editor,conference abstracts,comments,study protocols,and articles focusing on pancreatic fluid collection drainage using ECE-LAMS were excluded.For overlapping studies,only the most recent publication was included.The inclusion criteria were: (1) EUS-BD involving ECE-LAMS;and (2) Technical success rate reported.We manually retrieved full text for further evaluation.Any discrepancy was discussed with a senior author (Lu XX) in consultation.
Data extraction
Two authors (Peng ZX and Wen T) independently extracted data from each study using a predetermined data extraction sheet,with differences resolved through discussion.Extracted data included: Study characteristics (i.e.,first author,country,publication year,study design,and period of recruitment);study population (i.e.,total analyzed number of patients,patient demographics,etiology,and common bile duct diameter);treatment characteristics (i.e.,stent diameter,and causes of ERCP failure);and outcomes (i.e.,technical success rate,clinical success rate,reintervention rate,and details of adverse events).
Outcomes and definitions
The primary outcome was pooled technical success rate.The secondary outcomes were pooled rates of clinical success,reintervention,and adverse events.In most of the included studies,technical success was defined as accurate deployment of ECE-LAMS between the common bile duct and duodenal wall.However,the definitions of clinical success were variable among the included studies (Supplementary Table 1).Unfortunately,we could not redefine clinical success using a uniform definition in the present study.We thus stipulated the definitions of the clinical success based on the individual studies.Based on the American Society for Gastrointestinal Endoscopy lexicon,the adverse events were classified into three categories: Intraprocedural,postprocedural (up to 14 d),and late (any time after 14 d)[19].
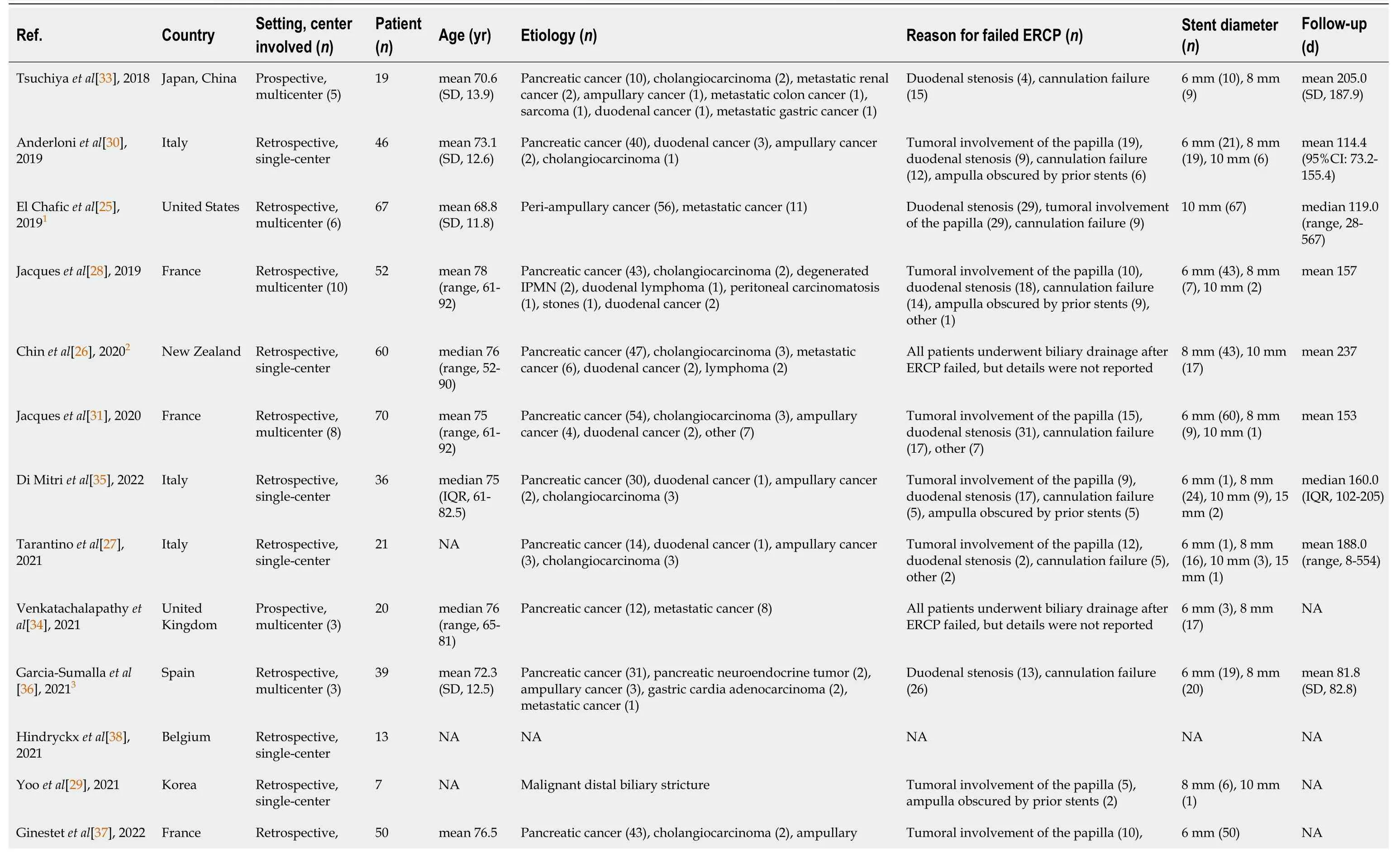
Table 1 Characteristics of studies included in the meta-analysis
Assessment of study quality
Two authors (Chen FF and Wen T) independently evaluated the quality of the included studies using the Methodological Index for Non-randomized Studies (MINORS)[20].Any differences were resolved by discussion.Eight items for noncomparative studies were evaluated: Clearly study aim;inclusion of consecutive patients;prospective data collection;appropriate endpoints;unbiased assessment of study;appropriate follow-up strategy;< 5% loss to follow-up;and calculation of study size.A score of 0 (not reported),1 (reported but inadequate),or 2 (reported and adequate) was provided.Studies with 10-16 and 0-9 points were identified as high and low quality,respectively.
Statistical analysis
We calculated the pooled rate of technical success,clinical success,reintervention,adverse events and its 95% confidence intervals (CIs).Considering that zero-event studies were also included in the present meta-analysis,we transformed the data using the Freeman-Tukey double-arcsine transformation to stabilize variance[21].The random-effects model was used to calculate the pooled rate since the heterogeneity existed in each outcome[22].The heterogeneity between studies was assessed usingτ2andI2statistics[23].TheI2values < 50%,50%-75%,and > 75% were suggestive of low,moderate,and high heterogeneity,respectively[24].We ran subgroup analysis comparing studies of different study quality (lowvshigh),region of origin (Europevsothers),year of publication (before 2021vs2021 onwards),cohort size (> 50vs< 50),and study scale (single-centervsmulticenter).Funnel plot asymmetry and Egger’s test were used to assess publication bias.Statistical analyses were performed using R software (version 4.1.3) andP< 0.05 was considered significant.
RESULTS
Study selection
The initial search from PubMed,Scopus and EMBASE yielded 130,125 and 307 articles,respectively.After removal of duplicates and initial screening of titles and abstracts,29 studies were selected for full-text assessment.Finally,a total of 14 studies were included in the meta-analysis[25-38].The flow diagram of the selection process is shown in Figure 1.
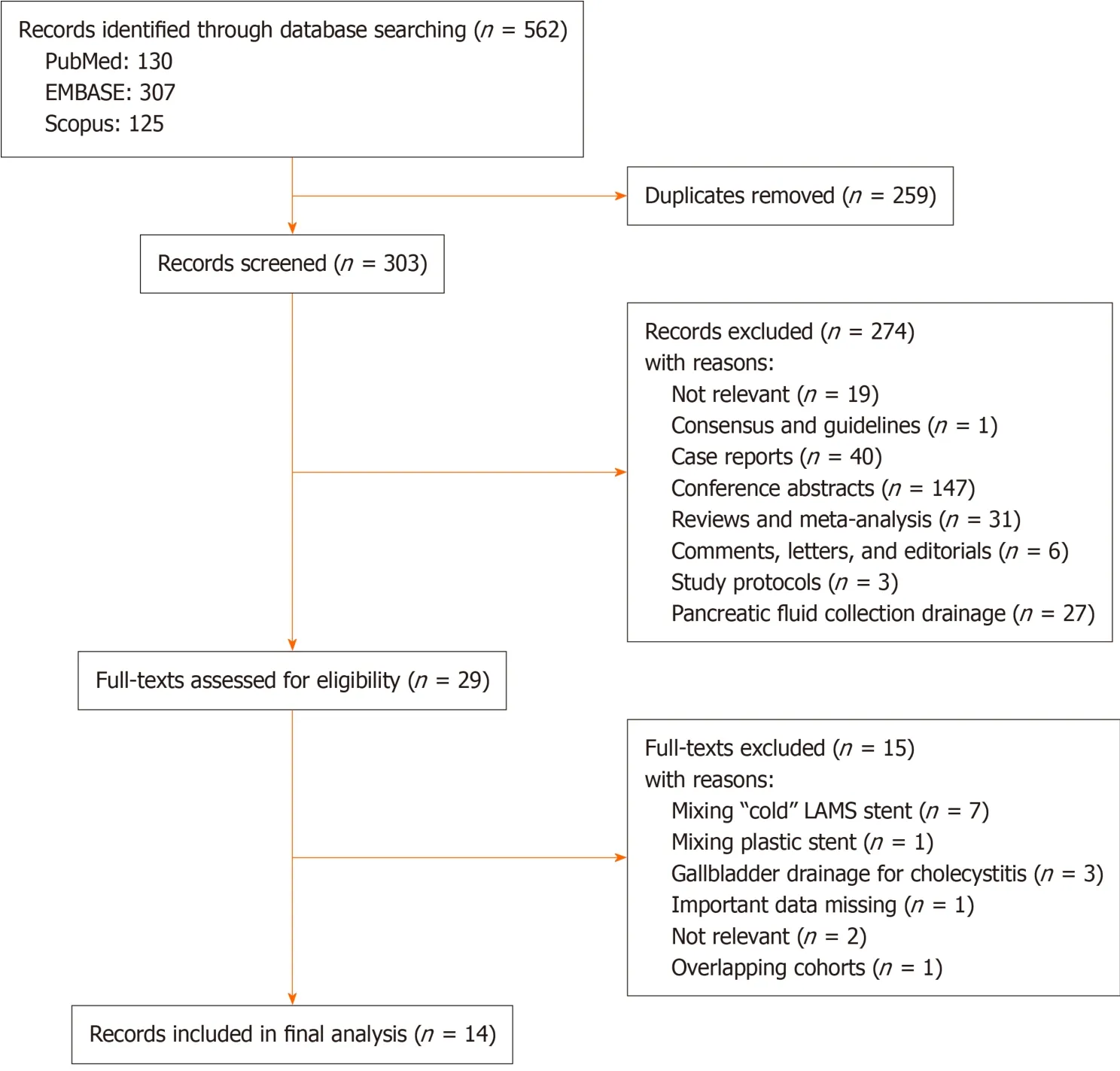
Figure 1 Flow diagram of study selection.
Study characteristics
The present meta-analysis comprised 620 participants (319 male,51.5%).Table 1 summarizes the characteristics of the 14 included studies from tertiary centers in Italy (n=3)[27,30,35],France (n=3)[28,31,37],United Kingdom (n=2)[32,34],Belgium (n=1)[38],Japan and China (n=1)[33],South Korea (n=1)[29],New Zealand (n=1)[26],Spain (n=1)[36] and United States (n=1)[25].Seven studies were multicenter,and seven were single center[25,28,31-34,36].Two studies were prospective and 12 were retrospective cohort studies[33,34].All studies were published from 2018 onwards.
Pancreatic cancer was the most common disease,and the others included cholangiocarcinoma,metastatic cancer and ampullary cancer.One study did not report whether the failed ERCP attempt was prior to EUS-BD[38],and two studiesdid not report the reason for failed ERCP[26,34].Duodenal stenosis was the most common cause for ERCP failure.
In most of the studies,the choice of the stent diameter was at the discretion of the operator.Thirteen studies including 607 cases reported the diameter of the stent in the EUS-BD procedure[25-37]: 6-,8-,10-and 15-mm stents were used in 254 (41.9%),238 (39.3%),111 (18.3%) and three (0.5%) cases,respectively.ECE-LAMS was manufactured by Boston Scientific in 13 studies[25-28,30-38] and Taewoong Medical Corporation in the other study[29].Four studies with 109 cases received routine double stent placement with a pigtail plastic stent[25,26,32,36].
Quality of studies
The results for study quality are shown in Supplementary Table 2.None of the studies reported the cohort size calculation.The median MINORS quality score of the 14 studies was 11,with a range of 6-13.The score was ≥ 10 in nine studies (high quality)[26-28,30-34,36],and < 10 in five (low quality)[25,29,35,37,38].
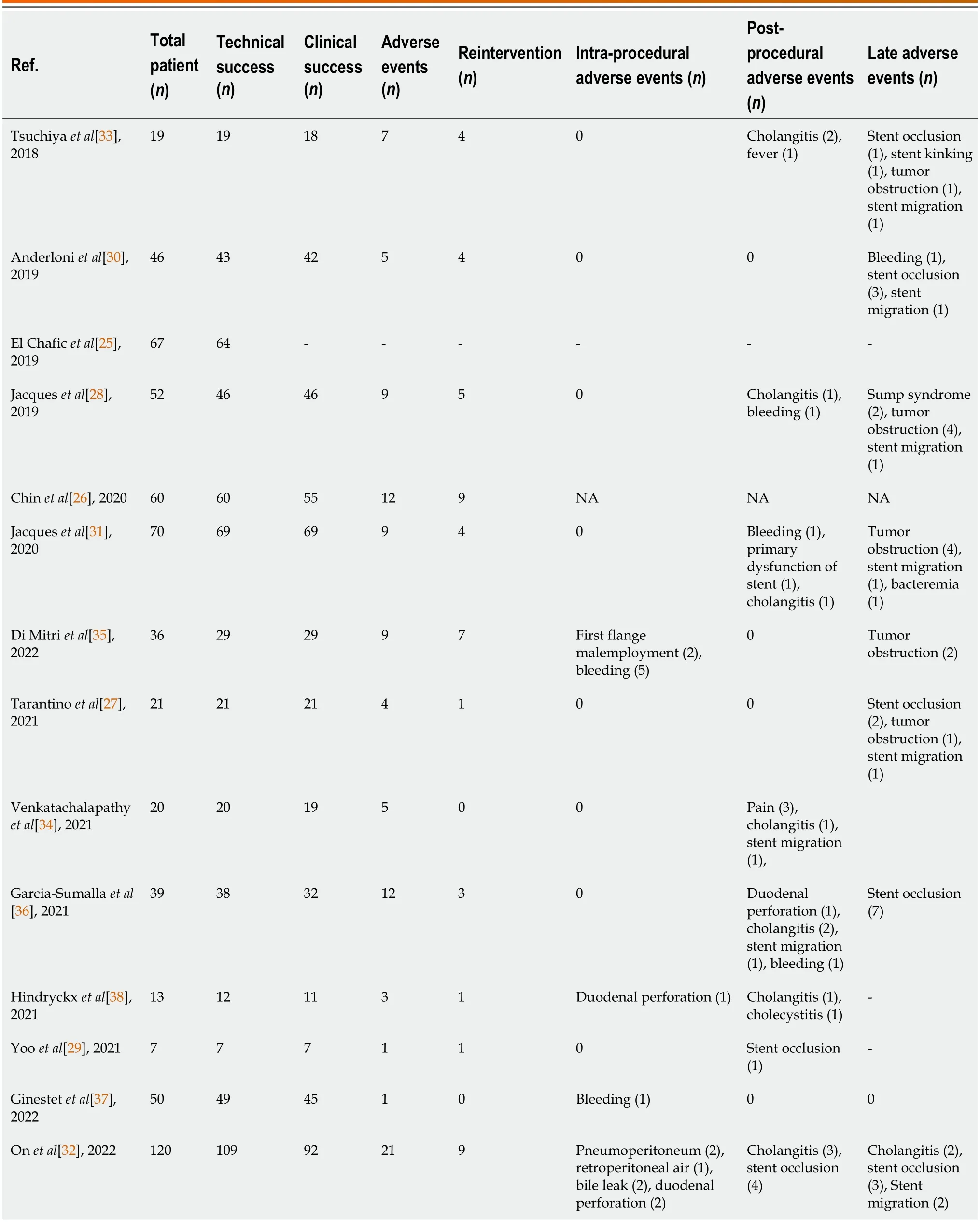
Table 2 Outcomes of included studies in the meta-analysis
Meta-analysis of outcomes
The outcomes of included studies are summarized in Table 2.The pooled rate of technical success was 96.7% (95%CI: 93.5%-98.9%;I2=55%;τ2< 0.01,P< 0.01),which included 620 patients from 14 studies (Figure 2A).Moderate heterogeneity in this outcome was observed.We carried out subgroup analyses focusing on the region of origin,size of study,year of publication,and study scale (single center or multicenter) and found that results were generally consistent across subgroups (Table 3;Supplementary Figure 1).The funnel plot showed no asymmetry.No significant publication bias assessed by Egger’s test (P=0.55) was observed (Figure 3A).
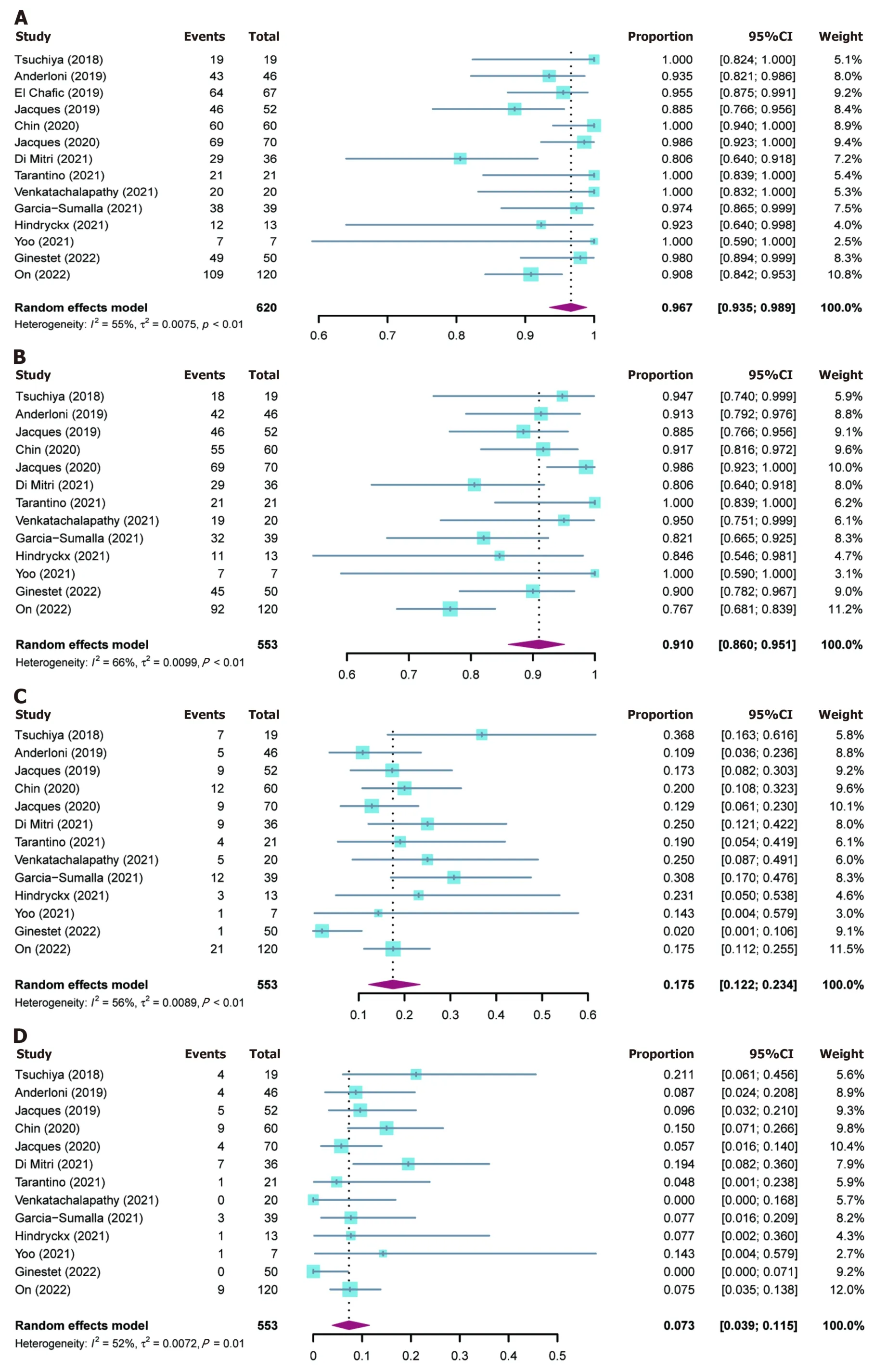
Figure 2 Forest plots for outcomes. A: Technical success rate;B: Clinical success rate;C: Adverse events rate;D: Reintervention rate.CI: Confidence interval.
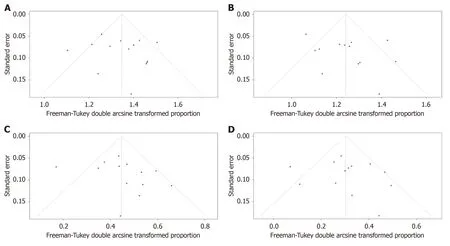
Figure 3 Funnel plots to evaluate publication bias. A: Studies of technical success rate;B: Studies of clinical success rate;C: Studies of adverse events rate;D: Studies of reintervention rate.
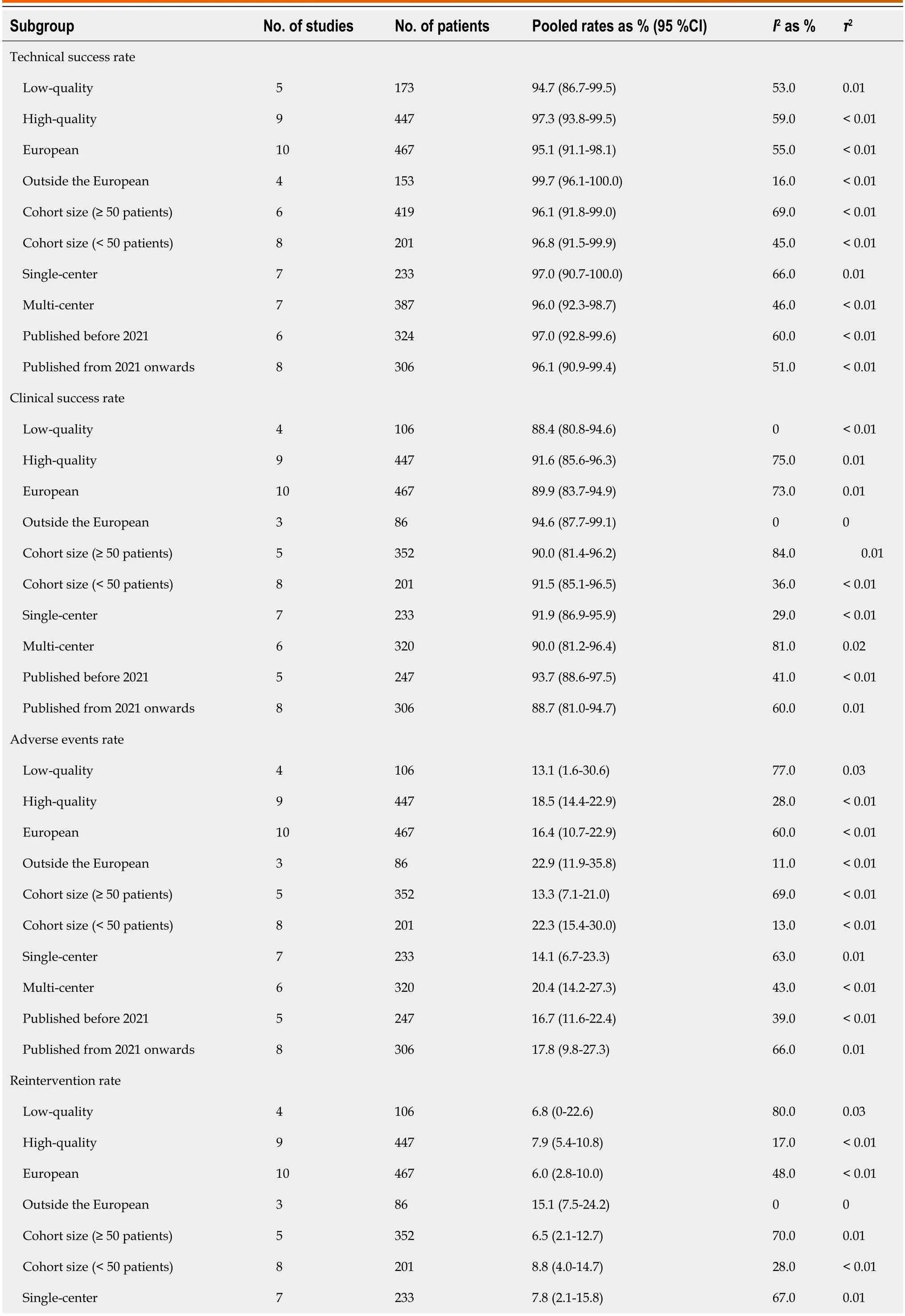
Table 3 Subgroup analysis
Thirteen studies reported clinical success rates[26-38].The remaining study was excluded due to a high rate of loss to follow-up (35.8%)[25].The pooled clinical success rate was 91.0% (95%CI: 86.0%-95.1%;I2=66%;τ2< 0.01,P< 0.01) (Figure 2B).Similarly,we did encounter moderate heterogeneity in this outcome and we were unable to explain the heterogeneity by subgroup analysis (Table 3;Supplementary Figure 2).Furthermore,no significant publication bias was observed (Figure 3B).
As mentioned above,one study was excluded from the analysis of adverse event rates[25].The pooled rate of adverse events was 17.5% (95%CI: 12.2%-23.4%;I2=56%;τ2< 0.01,P< 0.01;Egger’s test for bias,P=0.27) (Figures 2C and 3C).The most common intraprocedural,postprocedural,and late adverse events were bleeding,cholangitis,and stent occlusion,respectively (Table 2).
The pooled reintervention rate was 7.3% (95%CI: 3.9%-11.5%) (Figure 2D),which included 553 patients from 13 studies (I2=52%;τ2< 0.01,P< 0.01;Egger’s test for bias,P=0.59) (Figure 3D).Results of subgroup analyses on the rates of adverse events and reintervention are given in Table 3 and Supplementary Figures 3 and 4.
DlSCUSSlON
This meta-analysis pooled data from 14 eligible studies involving 620 patients with biliary obstruction,and we analyzed the pooled rates of technical success,clinical success,adverse events,and reintervention for ECE-LAMS for palliation of biliary obstruction.This updated meta-analysis had more than twice as many patients as previous analyses.
EUS-BD with dedicated ECE-LAMS is a viable approach after ERCP failure and has potential for reproducible and generalized clinical application.For instance,a recent meta-analysis of five studies with 201 patients revealed a 93.8% technical and 88.8% clinical success rate for EUS-BD using ECE-LAMS for biliary drainage[39].Our former meta-analysis (6 studies,270 participants) also published in 2020 demonstrated comparable rates[16].Furthermore,this updated metaanalysis consistently indicated similar pooled rates of technical success (96.7%) and clinical efficacy (91.0%).Although ECE-LAMS is being used in increasingly more medical centers worldwide,its reproducibility seemed not to be compromised,as evident by the consistent efficacy outcomes among subgroups (Supplementary Figures 1E and 2E).Moreover,the electrocautery system appears to be easy for training endoscopists.One recent study indicated that ECE-LAMS is even successful when used by less-experienced endoscopists,probably owing to strict compliance with the manufacturer’s recommended protocol[28].
ECE-LAMS is also a safe technique.The low incidence of adverse events makes the ECE delivery system an ideal device for LAMS,with a pooled rate of only 17.5% in this meta-analysis,which is comparable to results reported in other studies (13.6%-17.1%)[16,40,41].To reduce the adverse event rate of EUS-BD,accumulation of endoscopic experience is usually advocated since the procedures are highly operator dependent[42-44].However,the adverse event rate of the ECE-LAMS procedures performed by nonexperts was similar to that of experts[32].The similar satisfactory success rate for expert and nonexpert operators[28] suggests that the ECE-LAMS technique is reproducible and generalizable.
Many attempts have been made to reduce the incidence of adverse events in ECE-LAMS placement.Anchoring a coaxial double-pigtail plastic stent (DPS) through ECE-LAMS may prevent stent migration.Several recent studies have found that placement of a DPS within the ECE-LAMS is superior to ECE-LAMS alone in reducing the reintervention rate[25,26,32].In this meta-analysis,the pooled reintervention rate was 7.3% (95%CI: 3.9%-11.5%).However,a retrospective study of 41 participants from three Spanish tertiary centers demonstrated no significant difference in reintervention rates between DPS within ECE-LAMS and ECE-LAMS alone (9.1%vs5.8%,P=0.99)[36].Thus,the potential of positioning a DPS through ECE-LAMS for reducing the need for reintervention should be evaluated further in large-scale prospective studies,such as the ongoing BAMPI trial initiated in November 2020[45].
ECE-LAMS sizes ranging from 6 mm to 8 mm in diameter were used in about 80% of cases in the present metaanalysis.It should be noted that small stent diameter is more likely to be associated with increased rates of specific adverse events (e.g.,stent lumen occlusion because of sludge accumulation and food waste)[32,33].These adverse events relating to stent diameter could be prevented by the use of a larger-bore stent but may at the expense of technical success[32].Hence,choosing an optimal stent size necessitates considering the balance between the reduction in risk of adverse events and superior technical success.
EUS-BD using ECE-LAMS has been previously considered as a palliative treatment,yet now this is being recognized as a bridge to surgery approach.Several recent studies have provided evidence that ECE-LAMS as a bridge to elective surgery (e.g.,pancreatoduodenectomy,PD) in malignant biliary obstruction is feasible and safe[32,46].Considering influences of preoperative BD on the eventual surgical removal of the obstruction,we suggest that: (1) ECE-LAMS deployment from the duodenal bulb to the common bile duct is preferable to stent placement between the stomach and left intrahepatic bile duct,given the extent of PD;(2) Insofar as possible postprocedural cholangitis should be avoided,because it is an independent risk factor for mortality following PD[47];and (3) Treatment strategy involving multidisciplinary consultation is recommended.
The present study had several limitations.The lack of a uniform definition for clinical success is a potential contributor to study heterogeneity although the heterogeneity between the included studies was moderate in this meta-analysis.We were not able to identify sources of heterogeneity by conducting subgroup analyses because of the original study design (i.e.,incidence study).Additionally,most of the included studies were retrospective,which may have caused selection bias.A final limitation of this study was that all the included studies were drawn from tertiary referral centers,which may preclude generalization of the results to patients admitted to local hospitals or lower-level facilities.Notwithstanding these limitations,this work had important strengths.This was a more updated meta-analysis including 620 patients with the inclusion of more recent studies than the previous meta-analyses (14vs5 studies),even with the use of more stringent inclusion and exclusion criteria[39].
CONCLUSlON
The ECE-LAMS is an effective and safe approach for patients who bear biliary obstruction when ERCP is impossible.This approach could be generalizable and extensible to the practitioners in this field.Therefore,this should be acknowledged as a standard core component of management of malignant biliary obstruction.Large and prospective observational studies are needed to further investigate and confirm these findings.Further investigations regarding the use of ECELAMS as the first-line intervention for biliary drainage or the “bridge to surgery” approach should be performed to invigorate more practical applications with hopefully favorable outcomes.
ARTlCLE HlGHLlGHTS
Research background
Endoscopic-ultrasound-guided biliary drainage (EUS-BD) with electrocautery-enhanced lumen-apposing metal stent (ECE-LAMS) has recently been reported as an alternative treatment approach for malignant biliary obstruction after endoscopic retrograde cholangiopancreatography (ERCP) failure.
Research motivation
In 2021,we conducted a meta-analysis focusing on the EUS-guided choledochoduodenostomy with ECE-LAMS for BD.However,only six studies involving 270 patients were included in the analyses.Intriguingly,studies regarding EUS-BD using ECE-LAMS have been widely performed in the last 2 years.Hence,an updated meta-analysis was warranted to determine further the feasibility and safety of ECE-LAMS for palliation of biliary obstruction when ERCP is impossible.
Research objectives
To evaluate the efficacy and safety of EUS-BD with ECE-LAMS for treatment of biliary obstruction after ERCP failure.
Research methods
We searched PubMed,EMBASE and Scopus databases from January 1,2012 to May 13,2022.The following search keywords were used: “endoscopic ultrasound”,“EUS”,“lumen-apposing metal stent”,“LAMS”,“electrocauteryenhanced”,“electrocautery-enabled”,“biliary drainage”,“transmural drainage”,“biliary obstruction”,“bile duct obstruction”,and “obstructive jaundice”.The primary outcome of our study was pooled technical success rate.The secondary outcomes were pooled rates of clinical success,reintervention,and adverse events.We ran subgroup analysis comparing studies of different study quality (lowvshigh),region of origin (Europevsothers),year of publication (before 2021vs2021 onwards),cohort size (> 50vs< 50),and study scale (single centervsmulticenter).Funnel plot asymmetry and Egger’s test were used to assess the publication bias.Meta-analysis was performed using a random-effects model following Freeman-Tukey double-arcsine transformation in R software.
Research results
Fourteen studies involving 620 participants were included in the analysis.The pooled rate of technical success was 96.7%,and clinical success was 91.0%.Adverse events were reported in 17.5% of patients.Overall reintervention rate was 7.3%.Subgroup analyses showed results were generally consistent.
Research conclusions
EUS-BD using ECE-LAMS is an effective and safe approach for patients with biliary obstruction when ERCP is impossible.This approach could be generalizable to practitioners in this field.
Research perspectives
The present meta-analysis adopted strict inclusion and exclusion criteria to ensure appropriate methodological quality to evaluate the efficacy and safety of EUS-BD using ECE-LAMS.Large and prospective observational studies are needed to further investigate and confirm our findings.
FOOTNOTES
Co-first authors:Zu-Xiang Peng and Fang-Fang Chen.
Co-corresponding authors:Hong-Ming Liu and Xiao-Xiao Lu.
Author contributions:Peng ZX,Chen FF,Tang W,Liu HM,and Lu XX contributed to the conceptualization of the study;Peng ZX,Chen FF,Tang W,Zeng X,Du HJ,Pi RX,Liu HM,and Lu XX were involved in the data curation;Peng ZX,Chen FF,Tang W,and Zeng X participated in the formal analysis;Peng ZX,Chen FF,Tang W,Du HJ,and Lu XX contributed in the investigation of the manuscript;Peng ZX was involved in the validation of this article;Peng ZX and Chen FF contributed to the visualization;Peng ZX,Chen FF,Tang W,Liu HM,and Lu XX wrote the original draft;Tang W and Lu XX participated in the methodology;Liu HM and Lu XX contributed to the supervision,project administration,and writing review and editing.Peng ZX and Chen FF contributed equally to this work as co-first authors.The research was performed as a collaborative effort,and the designation of co-first authors authorship accurately reflects the distribution of responsibilities and burdens associated with the time and effort required to complete the study and the resultant paper.This also ensures effective communication and management of post-submission matters,ultimately enhancing the paper's quality and reliability.Peng ZX and Chen FF contributed efforts of equal substance throughout the research process.The choice of these researchers as co-first authors acknowledges and respects this equal contribution,while recognizing the spirit of teamwork and collaboration of this study.Lu XX and Liu HM have made equal contributions to this work as co-corresponding authors.It has been decided to designate Lu XX and Liu HM as co-corresponding authors for two main reasons.First,the co-corresponding authors from the research team possess diverse expertise and skills from different fields,and their designation best reflects this diversity.It also facilitates the most comprehensive and in-depth exploration of the research topic,ultimately enriching readers’ understanding by providing various expert perspectives.Second,Lu XX and Liu HM have made substantial and equal contributions throughout the research process.Selecting these researchers as co-corresponding authors acknowledges and respects their equal contributions,showcasing the collaborative and teamwork spirit within this study.We believe that designating Lu XX and Liu HM as co-corresponding authors is fitting for our manuscript as it accurately reflects the collaborative spirit,equal contributions,and diversity within our team.
Conflict-of-interest statement:All the authors report having no relevant conflicts of interest for this article.
PRlSMA 2009 Checklist statement:The authors have read the PRISMA 2009 Checklist,and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Zu-Xiang Peng 0000-0003-3361-0035;Hong-Ming Liu 0000-0001-5800-6307;Xiao-Xiao Lu 0009-0003-8244-6662.
S-Editor:Wang JJ
L-Editor:Filipodia
P-Editor:Xu ZH
杂志排行
World Journal of Gastrointestinal Surgery的其它文章
- lndocyanine green: The guide to safer and more effective surgery
- Endoscopic ultrasound-guided lauromacrogol injection for treatment of colorectal cavernous hemangioma: Two case reports
- Abdominal cocoon syndrome-a rare culprit behind small bowel ischemia and obstruction: Three case reports
- Link between mutations in ACVRL1 and PLA2G4A genes and chronic intestinal ulcers: A case report and review of literature
- Clinical efficacy and safety of erlotinib combined with chemotherapy in the treatment of advanced pancreatic cancer: A meta-analysis
- lmpact of frailty on short-term postoperative outcomes in patients undergoing colorectal cancer surgery: A systematic review and meta-analysis
