The outcomes and safety of patients undergoing endoscopic retrograde cholangiopancreatography combining a single-use cholangioscope and a single-use duodenoscope: A multicenter retrospective international study
2024-03-04AssnroFuzzMttoCoomoRmnMutusmyBnmnWmLmnCrmoBrrCroFrJosNtoMonRmnnAmyTyrHroonAvSrrDnErSturtSrmnBnMroSpnAnrInnonKrmNswrRyAnrAnronAssnroRp
Assnro Fuzz ,Mtto Coomo ,M K ,V.Rmn Mutusmy ,B Bnmn ,Wm Lmn ,Crmo Brr ,Cro Fr ,Jos Nto ,A A-L ,Mon Rmnn ,Amy Tyr ,Hroon S ,Av Srr ,Dn Er ,Sturt Srmn ,C Bn ,Mro Spn ,Anr Innon ,Krm K ,Nswr Ry ,Anr Anron ,Assnro Rp ,
a Division of Gastroenterology and Digestive Endoscopy, Humanitas Research Hospital - IRCCS, Via Manzoni 56, 20089, Rozzano, Milano, Italy
b Gastroenterology, Rutgers Robert Wood Johnson; New Brunswick, New Jersey, USA
c Vatche and Tamar Manoukian Division of Digestive Diseases, University of California, Los Angeles School of Medicine at UCLA, Los Angeles, California, USA
d Division of Digestive and Liver Disorders; Indiana University School of Medicine, Indianapolis, IN, USA
e Department of Gastroenterology and Hepatology, University Hospitals Leuven, KU Leuven, 3000 Leuven, Belgium
f U.O.C. di Gastroenterologia ed Endoscopia Digestiva, Ospedale Giuseppe Mazzini, ASL Teramo, Italy
g Gastroenterology and Digestive Endoscopy Unit, Forlì-Cesena Hospitals, AUSL Romagna, Forlì-Cesena, Italy
h Borland Groover Clinic, Jacksonville, Florida, USA
i Department of Gastroenterology and Hepatology, King Fahad Medical City, Riyadh, Saudi Arabia
j Asian Institute of Gastroenterology, Hyderabad, India
k Section of Gastroenterology, Department of Emergency and Organ Transplantation, University of Bari, Bari 70124, Italy
l Department of Biomedical Sciences, Humanitas University, Via Rita Levi Montalcini 4, 20090, Pieve Emanuele, Milan, Italy
Keywords: Single-operator cholangioscopy Single-use duodenoscope Endoscopic retrograde cholangiopancreatography Indeterminate biliary stricture Difficult biliary stones
ABSTRACT Background: Duodenoscope-related multidrug-resistant organism (MDRO) infections raise concerns.Disposable duodenoscopes have been recently introduced in the market and approved by regulatory agencies with the aim to reduce the risk of endoscopic retrograde cholangiopancreatography (ERCP) associated infections.The aim of this study was to evaluate the outcome of procedures performed with single-use duodenoscopes in patients with clinical indications to single-operator cholangiopancreatoscopy.Methods:This is a multicenter international,retrospective study combining all patients who underwent complex biliopancreatic interventions using the combination of a single-use duodenoscope and a singleuse cholangioscope.The primary outcome was technical success defined as ERCP completion for the intended clinical indication.Secondary outcomes were procedural duration,rate of cross-over to reusable duodenoscope,operator-reported satisfaction score (1 to 10) on performance rating of the single-use duodenoscope,and adverse event (AE) rate.Results:A total of 66 patients (26,39.4% female) were included in the study.ERCP was categorized according to ASGE ERCP grading system as 47 (71.2%) grade 3 and 19 (28.8%) grade 4.The technical success rate was 98.5% (65/66).Procedural duration was 64 (interquartile range 15-189) min,cross-over rate to reusable duodenoscope was 1/66 (1.5%).The satisfaction score of the single-use duodenoscope classified by the operators was 8.6 ± 1.3 points.Four patients (6.1%) experienced AEs not directly related to the single-use duodenoscope,namely 2 post-ERCP pancreatitis (PEP),1 cholangitis and 1 bleeding.Conclusions:Single-use duodenoscope is effective,reliable and safe even in technically challenging procedures with a non-inferiority to reusable duodenoscope,making these devices a viable alternative to standard reusable equipment.
Introduction
The major therapeutic endoscopic strategy for several malignant and benign illnesses of the pancreatobiliary system is endoscopic retrograde cholangiopancreatography (ERCP).Multidrugresistant organism (MDRO) infection outbreaks associated with ERCP have been documented in Europe and USA with increasing prevalence in the last decade [1,2].
ERCP is generally performed by a duodenoscope,a complex instrument with a distinctive design in the distal tip where the elevator mechanism is located.Unlike standard endoscopes,this specific design makes the reprocessing process difficult.Insufficient manual cleaning due to limited access in this recessed space is associated with possible surface defects,thus enabling biofilm formation and increasing the risk of duodenoscope crosscontamination [3,4].
Consequently,the USA Food and Drug Administration (FDA)asked endoscope manufacturers to produce duodenoscopes with innovative designs including instruments with disposable caps or distal ends,to improve cleaning and disinfection or eliminate the need for reprocessing [5].In response,three duodenoscopes manufacturers marketed endoscope with removable and disposable distal end devices.Regrettably,persistent bacterial contamination in areas of the duodenoscope remained when reprocessing.In a multicenter study spanning 67 Dutch centers,155 duodenoscopes that had been reprocessed and were ready for use were used to sample various locations.Microbial growth was reported in the biopsy channel,suction channel,protection cap,and air/water channel in addition to the distal elevator mechanism [6].
Being the first FDA approved single-use sterile disposable duodenoscope,the EXALT Model D (Boston Scientific Corporation,Marlborough,Massachusetts,USA) was developed to eliminate the risk of infectious transmission.The performance of this singleuse duodenoscope was initially assessed in a bench study performed on an anatomical model by ERCP experts showing similar outcomes in comparison to 3 different reusable duodenoscopes [7].Experts have demonstrated that single-use duodenoscopes are an alternative to reusable duodenoscopes in several studies examining this new scope,including a randomized clinical trial (RCT) [8–10].However,the majority of the enrolled patients in these studies required only low complexity ERCP according to American Society for Gastrointestinal Endoscopy (ASGE) ERCP complexity level [11].Moreover,there is a complete lack of data about efficacy,safety,and adverse events (AEs) of the combination of the new single-use duodenoscope and the single-use cholangioscope.Cholangioscopy offers a highly therapeutic and diagnostic yield procedure that remains to be classified as a high-level complex procedure.
We conducted a retrospective international multicenter study to assess the feasibility,outcome,and safety of the single-use duodenoscope in combination with the single-use cholangioscope for ERCP with high ASGE complexity level in order to better understand the outcomes of the new single-use duodenoscope in a high complexity level ERCP with a large cohort of patients.
Methods
This is a multicenter,international,retrospective study from 10 tertiary referral centers (4 in Europe,4 in USA,and 2 in Asia) on all consecutive patients with clinical indications for cholangioscopy or pancreatoscopy who have been managed using the combination of the single-use duodenoscope (EXALT Model D) and singleuse cholangioscope (SpyGlass DS II,Boston Scientific Corporation)(Fig.1) between May 2020 and September 2021.
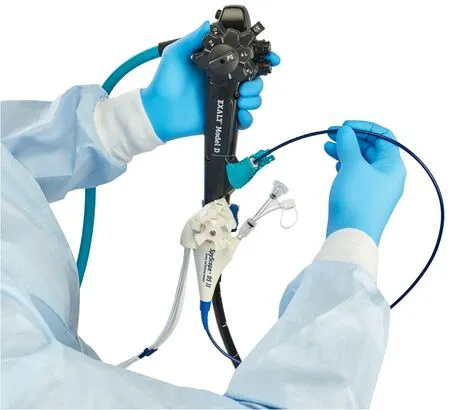
Fig.1. Single-use duodenoscope EXALT Model D (Boston Scientific Corporation,Marlborough,Massachusetts,USA) combined with single-use cholangioscope Spy-Glass DS II (Boston Scientific Corporation).
The institutional review board (IRB) of each participating center approved the study,and the protocol was performed in accordance with theDeclarationofHelsinki.Moreover,all patients enrolled in the present study provided signed informed consent.
Study device
The single-use duodenoscope EXALT Model D is a sterile fully disposable endoscope containing some recyclable plastic components.
The duodenoscope presents a distal end outer diameter of 15.1 mm,an insertion tube outer diameter of 11.3 mm,a working length of 1240 mm,a working channel inner diameter of 4.2 mm,a field of view of 108 ° and a weight of 700 g.After connecting the water and suction bottle,the scope is plugged into its dedicated controller and is ready to use.The single-use cholangioscope Spy-Glass DS II is a sterile disposable catheter of 10.5 Fr diameter with two dedicated irrigation channels and one 1.2 mm diameter working channel for accessory devices and aspiration.
Accessory devices used in this study include probes for electrohydraulic (EHL) or laser lithotripsy (LL) and dedicated biopsy forceps (SpyBiteTM;Boston Scientific Corporation) for intraductal tissue acquisition.
Procedure
All ERCP procedures were performed with the single-use duodenoscope in combination with the single-use cholangioscope by experienced endoscopists (>200 ERCP/year) using CO2insufflation under deep sedation or under general anesthesia managed by the anesthesiologist in accordance with local sedation policies.At each center,retrospective data collection was completed immediately upon the local IRB approval.
We included all consecutive patients with bilio-pancreatic diseases requiring cholangioscopy or pancreatoscopy during the study period.Exclusion criteria were age less than 18 years,lack of follow-up data less than 30 days,less than 3 ERCP cases with single-use duodenoscope in combination with the single-use cholangioscope performed during the study period by each participating endoscopist,international normalized ratio>1.5,and/or platelet count<50×103/mm3.
The procedural steps performed during the ERCP were left to the discretion of the endoscopist,depending on the procedure indication.All procedures started with the use of the single-use duodenoscope with the single-use cholangioscope.In case of failure of any necessary maneuvers with the single-use duodenoscope,the endoscopist had to cross-over to use a reusable duodenoscope.In case of cross-over,the reason of the inability to complete the ERCP with the single-use duodenoscope and the ability to complete the ERCP with the reusable duodenoscope were documented.
Rectal indomethacin (100 mg),hydration with Ringer’s lactate and prophylactic intravenous antibiotics were administered in all patients if not contraindicated [12].
Data
Data were collected and recorded in an excel sheet.For each patient,demographics [age,sex,body mass index (BMI)],baseline characteristics (e.g.,immunosuppression due to underlying disease or due to chemotherapy or post-transplantation maintenance immunosuppression),indication for the procedure,ERCP complexity level according to ASGE grading system [11],previous sphincterotomy,procedure duration (from duodenoscope insertion to duodenoscope removal),details of procedures performed (sphincterotomy,stone extraction,lithotripsy,biopsies,etc),list of ancillary devices used,need for cross-over to a reusable duodenoscope,single-use duodenoscope performance rating,and AEs with severity graded according to the ASGE lexicon’s severity grading system [13]and their management were collected.
AEs were classified as immediate if occurred during the procedure,early when presenting within 14 days,and late when presenting after 14 days from the procedure.
All operators were asked to formulate a judgment on the overall performance of the single-use duodenoscope,from 1 (unsatisfied)to 10 (very satisfied) regarding navigation/pushability from the insertion to the duodenum,stability during the cannulation of the papilla,maneuverability and torquability of the scope,ease and ability to advance ancillary devices through the working channel,and image quality.
Patients were followed up with periodic blood tests and clinic visits at the discretion of the responsible physician for each participating hospital.
Outcomes
The primary outcome of the study was technical success defined as the ability to successfully complete the entire procedure(completed without cross-over to a reusable duodenoscope) with the intended diagnostic or therapeutic maneuvers according to the clinical indication (i.e.stone fragmentation/removal,biopsies from the targeted lesion,etc).The secondary outcomes were procedural duration,rate of cross-over,and AEs rate.
The rate of cross-over to the reusable duodenoscope was analyzed,stratifying the cross-over into three groups: 1) cross-over to a reusable duodenoscope after inability to complete the procedure with the single-use duodenoscope,followed by completion of the procedure with a reusable duodenoscope;2) cross-over to a reusable duodenoscope after inability to complete the procedure with the single-use duodenoscope,followed by inability to complete the procedure with a reusable duodenoscope;3) cross-over to another type of endoscope because an ERCP is no longer indicated based on intra-procedural findings (for example switching the single-use duodenoscope with an endoscopic ultrasound(EUS) scope for an EUS-guided bile duct drainage due to inability to reach the papilla because of duodenal stricture/narrowing).Special attention was paid to collect data about clinical or biochemical signs of post-procedural infection and need for antibiotic therapy.
Statistical analysis
The distribution of continuous parameters was assessed using the Shapiro-Wilk test,then these variables were expressed as mean and standard deviation (SD) or median and interquartile range(IQR),as appropriate.Categorical variables were reported as counts and percentages.Statistical analyses were performed using the Statistical Analysis Software (SAS Institute Inc.,Cary,NC,USA).
Results
The population of this study composed 66 patients [26 (39.4%)females,with a mean age of 64.6 ± 16.3 years]treated with the single-use duodenoscope plus single-use cholangioscope.Characteristics of the patients and main indications of the procedures are reported in Table 1.
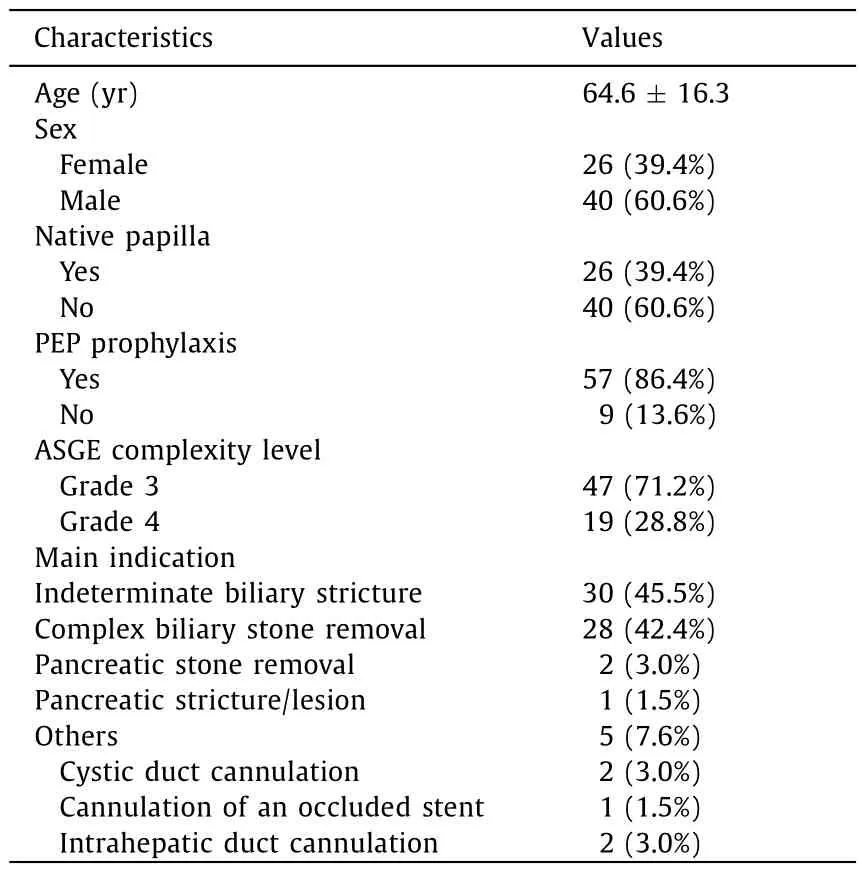
Table 1Patients characteristics and main indications of the procedures(n=66).
In our cohort,patients with native papilla represented 39.4%(26/66) of the patients.The reasons for choosing a single-use duodenoscope are listed in Table 2: the most common indicator was high risk of cholangitis (27,40.9%),followed by the need to protect reusable duodenoscope from MDRO infection (10,15.2%).The ASGE complexity level for ERCP for our study procedures were categorized as 47 (71.2%) grade 3 and 19 (28.8%) grade 4;no grade 1 or 2 procedures were performed.
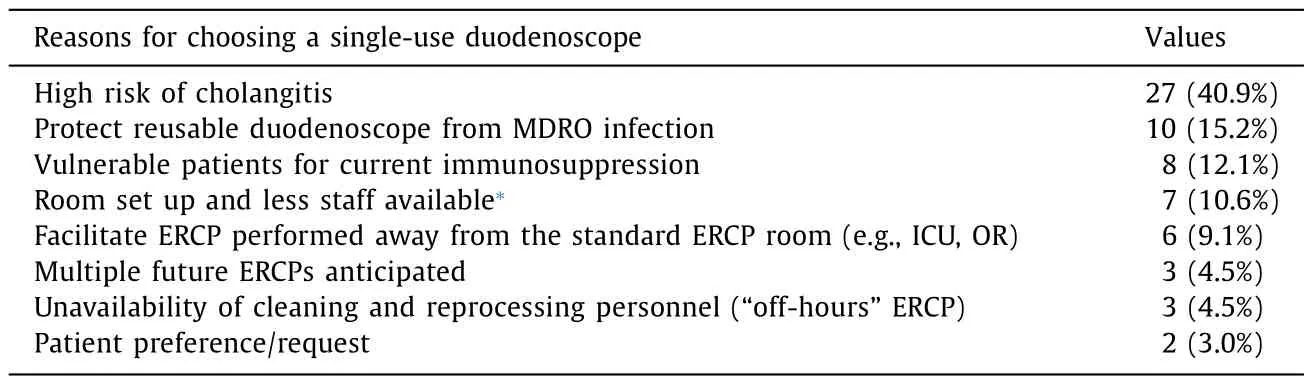
Table 2List of common reasons for choosing the EXALT Model D single-use duodenoscope.
The technical success rate was 98.5% (65/66) with only one(1.5%) cross-over to reusable duodenoscope due to the inability to complete the procedure with the single-use duodenoscope for an unstable scope position in the duodenum.The indication for ERCP in this cross-over case was biliary drainage for indeterminate common bile duct stricture.The median procedural time was 64 (IQR 15-189) min.
Focusing on the most frequent indication,namely indeterminate biliary stricture in 45.5% of the patients,the possibility to obtain tissue for histological analysis using a dedicated biopsy forceps was achieved in 83.3% (25/30) of the patients (Fig.2).In 24/25 (96.0%)samples the material obtained was considered as adequate by the pathologist.In the remaining 5 patients,biopsies were no longer deemed necessary due to intra-procedural findings of biliary stones(n=1),and of strictures with benign visual features under direct cholangioscopy (n=4).
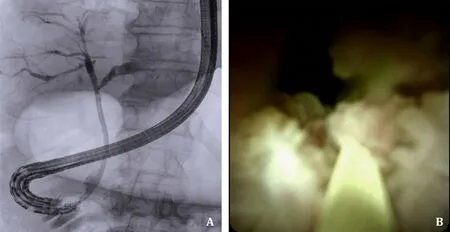
Fig.2. Fluoroscopic view (A) and direct endoscopic view (B) of an indeterminate biliary stricture managed using the combination of single-use duodenoscope and single-use cholangioscope.
In the second most common indication for ERCP,namely the treatment of complex biliary stones in 28 patients,EHL was performed in 24 (85.7%) of the patients and LL was used in the remaining 4 (14.3%) procedures (Fig.3).Complete stone removal was reached in all the index ERCPs performed for complex biliary stones.

Fig.3. Electrohydraulic lithotripsy under direct endoscopic control with single-use cholangioscope.
In our population only four patients (6.1%) experienced AEs,namely 2 post ERCP pancreatitis (PEP),1 cholangitis and 1 bleeding.The latter one was an intra-procedural bleeding occurring from the sphincterotomy that was successfully treated in the same session with endoscopic hemostasis.The other 3 AEs were early and managed conservatively.Regarding the ASGE severity level,the AEs were classified as severe for one case of PEP,moderate for one case of cholangitis,and mild for the other case of PEP and the bleeding case.
The corresponding results of our study for the main outcomes in aggregate literature are summarized in Table 3 [14–18].The mean score on the overall performance of the single-use duodenoscope classified by the operators was 8.6 ± 1.3 points.
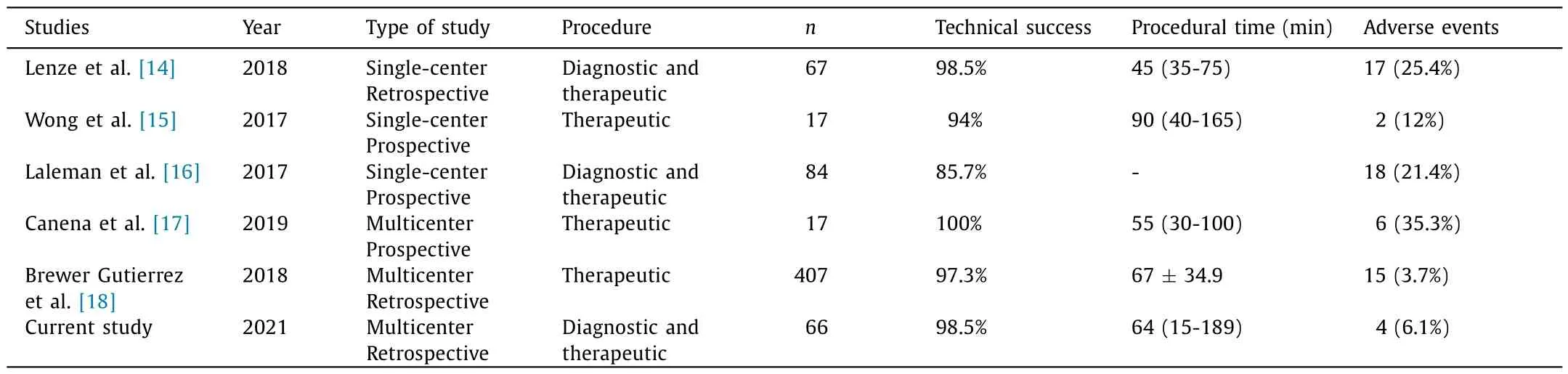
Table 3Overview of overall main outcomes in our cohort and the aggregated literature using Spyglass DS with reusable duodenoscope.
Discussion
The present study aimed to evaluate how one of the new disposable duodenoscope (EXALT model D) performed in a high complexity level ERCP in a real-life setting.Our data showed that the single-use duodenoscope combined with a single-use cholangioscope obtained excellent results in terms of technical success(98.5%),with low rates of cross-over to a standard reusable endoscope (1.5%).
Our results confirmed the efficacy and safety of single-use duodenoscopes,which was in line with previously published studies [8,9,19].Nevertheless,these studies were based on a small number of patients treated with low-complexity ERCP.
In our study we considered procedures performed in different centers located around the world.Our findings confirmed the excellent procedural success provided by these instruments and reproducibility of the results,even in a setting of high complexity procedures (ASGE grade 3 or 4).
The single-use device offers comparable results to the reusable duodenoscope in terms of technical success rate,procedural duration,and AEs rate when compared to cholangioscopy ERCPs performed with the reusable duodenoscope in the literature,in patients with similar baseline characteristics and indications.Similar results were recently obtained in a randomized clinical trial by Bang et al.[9],including patients with mainly (>80%) lowcomplexity ERCP procedures performed by experts.The authors reported similar technical performance between single-use duodenoscopes and reusable duodenoscope when performing lowcomplexity ERCP.
In order to lower the risk of infections linked to duodenoscopes,it would be of great importance to determine whether these outcomes could be generalized to a population of patients who need more sophisticated procedures.The real benefit in using this novel device might also result from a tailored use in patients with highrisk conditions,like immunocompromised subjects or those carrying an MDRO infection,taking into account the similar results in terms of efficacy and safety as well as the costs of a disposable duodenoscope at the moment [20].It could be speculated that in addition to considering the procedural duration,the faster disposal of the used duodenoscope and the lack of need for processing may lead to a reduction in overall work time for endoscopy operators and on optimization of resources [21];the possibility of reducing to the point of eliminating the personnel needed for instrument reprocessing would also provide cost savings.Another thing to consider is the outstanding overall impression of the operators’use of the single-use duodenoscope.Over 80% of endoscopists gave extremely positive ratings in terms of stability,maneuverability,and ease of use of various accessories in highly complex procedures.The instrument’s light weight is essential to avoid overexerting the operator during advanced and labor-intensive complex bilio-pancreatic endoscopy,which was one of the features that pleased operators.A further characteristic was the greater simplicity of passing the different ancillary devices through the operating channel (e.g.,single-use cholangioscope with EHL),compared with reusable duodenoscopes.In addition,as recently emphasized [9],the single-use scope due to its stiffness leads to a more straight position into the duodenum providing access to the papilla from a superior or horizontal angle.
Similar to using instruments made by different manufacturers,these minor variations may make the instrument feel less "familiar" during initial usage.Another feature that is not currently used but might be in the future is the ability to design disposable instruments specifically for each endoscopist with a handle that is ergonomically designed and sized for each individual hand size.This would further enhance ergonomics and lessen operator fatigue.The very few patients engaged in this study and its retrospective methodology are two of its drawbacks;nonetheless,it must be mentioned that there are currently no studies with a larger population on this particular topic in the literature.Additionally,it may be challenging to generalize the findings since procedures carried out by highly skilled endoscopists in tertiary referral facilities were taken into account.
In conclusion,the single-use duodenoscope is effective,dependable,and safe even during technically complicated procedures with a high level of complexity,according to our data,which also supports the non-inferiority of a disposable device compared to a reusable one.
Acknowledgments
None.
CRediT authorship contribution statement
Alessandro Fugazza:Conceptualization,Data curation,Formal analysis,Writing– original draft,Writing– review &editing.Matteo Colombo:Conceptualization,Data curation,Formal analysis,Writing– original draft.Michel Kahaleh:Data curation,Formal analysis.V.Raman Muthusamy:Data curation,Formal analysis.Bick Benjamin:Data curation,Formal analysis.Wim Laleman:Data curation,Formal analysis.Carmelo Barbera:Data curation,Formal analysis.Carlo Fabbri:Supervision.Jose Nieto:Data curation,Formal analysis.Abed Al-Lehibi:Data curation,Formal analysis.Mohan Ramchandani:Data curation,Formal analysis.Amy Tyberg:Data curation,Formal analysis.Haroon Shahid:Data curation,Formal analysis.Avik Sarkar:Data curation,Formal analysis.Dean Ehrlich:Data curation,Formal analysis.Stuart Sherman:Data curation,Formal analysis.Cecilia Binda:Data curation,Formal analysis.Marco Spadaccini:Data curation,Formal analysis,Writing– original draft.Andrea Iannone:Data curation,Formal analysis,Methodology,Writing– original draft.Kareem Khalaf:Conceptualization,Writing– original draft.Nageshwar Reddy:Supervision.Andrea Anderloni:Supervision,Writing– review &editing.Alessandro Repici:Supervision.
Funding
None.
Ethical approval
This study was approved by the Institutional Review Board of each participating center and the protocol was performed in accordance with theDeclarationofHelsinki.Written informed consent was obtained from all participants.
Competing interest
Alessandro Fugazza is the consultant for Boston Scientific and Olympus.Michel Kahaleh is the consultant for Boston Scientific,Abbvie,and Interscope Med.Wim Laleman is the consultant and research support for Boston Scientific.Carlo Fabbri is the consultant for Boston Scientific,speaker for Steris.Jose Nieto is the consultant for Boston Scientific.Andrea Anderloni is the consultant for Boston Scientific and Olympus.Alessandro Repici is the consultant for Boston Scientific,ERBE and Fujifilm.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Recent advances in promising drugs for primary prevention of gastroesophageal variceal bleeding with cirrhotic portal hypertension
- Stereotactic body radiotherapy in pancreatic adenocarcinoma
- Application of ultrasonography-elastography score to suspect porto-sinusoidal vascular disease in patients with portal vein thrombosis
- Polydatin ameliorates hepatic ischemia-reperfusion injury by modulating macrophage polarization
- Hypomethylation of glycine dehydrogenase promoter in peripheral blood mononuclear cells is a new diagnostic marker of hepatitis B virus-associated hepatocellular carcinoma
- AGK2 pre-treatment protects against thioacetamide-induced acute liver failure via regulating the MFN2-PERK axis and ferroptosis signaling pathway
