Polydatin ameliorates hepatic ischemia-reperfusion injury by modulating macrophage polarization
2024-03-04HiLiBoChunZhiChenChngZhenRenKeYnSunHoLiuShoHuSongZhiRenFu
Hi-Li Bo ,Chun-Zhi Chen ,Chng-Zhen Ren ,Ke-Yn Sun ,Ho Liu ,Sho-Hu Song ,Zhi-Ren Fu ,
a Department of Organ Transplantation, Shanghai Changzheng Hospital, Navy Military Medical University, Shanghai 200003, China
b Department of Surgical Oncology, the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China
c Department of Cardiology, Shanghai Changzheng Hospital, Naval Military Medical University, Shanghai 200003, China
d Department of General Surgery, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China
Keywords: Hepatic ischemia-reperfusion injury Polydatin Macrophage Polarization Inflammation
ABSTRACT Background: Polydatin,a glucoside of resveratrol,has shown protective effects against various diseases.However,little is known about its effect on hepatic ischemia-reperfusion (I/R) injury.This study aimed to elucidate whether polydatin protects liver against I/R-induced injury and to explore the underlying mechanism.Methods: After gavage feeding polydatin once daily for a week,mice underwent a partial hepatic I/R procedure.Serum alanine aminotransferase (ALT)/aspartate aminotransferase (AST),hematoxylin-eosin (H&E)and TdT-mediated dUTP nick-end labeling (TUNEL) staining were used to evaluate liver injury.The severity related to the inflammatory response and reactive oxygen species (ROS) production was also investigated.Furthermore,immunofluorescence and Western blotting were used to detect macrophage polarization and the NF-κ B signaling pathway in macrophages.Results: Compared with the I/R group,polydatin pretreatment significantly attenuated I/R-induced liver damage and apoptosis.The oxidative stress marker (dihydroethidium fluorescence,malondialdehyde,superoxide dismutase and glutathione peroxidase) and I/R related inflammatory cytokines (interleukin-1β,interleukin-10 and tumor necrosis factor-α) were significantly suppressed after polydatin treatment.In addition,the result of immunofluorescence indicated that polydatin reduced the polarization of macrophages toward M1 macrophages both in vivo and in vitro.Western blotting showed that polydatin inhibited the pro-inflammatory function of RAW264.7 via down-regulating the NF-κ B signaling pathway.Conclusions: Polydatin protects the liver from I/R injury by remodeling macrophage polarization via NFκB signaling.
Introduction
Hepatic ischemia-reperfusion (I/R) injury is a common complication after liver surgery,especially liver transplantation,which increases risk of transplant rejection and acute liver failure [1].To date,there is no effective treatment for liver damage after I/R injury.
Hepatic I/R injury is a multifactorial and complex disease in which oxidative stress and the inflammatory response are crucial.During the I/R process,excessive production of reactive oxygen species (ROS) interferes with intracellular redox homeostasis,which can directly lead to severe cellular damage [2].Kupffer cells,the liver resident macrophages rapidly respond to the damage and excessive ROS in the liver and subsequently secrete pro-inflammatory cytokines and chemokines to remove cell debris.Meanwhile,macrophages secrete anti-inflammatory cytokines and growth factors to promote wound healing [3,4].In this process,Kupffer cells (macrophages) play a major role in modulating inflammation caused by I/R [5].Hence,regulation of the balance between M1 and M2 macrophages,that is,decreasing the proportion of M1 macrophages or promoting more M2 macrophage infiltration,might be useful for treating liver I/R injury.
Polydatin is a glucoside form of resveratrol purified fromPoly-gonumcuspidatumSieb roots [6].Many studies have validated that polydatin exhibits beneficial properties,such as antitumor [7],antioxidant [8]and anti-inflammatory effects [9].Nevertheless,as an effective antioxidant,the effect of polydatin on hepatic I/R injury still needs to be elucidated.This study aimed to evaluate the protective effect of polydatin on hepatic I/R injury and the underlying mechanisms.
Methods
Reagents
Polydatin was purchased from MedChem Express (Monmouth Junction New Jersey,USA,HY-N0120A).Lipopolysaccharide (LPS),a hematoxylin-eosin (H&E) staining kit,a TdT-mediated dUTP nickend labeling (TUNEL) fluorescence detection kit,and tumor necrosis factor-α(TNF-α),interleukin-1β(IL-1β) and IL-10 enzymelinked immunosorbent assay (ELISA) kits were obtained from Beyotime (Shanghai,China).The primary antibodies used for Western blotting and immunofluorescence included cleaved Caspase-3 (Cell Signaling Technology (CST),Danvers,Massachusetts,USA,#9664),Bcl-2 (CST,#15071),Bax (CST,#14796),IκBα(CST,#4814),p-IκBα(CST,#2859),GAPDH (CST,#5174),F4/80 (Thermo Fisher Scientific,Waltham,Massachusetts,USA,#14-4801-82),iNOS (Abcam,Cambridge,UK,#ab15323),CD206 (Abcam,#ab64693),P65 (CST,#8242),and myeloperoxidase (MPO,Beyotime,#A1374).
Animals
Male C57BL/6 mice (weighting 20–26 g) were supplied by the Joint Ventures Sipper BK Experimental Animal (Shanghai,China) and raised in a specific pathogen-free (SPF) environment at 24 ± 2 °C and a 12 h light/dark cycle.All mice accessed to food and wateradlibitum.All experimental protocols were conducted following the guidelines of the National Institute of Health for the Care and Use of Laboratory Animals and approved by the Scientific Investigation Board of Shanghai Changzheng Hospital (2019071106).
Model establishment
We randomly divided 48 mice into four groups (6 mice each group): sham+vehicle,sham+polydatin,I/R+vehicle,and I/R+polydatin.For polydatin pretreatment,mice were administered 200μL of corn oil containing polydatin (40 mg/kg) by gavage once a day for a week before the operation.This dosing treatment has been previously shown to protect against kidney injury and extend the lifespan of mice [10].The other two groups were administered an equal volume of corn oil as a vehicle control.A partial liver ischemia model was established based on a previous study,with slight modifications [11].Mice were anesthetized with pentobarbital (1%,50 mg/kg) intraperitoneally.A nontraumatic clip was employed to block the portal vein and hepatic artery blood supply to the left lateral and median lobes of the liver (approximately 70%) after a midline laparotomy.The incision was temporarily closed during ischemia.Ninety minutes later,the clip was removed to initiate reperfusion.Mice from the two sham groups received the same procedure under the condition without vascular occlusion.During the postoperative period,mice were kept in a warm environment using a heating pad until they were awake.Six hours after reperfusion,blood and ischemic liver lobes were collected from all mice after euthanasia.
Cell culture
RAW264.7 cells were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai,China) and were cultured in RPMI 1640 medium containing 10% fetal bovine serum (FBS),100 U/mL penicillin and 100 mg/mL streptomycin at 37 °C with 5% CO2.Specific to the cell experiments,RAW264.7 cells received 24 h of treatment with LPS (100 ng/mL) together with polydatin(400μmol/L) or vehicle.The dose of polydatin was verified in a previous study [12].
Serum biochemical analysis
The mice were sacrificed at the indicated time points (0,3,6,12,and 24 h post-reperfusion).All serum samples were collected and isolated by a 1200 g centrifuge for 15 min.A Fuji-DRYCHEM chemical analyzer (Fuji Film,Tokyo,Japan) was used to measure serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) according to the manufacturer’s instructions.
ELISA
Commercial ELISA kits were used to assess the cytokine (TNF-α,IL-1βand IL-10) concentrations in serum and cell culture supernatants according to the manufacturer’s instructions.
Western blotting analysis
The proteins from liver tissue or RAW 264.7 cells were lysed in RIPA Lysis Buffer (Roche,Basel,Switzerland).The protein concentration in the extracts was measured by the bicinchonininc acid (BCA) method (KeyGEN Biotech,Nanjing,China).After boiling with sodium dodecyl sulfate (SDS) loading buffer,sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was used to separate the same protein concentration of samples,with the obtained liquids transferred to polyvinylidene fluoride membranes for immunoblot analysis.Membranes were incubated with cleaved Caspase-3 (1:1000),Bcl2 (1:1000),Bax (1:1000),IκBα(1:1000),p-IκBα(1:1000) and GAPDH (1:1000) antibodies.Following incubation with secondary antibodies,ECL reagents (Share-bio,Shanghai,China) were used to detect the fluorescence intensity.The relative quantity of proteins was analyzed with ImageJ (National Institutes of Health,Bethesda,Maryland,USA).
Histopathological evaluation
After reperfusion,the livers of the mice were fixed in 4%paraformaldehyde (PFA) immediately after collection and embedded in paraffin.H&E stained the tissue sections (4μm) to assess histological damage.The modified Suzuki’s criteria were used for severity grading of hepatic pathological impairment [13].
Immunofluorescence analysis
Briefly,frozen liver tissues were cut and blocked using 10%bovine serum albumin (BSA) for no less than 1 h,followed by one night of incubation at 4 °C with primary antibodies (anti-MPO 1:10 0,anti-F4/80 1:20 0,anti-iNOS 1:20 0,and anti-CD206 1:200).PBS was used to wash the sections three times,and the sections were incubated for 1 h with the corresponding secondary antibodies and counterstained with 4′,6-diamidino-2-phenylindole(DAPI).We counted the numbers of positive cells in 10 high-power fields (HPF) per section.For cellular immunofluorescence staining,RAW264.7 cells were fixed for 1 h with 4% PFA and blocked with 5% BSA.The cells underwent one night of incubation with primary antibodies (anti-iNOS 1:500,anti-CD206 1:500 and anti-P65 1:100)at 4 °C and then received 1 h of incubation with the corresponding secondary antibodies at room temperature.The nuclei were then counterstained with DAPI for 2 h,and a fluorescence electron microscope (Olympus DX51,Tokyo,Japan) was used to obtain images.
TUNEL assays
For apoptosis assessment,we used a TUNEL fluorescence detection kit to stain liver sections following the instructions of the manufacturer.
Determination of oxidative stress
ROS levels in liver tissue were evaluated with dihydroethidium (DHE,KeyGEN BioTECH,Nanjing,China) according to the instructions.Assessment of the activities exhibited by superoxide dismutase (SOD),malondialdehyde (MDA) and glutathione peroxidase (GSH-Px) in liver tissue was performed following the manufacturer’s instructions of commercial kits (Jiancheng Bioengineering Institute,Nanjing,China).
qPCR analysis
TRIzol reagent (Invitrogen,Carlsbad,California,USA) was employed to extract total RNA from mouse livers or RAW264.7 cells,and the extracted total RNA was then subjected to reverse transcription with a Superscript III Reverse Transcriptase Kit(Invitrogen,Carlsbad,California,USA) according to the manufacturer’s instructions.SYBR RT-PCR kit (Takara,Tokyo,Japan) and a Step One Real-Time PCR System (Applied Biosystems,Waltham,Massachusetts,USA) were applied in the qPCR.We normalized the relative gene expression levels according to that of GAPDH.Table 1 lists the primer sequences used.

Table 1The sequence of the primers for qPCR.
Statistical analysis
SPSS software (Version 20.0;SPSS Inc.,Chicago,IL,USA) was adopted.Differences between two groups and more than two groups received Dunnett’s multiple comparison tests and were then analyzed by a two-tailed Student’st-test and one-way analysis of variance,respectively.All data were expressed as mean ±standard deviation.At least three independent replications for each experiment were performed for verification.The results were considered statistically significant whenP<0.05.
Results
Polydatin alleviated hepatic I/R injury in mice
The serum ALT and AST levels of both the I/R+vehicle and I/R+polydatin groups peaked at 6 h following reperfusion (Fig.1 A).Serum ALT and AST presented significantly higher levels in the I/R+vehicle group than in the I/R+polydatin group (5006.67 ± 1271.64 vs.2400.00 ± 807.50 U/L for ALT and 4689.33 ± 955.54 vs.2132.00 ± 795.59 U/L for AST,respectively,bothP<0.05).In addition,the liver sections collected at the same time point exhibited a much larger area of necrosis in the mice of the I/R+vehicle group than in the I/R+polydatin group,and the hepatic architecture of the liver in the I/R+polydatin group was much better preserved (Fig.1 B).As shown in Fig.1 C,Suzuki’s score was obviously decreased in the I/R+polydatin group compared with that in the I/R+vehicle group (P<0.01).These results suggested that polydatin pretreatment could protect against hepatic I/R injury.

Fig.1. Polydatin alleviated hepatic I/R injury in mice.A: Serum ALT and AST levels in the I/R+vehicle and I/R+polydatin groups (n=6 per group) at 0,3,6,12,and 24 h post-reperfusion;B: representative H&E staining at 6 h post-reperfusion in different groups (n=6 per group;scale bar: 100 μm);C: hepatic injury was scored according to Suzuki’s criteria.The results are presented as mean ± standard deviation.∗: P < 0.05;∗∗: P < 0.01.ALT: alanine aminotransferase;AST: aspartate aminotransferase;I/R:ischemia/reperfusion;H&E: hematoxylin-eosin.
Polydatin ameliorated I/R-induced apoptosis in the liver
The number of TUNEL-positive cells as the apoptotic hepatocytes were significantly increased in the I/R+vehicle group,and polydatin significantly decreased these cells (27.84 ± 4.05 vs.4.47 ± 1.37 in percentages,P<0.01;Fig.2 A,B).Western blotting also demonstrated that the expression levels of the proapoptotic proteins cleaved caspase-3 and Bax were significantly lower in the I/R+polydatin group than in the I/R+vehicle group,while the ratio of Bcl-2/Bax was considerably higher (P<0.05 for cleaved caspase-3 andP<0.01 for Bcl-2/Bax,Fig.2 C-E).As expected,polydatin pretreatment significantly attenuated apoptosis after liver I/R injury.
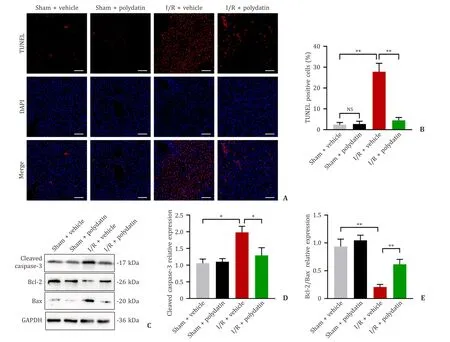
Fig.2. Polydatin ameliorated I/R-induced apoptosis in the liver.A: Fluorescence-stained TUNEL-positive cells in mice from the four groups at 6 h post-reperfusion (n=6 per group;scale bar: 100 μm);B: quantification of the number of TUNEL-positive cells in the four groups;C: the protein expression levels of cleaved caspase-3,Bcl-2 and Bax were determined by Western blotting (n=5 per group);D: the relative densities of cleaved caspase-3 were analyzed and normalized to GAPDH;E: Bcl-2/Bax protein levels in the livers of each group.The results are presented as mean ± standard deviation.∗: P < 0.05;∗∗: P < 0.01.TUNEL: TdT-mediated dUTP nick-end labeling;GAPDH:glyceraldehyde-3-phosphate dehydrogenase.
Polydatin suppressed oxidative stress in the liver
Oxidative stress plays an initiating and critical role during liver I/R injury.DHE staining showed that the oxidative stress was significantly increased after hepatic I/R injury,and that polydatin significantly alleviated the oxidative stress in I/R model (Fig.3 A,B).MDA,another index of oxidative stress,paralleled the changes as DHE stains.The SOD and GSH-Px,the antioxidant defense enzymes,were significantly decreased in the I/R+vehicle group,and antecedent treatment with polydatin significantly increased SOD and GSH-Px activities (Fig.3 C-E).
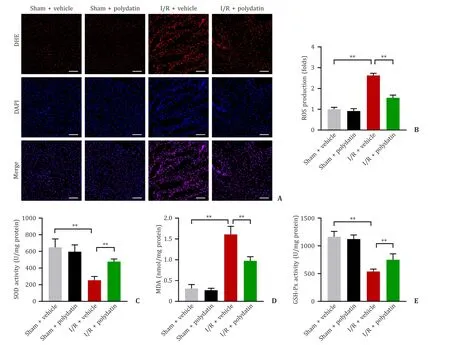
Fig.3. Polydatin suppressed oxidative stress in the liver.A: Representative images of DHE staining in liver sections of the four groups (n=6 per group;scale bar: 100 μm);B: the DHE mean density of ROS production;C-E: SOD,MDA and GSH-Px activities (n=6 per group).Data are presented as mean ± standard deviation.∗∗: P < 0.01.SOD:superoxide dismutase;MDA: malondialdehyde;GSH-Px: glutathione peroxidase;DHE: dihydroethidium.
Polydatin mitigated the inflammatory response in the liver
MPO,a delegate of neutrophil infiltration,was significantly increased in the I/R+vehicle group,and the MPO-positive cells in the I/R+polydatin group were significantly decreased (Fig.4 A,B).In addition,the levels of the pro-inflammatory cytokines,TNFαand IL-1β,were significantly decreased in the serum of the I/R+polydatin group compared with those in the I/R+vehicle group (Fig.4 C,D).IL-10,an essential anti-inflammatory cytokine,was significantly increased in polydatin pretreatment mice compared to that in the I/R+vehicle group (Fig.4 E).
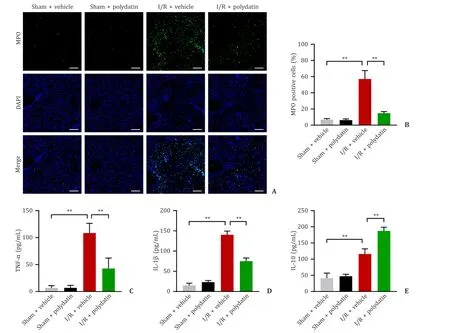
Fig.4. Polydatin mitigated the inflammatory response in the liver.A: Representative images of immunofluorescence-stained MPO-positive cells in liver tissues at 6 h postreperfusion (n=6 per group;scale bar: 100 μm);B: MPO activity in each group;C-E: the serum levels of TNF-α,IL-1 β,and IL-10 (n=6 per group).The results are presented as mean ± standard deviation.∗∗: P < 0.01.MPO: myeloperoxidase;DAPI: 4′,6-diamidino-2-phenylindole;IL-1 β: interleukin-1beta;IL-10: interleukin-10.
Polydatin modulated the polarization of macrophages in vivo
As mentioned above,both oxidative stress and inflammation were enhanced during hepatic I/R injury.Under different conditions,macrophages are capable of switching between M1 and M2 [14].The infiltration of macrophages exhibited a significant increase in the I/R+vehicle group.The I/R+polydatin group exhibited a significant decrease of macrophage compared with the I/R+vehicle group.Moreover,the group treated with vehicle presented obviously activated M1-type macrophages during I/R,and polydatin pretreatment decreased the ratio of M1-polarized macrophage activation (Fig.5 A-C).Furthermore,the expression levels of representative genes of M1 (iNOS,TNF-α,and IL-1β) and M2 (CD206,TGF-β,and Arg1) macrophages in the liver were also measured.The findings confirmed our study that polydatin pretreatment weakened M1 macrophage activation during hepatic I/R(Fig.5 D,E).
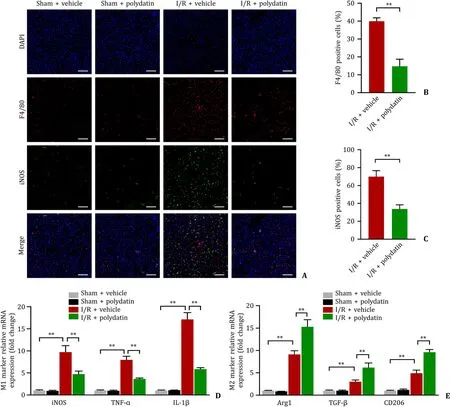
Fig.5. Polydatin modulated macrophage polarization in vivo.A: Representatives of immunofluorescence staining of F4/80 (red) and iNOS (green) in liver sections of the four groups (n=6 per group;scale bar: 100 μm);B and C:the numbers of F4/80-and iNOS-positive cells in the I/R ± polydatin groups,representing pro-inflammatory and anti-inflammatory macrophages,respectively;D: levels of mRNAs encoding iNOS,TNF-α,and IL-1 β (M1 markers) in the liver (n=5 per group);E: levels of mRNAs encoding Arg1,TGF-β and CD206 (M2 markers) in the liver (n=5 per group).Data are presented as mean ± standard deviation.∗: P < 0.05;∗∗: P < 0.01.iNOS: inducible nitric oxide synthase;TNF-α: tumor necrosis factor-alpha;IL-1 β: interleukin-1beta;DAPI: 4′,6-diamidino-2-phenylindole.
Polydatin promoted anti-inflammatory polarization of macrophages in vitro
To further verify the role of polydatin in regulating macrophage polarization,we analyzed the polarization phenotype alteration in RAW264.7 cells.Our results suggested that M2 phenotypic transformation was promoted,while the M1 phenotype was suppressed by polydatin (Fig.6 A).qPCR showed that the levels of classical pro-inflammatory markers,such as iNOS,TNF-α,and IL-1β,presented a remarkable decrease,while the anti-inflammatory markers (CD206,TGF-β,and Arg1) presented an increase in the LPS+polydatin group than in the LPS+vehicle group (Fig.6 B,C).
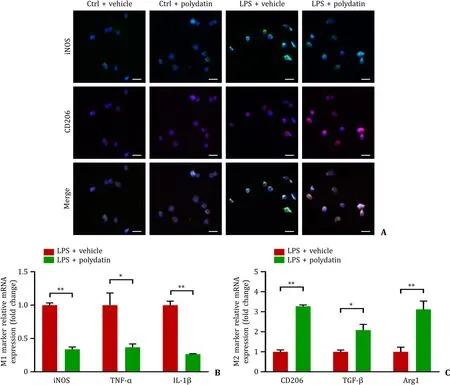
Fig.6. Polydatin promoted anti-inflammatory polarization of macrophages in vitro.A: Representative images of immunofluorescence staining of iNOS (green) and CD206(red) in Raw264.7 cells of the four groups.Scale bar: 20 μm;B: levels of mRNAs encoding iNOS,TNF-α,and IL-1 β (M1 markers) in Raw264.7 cells treated with LPS with or without polydatin (n=3 per group);C: levels of mRNAs encoding CD206,TGF-β and Arg1 (M2 markers) in Raw264.7 cells treated with LPS with or without polydatin(n=3 per group).Data are presented as mean ± standard deviation.∗: P < 0.05;∗∗: P < 0.01.TNF-α: tumor necrosis factor-alpha;IL-1 β: interleukin-1beta;iNOS: inducible nitric oxide synthase;LPS: lipopolysaccharide.
Polydatin alleviated the release of pro-inflammatory cytokines via the NF- κB signaling pathway
The NF-κB signaling pathway shows a close association with the functional phenotype of macrophages [15,16].Correspondingly,some reports have indicated that polydatin inhibits the NF-κB signaling pathway [7,9].Inhibition of the NF-κB signaling pathway also decreases pro-inflammatory M1 macrophage polarization [17].Our results showed that polydatin pretreatment lowered the phosphorylated IκBαexpression level when exposed to LPS (Fig.7 A,B).Immunofluorescence showed that more p65,a major element of NF-κB,was distributed in the nucleus of macrophages in the LPS group,whereas less nuclear localization of p65 was observed in the polydatin group than in the control group (Fig.7 C).The inflammatory cascade response is closely related to the release of the pro-inflammatory cytokines TNF-αand IL-1βand is associated with the NF-κB signaling pathways [18].As indicated in Fig.7 D,E,the protein levels of TNF-αand IL-1βwere markedly decreased in the conditioned medium of the RAW264.7 cells in the LPS+polydatin group compared with the LPS+vehicle group.
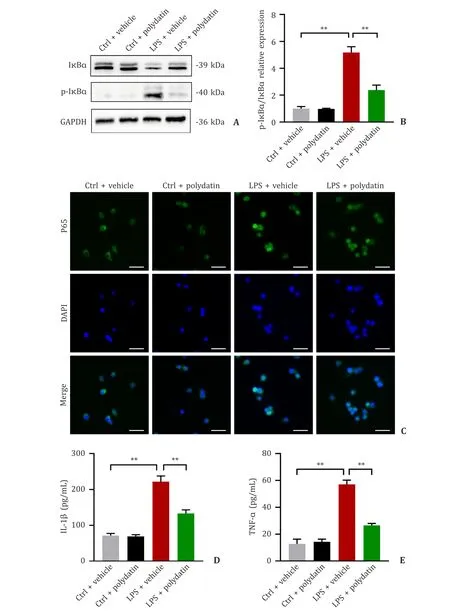
Fig.7. Polydatin alleviated the release of pro-inflammatory cytokines via the NF-κB signaling pathway.A and B: Immunoblot images showing the expression of I κB α and p-I κB α in Raw264.7 cells treated with LPS with or without polydatin (n=3 per group);C: the distribution of p65 (green) in macrophages.The cell nuclei were stained with DAPI (blue).Scale bar: 20 μm;D &E: the amounts of IL-1 β and TNF-α secreted into the supernatants of cultured RAW264.7 cells (n=3 per group).Data are presented as mean ± standard deviation.∗: P < 0.05;∗∗: P < 0.01.DAPI: 4′,6-diamidino-2-phenylindole;GAPDH: glyceraldehyde-3-phosphate dehydrogenase;IL-1 β: interleukin-1beta;TNF-α: tumor necrosis factor-alpha.
Discussion
Hepatic I/R injury is a common complication after hepatic resection and liver transplantation [19].In recent decades,researchers have been searching for effective strategies to mitigate I/R injury.The present study first demonstrated that polydatin pre-administration had protective effect on hepatic I/R injury.Preadministration of polydatin effectively reduced the production of ROS and pro-inflammatory factors,thereby reducing liver injury and cell apoptosis.Further investigation of the underlying mechanisms of these effects showed that polydatin inhibited the polarization of macrophages toward the M1 phenotype by preventing NF-κB pathway activation induced by ischemia,thus alleviating the hepatic I/R-induced inflammatory cascade response and improving the recovery of liver function after reperfusion.
During the early stage of the I/R process,excessive ROS production leads to intracellular oxidative stress,causing DNA damage and protein denaturation,eventually resulting in cell death and tissue damage [20].ROS can also act as a second messenger to regulate immunity and activate liver resident macrophages(Kupffer cells) to differentiate toward their pro-inflammatory (M1)phenotype [21].At the same time,due to massive cellular injury and destruction,various necrotic cell-released damage-associated molecular patterns (DAMPs) are present inside the cells;thus,a severe inflammatory response develops [22,23].Nevertheless,the massive inflammatory cell infiltration and accumulation of proinflammatory cytokines cause extra damage to survived hepatocytes,which in turn result in a more severe inflammatory cascade response [24,25].This study detected the activities exhibited by SOD,MDA,and GSH along with the fluorescence intensity of DHE.SOD and GSH-Px are antioxidant defense enzymes,which are also known as ROS scavengers.Combining these results,we found that polydatin was effective in reducing ROS production and alleviating oxidative stress in the liver during hepatic I/R.Furthermore,the pro-inflammatory cytokine (TNF-αand IL-1β) levels were decreased by polydatin pre-administration.Thus,inhibition of ROS by polydatin may be responsible for the attenuated inflammatory responses in the liver.
Aseptic inflammation after I/R leads to more severe tissue damage,and macrophages are the primary factor for the initiation and regression of this inflammatory response [24].Numerous studies have highlighted the important role played by macrophages in hepatic I/R models [26,27].As a kind of highly plastic immune cell,macrophages mediate inflammatory response initiation and regression during liver I/R [28].In general,macrophages are categorized into two phenotypes,namely,classically activated macrophages(M1 polarization) and alternatively activated macrophages (M2 polarization),which play different roles in different microenvironments [29].Despite many studies that explore the mechanisms of macrophage plasticity,its complex mechanisms are still not fully understood.
Excessive ROS production is the initiator of the inflammatory cascade response in the process of hepatic I/R injury,leading to the recruitment of leukocytes and other immune cells and further liver injury [30].As a natural antioxidant,polydatin greatly inhibits ROS production.ROS has been shown to enhance the phosphorylation of the NF-κB inhibitor IκB,leading to NF-κB pathway activation [31].Obviously,the NF-κB signaling pathway participates in pro-inflammatory cytokine transcription and pro-inflammatory macrophage polarization [32].Zhao et al.reported that mesenchymal stromal cell-derived exosomes weaken myocardial I/R injury by modulating the macrophage phenotype by targeting the NF-κB pathway [17].After the NF-κB pathway is activated,macrophages polarize toward the M1 phenotype,promoting pro-inflammatory cytokine secretion,such as TNF-αand IL-1β,and participate in aggravating I/R injury [33].Therefore,NF-κB signaling pathway inhibition has been shown to weaken inflammation by inhibiting the polarization of macrophages toward the M1 phenotype.Our study discovered that polydatin effectively reduced macrophage polarization toward the M1 phenotype bothinvitroandinvivo.We have good reasons to believe that this effect of polydatin is achieved by reducing ROS production and inhibiting NF-κB pathway activation.Our study further suggests that polydatin can target ROS production and inhibit NF-κB pathway activation,thereby regulating macrophage polarization.
In conclusion,our study showed that polydatin attenuated hepatic I/R injury by suppressing the inflammatory phenotype polarization regarding macrophages via inhibiting NF-κB signaling pathway.
Acknowledgments
None.
CRediT authorship contribution statement
Hai-Li Bao:Conceptualization,Data curation,Formal analysis,Writing– original draft.Chuan-Zhi Chen:Data curation,Formal analysis,Writing– original draft.Chang-Zhen Ren:Data curation,Software,Writing– review &editing.Ke-Yan Sun:Data curation,Software,Writing– review &editing.Hao Liu:Data curation,Methodology,Writing– review &editing.Shao-Hua Song:Super vision,Writing– review &editing.Zhi-Ren Fu:Funding acquisition,Supervision,Writing– review &editing.
Funding
This study was supported by grants from the National Natural Science Foundation of China (No.81970563) and the Medical Health Science and Technology Project of Health Commission of Zhejiang Province (2019RC055).
Ethical approval
All experimental protocols were conducted following the guidelines of the National Institute of Health for the Care and Use of Laboratory Animals and approved by the Scientific Investigation Board of Shanghai Changzheng Hospital (2019071106).
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Recent advances in promising drugs for primary prevention of gastroesophageal variceal bleeding with cirrhotic portal hypertension
- Stereotactic body radiotherapy in pancreatic adenocarcinoma
- Application of ultrasonography-elastography score to suspect porto-sinusoidal vascular disease in patients with portal vein thrombosis
- Hypomethylation of glycine dehydrogenase promoter in peripheral blood mononuclear cells is a new diagnostic marker of hepatitis B virus-associated hepatocellular carcinoma
- AGK2 pre-treatment protects against thioacetamide-induced acute liver failure via regulating the MFN2-PERK axis and ferroptosis signaling pathway
- Circulating RNA ZFR promotes hepatocellular carcinoma cell proliferation and epithelial-mesenchymal transition process through miR-624–3p/WEE1 axis
