Clinical characteristics of patients with early-and late-onset optic neuromyelitis optica spectrum disease
2024-04-04LIFeiLIUTingYangYihaoLINHuixiaTONGjingyiLIZongjunLIANGBinjiLIQifu
LI Fei, LIU Ting, Yang Yi-hao, LIN Hui-xia, TONG jing-yi, LI Zong-jun, LIANG Bin-ji, LI Qi-fu✉
1. Department of Neurology, the First Affiliated Hospital of Hainan Medical College, Haikou 570102, China
2. Key Laboratory of Tropical Brain Science Research and Translation in Hainan Province, Haikou 570102, China
Keywords:
ABSTRACT Objective: To analyze the different clinical features of patients with early-onset (EO-NMOSDs)and late-onset neuromyelitis optica spectrum diseases (LO-NMOSDs).Methods: A total of 51patients with neuromyelitis optica spectrum disease who were diagnosed in our hospital for the first time from January 2015 to December 2022 were included in the First Affiliated Hospital of Hainan Medical College and divided into 22 cases in the EO-NMOSDs group and 29 cases in the LO-NMOSDs group according to whether the age of onset was 50 years old.The basic data, Extended Disability Status Scale (EDSS) score, blood and cerebrospinal fluid test indicators of the two groups were statistically analyzed.Results: There were no significant differences in demographic characteristics, clinical features and serum AQP-4 antibody positivity rate between the two groups (all P>0.05), and there were significant differences in triglycerides (TG), low-density lipoprotein (LDL), apolipoprotein A (APOA),apolipoprotein B (APOB) and lipoprotein a (P=0.010, P=0.048, P=0.014, P=0.061, P=0.001,respectively), and cerebrospinal fluid LDH, There were significant differences between microprotein quantification and EDSS score (P=0.018, P=0.034, P=0.025, respectively), and the level of microprotein quantification in cerebrospinal fluid of LO-NMOSDs had a certain correlation with the degree of disability (r=0.52, P<0.03).Conclusion: LO-NMOSDs and EONMOSDs group patients have similar demographic characteristics, serum AQP-4 antibody positive rate and clinical features, but compared with EO-NMOSDs, patients in LO-NMOSDs group are prone to abnormal lipid metabolism, higher trace proteins in cerebrospinal fluid and more likely to be disabled, and among LO-NMOSDs, the higher the trace protein in the cerebrospinal fluid, the more severe the disability status of patients.
1.Introduction
Neuromyelitis Optic Neuritis Spectrum Disorders (NMOSDs)are antibody-mediated inflammatory demyelinating autoimmune diseases involving the central nervous system, characterized by recurrent Optic Neuritis (ON) and Longitudinally Extensive Transverse Myelitis (LETM).NMOSDs are an immunologic disease with a high rate of recurrence and disability[1].Previous studies have suggested that NMOSDs are prevalent in young women[2, 3], but with the improvement of diagnostic methods for the elderly, the diagnosis of the disease is gradually increasing in the elderly[4].AQP-4 antibody titers have been found to be higher in older patients, but there has not been an associated increase in diagnosis, suggesting that there are more unrecognized cases in this population[5].Elderly people are prone to a diagnostic lag due to the relatively high number of underlying diseases, which can lead to a higher burden of disease due to the lack of timely and more effective treatment[4].Therefore, patients in the LO-NMOSDs group have a more insidious onset of disease, and clinicians should be more vigilant in the diagnosis of patients with late-onset disease.
This study was conducted by comparing the basic data, Expanded Disability Status Scale (EDSS) scores, and blood and cerebrospinal fluid laboratory index characteristics of patients with EO-NMOSDs and LO-NMOSDs.The correlation between age and clinical characteristics was analyzed and further explored whether there were age-related clinical factors that collectively influenced the patients’ disability status.This will provide a further basis for evaluating patients’ judgments of disease progression, as well as early intervention treatment to minimize the occurrence of severe disabling conditions when possible.
2.Information and Methods
2.1 Study subjects
Patients who were first diagnosed with NMOSDs from January 2016 to December 2022 in the First Affiliated Hospital of Hainan Medical College were included.Inclusion criteria: (1) Compliance with the International Consensus Diagnostic Criteria for NMOSDs proposed in 2015[6]; using 50 years of age as the grouping criterion,patients with the age of first onset <50 years of age were included in the early-onset NMOSDs group, and patients with the age of first onset 50 years of age were included in the early-onset NMOSDs group.(2) First onset.(3) Complete clinical data.Exclusion criteria:(1) Accompanied by other autoimmune diseases.(2) Patients who had been treated with hormones and immunosuppressants before admission.(3) Combined with other causes of vision loss ophthalmologic related diseases.
2.2 Research Methods
2.2.1 Collection of clinical data
General clinical data of patients previously hospitalized in our hospital for NMOSDs were collected, including age, first symptom,gender, EDSS, BMI, whether it was accompanied by self-immune antibody positivity, whether it was physically fit or not in the past,indexes of lipid levels in peripheral blood, indexes of cerebrospinal fluid biochemistry and positivity rate of serum AQP-4 antibody.Recently, 3-5 mL of peripheral venous blood was drawn in the morning on an empty stomach, and analyzed by Roche Cobas automatic analyzer, and the enzyme colorimetric method was used to detect high-density lipoprotein (HDL), serum total cholesterol(TC) and low-density lipoprotein (LDL).density lipoprotein (LDL);Apolipoprotein A (Apo A) and Apolipoprotein B (Apo B) by immunoturbidimetric assay; Triglyceride (TG) by colorimetric assay;and Triglyceride (TG) by ELISA or cell-based assay.ELISA or Cell Based Assay (CBA) was used to determine serum anti aquaporin 4 (AQP4) antibody; and 2 mL of cerebrospinal fluid (CSF) was collected by lumbar puncture prior to immunotherapy after admission, and cerebrospinal fluid (WBC) was analyzed by resistive antimicrobial assay, cerebrospinal fluid (CL) was analyzed by ionselective electrode assay, cerebrospinal fluid (LDH) was analyzed by enzyme rate assay, and cerebrospinal fluid (CSF) was analyzed by enzyme rate assay.Cerebrospinal fluid WBC was analyzed by resistance resistance method, cerebrospinal fluid CL by ion-selective electrode method, cerebrospinal fluid LDH by enzyme rate method,and cerebrospinal fluid Mill-Total Protein (MTP) by turbidimetric method of sodium sulfosalicyl sulfate.
2.2.2 Statistical methods
SPSS 25.0 was used for statistical studies, and Graphpad Prism 9 software was used for graphing.Quantitative data were first tested for normality.Data with the attributes of normal distribution were expressed as (±s) and data without the attributes of normal distribution were expressed as median (first quartile, third quartile).The nonparametric Mann-Whitney U test was used for data that did not have the properties of normal distribution, and the independent samples t test was used for data that had the properties of normal distribution.The number of cases (percentage) was used to express the count data, and the chi-square test or exact probability method was used to compare the data between the two groups in this experiment.Pearson correlation analysis was used to analyze the data with the attributes of normal distribution, and Spearman rank correlation was used to analyze the data without the attributes of normal distribution.P<0.05 was considered as the statistically significant difference (two-tailed).
3.Results
3.1 General information
A total of 51 patients with NMOSDs who were hospitalized in the Department of Neurology of the First Affiliated Hospital of Hainan Medical College due to NMOSDs and met the enrollment criteria of the present study were retrospectively collected from January 2015 to December 2022, of whom a total of 22 patients were eligible for the early-onset group, with 6 male patients, 16 female patients,aged 4-46 years, and with an average age of (31±11) years.There were 29 patients in the late-onset group, of which 2 were male and 27 were female, aged 50-75 years, with an average age of (50±7)years.There were no statistically significant differences between the patients in the EO-NMOSDs group and the LO-NMOSDs group in terms of BMI, whether they suffered from the underlying diseases,and the positivity rate of autoimmune antibodies (P>0.05 for all of them), suggesting that the two groups of patients were comparable.The first clinical manifestations in the EO-NMOSDs group were myelitis in 54.54% (12/22), isolated optic neuritis in 22.72% (5/22),optic neuritis combined with myelitis in 13.6% (3/22), posterior pole syndrome in 4.5% (1/22), and brainstem syndrome in 4.5% (1/22),while in the LO-NMOSDs group The first clinical manifestations were myelitis in 58.62% (17/29), isolated optic neuritis in 20.68%(6/29), optic neuritis combined with myelitis in 17.24% (5/29), and polar posterior region syndrome in 3.4% (1/29), and there was no statistically significant difference in the first symptoms and signs among the patients in the two groups (Fisher’s exact method of probability:P= 0.918, Table 1).Patients in the EO-NMOSDs group had an EDSS score of 1-4 and a median score of 1.5 (1, 1.5) on admission; patients in the LO-NMOSDs group had an EDSS score of 1-8 and a median score of 1.5 (1.5, 2) on admission; patients in the LO-MOSDs group had higher EDSS scores than those in the EO-NMOSDs group on admission (P = 0.025),see Table 1.
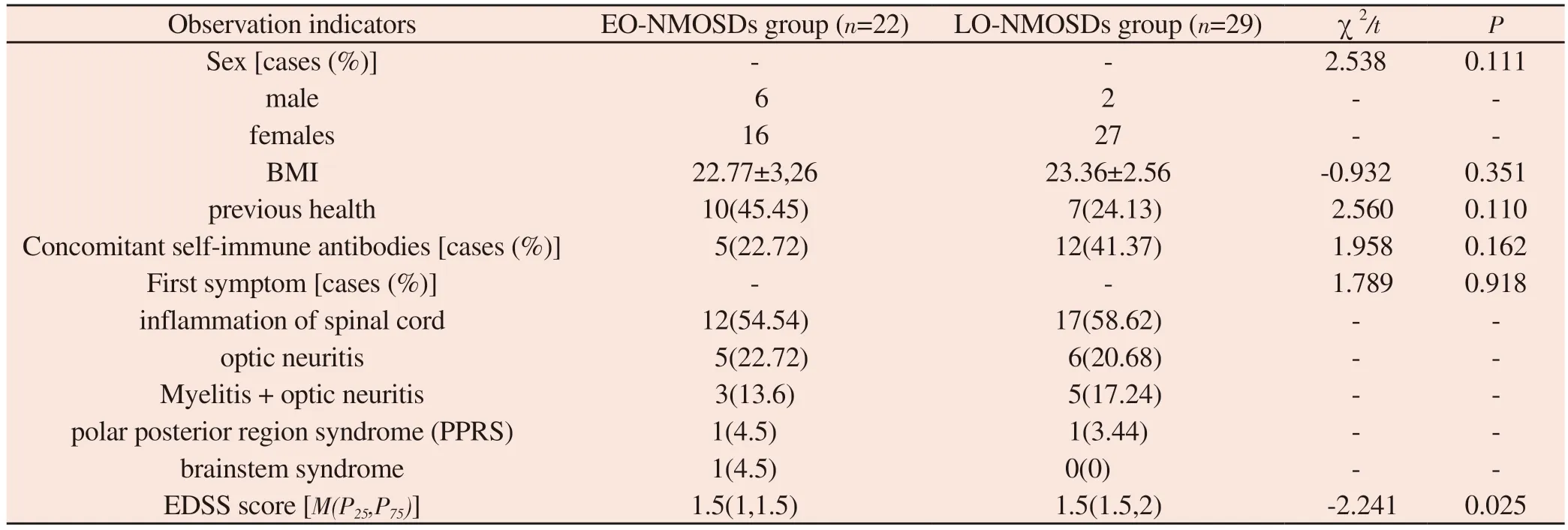
Tab 1 Comparison of clinical data of patients with EO-NMOSDs and LO-NMOSDs
3.2 Comparison of various laboratory indices
Comparison of lipid indices between patients with EO-NMOSDs and LO-NMOSDs suggested that there was a statistically significant difference between the two groups in TG, LDL, APOA, APOB, and lipoprotein a (P=0.010,P=0.048,P=0.014,P=0.061, andP=0.001,respectively).And there was no statistically significant difference between the two groups in TC and HDL (P>0.05) (see Table 2).Further comparison of serum AQP-4 antibody positivity between the two groups revealed no statistical difference between the two groups(P > 0.05).(See Table 3.) And in comparing the cerebrospinal fluid indexes between patients with EO-NMOSDs and LO-NMOSDs,it was found that there were statistically significant differences in cerebrospinal fluid LDH and cerebrospinal fluid microprotein quantification (MTP) between the two groups (P=0.018, P=0.034,respectively); whereas, there were no statistically significant differences between the two groups in cerebrospinal fluid WBC,cerebrospinal fluid GLU, and cerebrospinal fluid CL (P>0.05)(See Table 4.) Subsequently, we found no statistically significant differences between the two groups by the serum AQP-4 antibody positivity rate of the two groups (P> 0.05).are shown in Table 4.Subsequently, we concluded by Spearman’s rank correlation analysis that there was a positive correlation between cerebrospinal fluid micro-protein quantification and EDSS score.(r=0.52, P<0.03)Figure 1.

Tab 2 Comparison of lipid indices in patients with EO-NMOSDs and LONMOSDs

Fig 1 Correlation analysis between cerebrospinal fluid micro-protein quantification and EDSS score
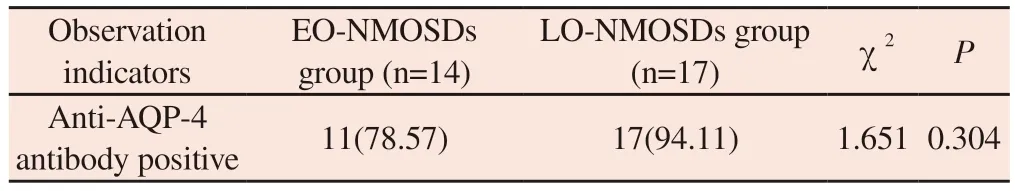
Tab 3 Comparison of serum AQP-4 antibody positivity indexes in patients with EO-NMOSDs and LO-NMOSDs

Tab 4 Comparison of cerebrospinal fluid indices in patients with EO-NMOSDs and LO-NMOSDs
4.Discussion
Optic neuromyelitis optica spectrum disorders (NMOSDs)are inflammatory diseases of the central nervous system, the pathogenesis of which is mainly related to AQP-4 antibodies.NMOSDs are characterized by high recurrence and disability rates,so early recognition and early intervention are particularly important.The disease is more common in young and middle-aged people, and there are more females than males[7].Some studies have shown that the overall recurrence rate of the disease is between 60% and 98%[8].Each recurrence may lead to severe visual and motor impairment,as well as a heavy burden for patients and their families later in life,reducing the quality of life[9].Some studies have shown that age is an important risk factor for visual and motor impairment in patients[10-12].However, it does not exclude the reason that elderly patients themselves have more underlying diseases[13].We hope to further investigate age-related risk factors leading to patient dysfunction by analyzing clinical case data.
General clinical information (1) Sex differences: according to the latest worldwide epidemiologic studies, it was confirmed that there are sex differences in the disease and that it is more prevalent in females, which is consistent with this study[2].In a study on the disease in women and patients of childbearing age, it was shown that it may be due to hormonal differences between men and women that cause women to have a susceptibility to the disease.In AQP-4 positive patients, the ratio of AQP-4 positivity in females to males was as high as 8-9:1, which increased the prevalence of NMOSDs in females[4, 14].(2) Age: In the past, we thought that NMOSDs were prevalent in young people, but in recent years, the prevalence of the disease has gradually increased in the elderly, and some studies have suggested that it may be due to the decrease in mortality rate in the elderly, which increases the number of confirmed diagnoses[2].It has also been suggested that the long interval between the onset of symptoms of optic neuritis and spondylitis in the early late-onset group of patients predisposes to a diagnostic lag[15].In the present study, we found a high proportion of elderly patients among patients enrolled in the same period, which is at variance with the latest epidemiologic studies, and we consider that this may be attributable to geographical aspects.(3) Geographic aspect A study found that people from Asian, African, Arab and Latin American backgrounds are susceptible to the disease, and this study considered that there might be a geographic factor in the development of NMOSDs [16,17].Bukhari[18] et al.in 2017, did a study on whether the incidence of the disease is influenced by environmental factors confirmed that in lower latitudes there is indeed an phenomenon of increased incidence[19].Hainan is located in the southernmost part of China and at low latitudes, and no increase in prevalence was found from the comparison of the number of existing cases in our hospital with the number of cases in other parts of China[20].However, the ratio of the EO-NMOSDs group to the LO-NMOSDs group in this study was not consistent with a cohort study on the disease in China in 2015[7].In this study, the prevalence was found to be higher in the elderly than in the young, so we suspect that the increased prevalence in the elderly may be related to geographical differences.(4) First symptom: Regarding the presentation of first symptom in patients of different age groups, some researchers believe that elderly people tend to start with acute myelitis, while young people start with optic neuritis[21].However, there are also findings suggesting that there is not much difference in the form of first symptoms between the EONMOSDs and LO-NMOSDs groups[10].In the present study 54.54%of patients in the EO-NMOSDs group and 58.62% of patients in the LO-NMOSDs group started with acute myelitis, and 22.72%of patients in the EO-NMOSDs group and 20.68% of patients in the LO-NMOSDs started with acute optic neuritis in comparable proportions, which is different from a portion of the previous studies,probably because the present study was conducted for neurologic patients only and the missing case data of patients attending ophthalmology department in our hospital caused bias in the results.(5) EDSS scores: some studies have shown that visual and motor deficits are more likely to be present in patients with advanced age of onset[22, 23], and the investigators considered the possibility that patients in the LO-NMOSDs group may have a reduced immune tolerance and be susceptible to comorbidities with other disorders[10],which may lead to dysfunctionality, but the study did not perform further correlation analysis.In the present study, we also obtained the same conclusion that patients in the LO-NMOSDs group were more likely to suffer from severe dysfunction (P<0.025), and we further investigated cerebrospinal fluid MTP on the basis of age, and we found that there was a positive correlation between EDSS scores and cerebrospinal fluid MTP in patients in the LO-NMOSDs group(r=0.52, P<0.03).
NMOSDs, as a neurologic immune disease, have been known to cause alterations in lipid metabolism when they initiate immune inflammation[24].It has been shown that significant abnormalities in lipid metabolism have been found in cerebrospinal fluid antibody-positive NMOSD patients with multiple sclerosis, and such abnormalities considered may be related to altered metabolic pathways as a result of demyelination, CNS inflammation, etc.[25].Some researchers have even found that certain changes in lipid levels can distinguish the damage in the corresponding areas, for example,Wang Jinyang et al.found that among the lipid-related indicators,serum Apo A1 has the role of distinguishing between different lesion involvement sites in NMOSD patients[26].And further study found that low HDL-C and high TG levels were correlated with disease activity and disability in NMOSDs[27].And through the present study, we observed statistically significant differences in TG, LDL, APOA, APOB and lipoprotein a between the two groups in EO-NMOSDs compared to LO-NMOSDs, but it could not be ruled out that the elderly population has its own predisposition to abnormal lipid metabolism.It has been reported that high levels of TG are positively associated with disabling status and recurrence of NMOSDs compared to patients with NMOSDs with normal levels of TG[28].However, the results of the present study did not find a correlation between lipid alterations and patients’ disabling conditions, which is different from previous studies, probably because of the small sample size of the present study.Since the focus of data collection in this study differed from previous studies, a specific study to analyze whether the site of patient involvement was metabolized with dyslipidemia could not be performed.
The Blood Brain Barrier (BBB) stabilizes the environment of the central nervous system (CNS) by preventing serum proteins,microorganisms, and fatty acids from entering the CNS.Albumin is produced only outside the CNS and does not normally cross the blood-brain barrier and enter the CNS.If albumin in the circulatory system instead enters the cerebrospinal fluid, it suggests that the blood-brain barrier is disrupted when inflammation occurs in the CNS[29].Some researchers have suggested that increased CSF protein is a result of blood-brain barrier disruption[30].Whereas cerebrospinal fluid MTP is normally present in the cerebrospinal fluid in very small amounts, it is only when there is inflammation in the tissues and organs that MTP increases accordingly[31].In the present study, we further investigated the cerebrospinal fluidrelated indexes between the two groups and found that there were significant differences in cerebrospinal fluid Lactic dehydrogenase(LDH) and cerebrospinal fluid MTP between the two groups, and that there was a positive correlation between MTP and EDSS scores only in the late-hair group, and no such correlation was found in the early-hair group, which may be a reflection of the fact that the higher the cerebrospinal fluid protein, the higher the cerebrospinal fluid protein, the higher the cerebrospinal fluid MTP, and the higher the cerebrospinal fluid MTP in the early-hair group.This may be a more detailed addition to the studies that higher cerebrospinal fluid protein is associated with more severe dyskinesia[32, 33].
In conclusion, patients in the late-onset group had higher EDSS scores and more severe movement disorders on admission than patients in the early-onset group, with significant differences in cerebrospinal fluid MTP between the two groups, and a positive correlation between MTP and EDSS scores only in the late-onset group, with no correlation results seen in the early-onset group.Since this study is a retrospective clinical study with a small number of patients enrolled, a more detailed study is needed in the future to learn the different clinical features between the LO-NMOSDs and EO-NMOSDs groups, and to look forward to a more comprehensive understanding of NMOSDs.
Description of authors’ contributions: Li Fei: data collection,statistical analysis and processing, and article writing; Liu Ting:topic selection and design; Yang Yi-Hao: article quality assessment,Li Qi-Fu: feasibility assessment of the topic selection, and article revision; Tong Jing-Yi, Lin Hui-Hsia, Lai Zong-Jun, and Leung Bun-Ki: data collection.
Conflict of interest
The content of the article does not involve relevant conflict of interest.
Authors’ declaration
The article is original, the content is true, the data is accurate, the content does not involve leakage, there is no double submission,there is no plagiarism, there is no plagiarized content, there is no dispute over authorship, and the authors are responsible for their own responsibility.
杂志排行
Journal of Hainan Medical College的其它文章
- Advances in microRNA and inflammatory bowel disease and their related mechanisms
- Pueraria isoflavones inhibit XOD and GLUT9 to decrease uric acid production and promote uric acid excretion, respectively
- Advances of N6-methyladenosine modification on circular RNA in hepatocellular carcinoma
- Research progress on the clinical diagnosis and treatment of COPD with pulmonary embolism
- Effect of different interventions on orthodontic tooth movement acceleration: A network meta-analysis
- To analyze the differentially expressed genes in chronic rejection after renal transplantation by bioinformatics
