First report of the histopathological effect of electrocautery using on the urethral taste rosea during glans penis injury by incision in rabbits
2024-02-25OzgurCglrAyhnKntMhmtDumluAyinNzihAkSvilyOzmn
Ozgur Cglr ,Ayhn Knt,Mhmt Dumlu Ayin ,Nzih Ak ,Svily Ozmn
a Ataturk University,Medical Faculty,Department of Pediatric Surgery,Erzurum,Turkey
b Recep Tayyip Erdogan University,Medical Faculty,Department of Neurosurgery,Rize,Turkey
c Ataturk University,Medical Faculty,Department of Neurosurgery,Erzurum,Turkey
d Recep Tayyip Erdogan University,Medical Faculty,Department of Urology,Rize,Turkey
e Ataturk University,Medical Faculty,Department of Pathology,Erzurum,Turkey
KEYWORDS Urethral taste rosea;Taste buds;Pudendal nerve;Degeneration;Penile surgery
Abstract Objective: Currently,electrocautery devices have frequently been used in penile surgical procedures.We hypothesized that electrocautery using during penile surgical procedures may harm the taste rosea and the dorsal nerve of the penis or clitoris.Methods: Eighteen young age male New Zealand rabbits were studied: five in the control(Group I,n=5),five in the penile surgery without using electrocautery (sham group,Group II,n=5),eight in the monopolar cautery(study group,Group III,n=8)groups under general anesthesia.The animals were followed for 3 weeks and sacrificed.Penile tissue-pudendal nerve root complexes and dorsal root ganglion of sacral 3 level were examined using stereological methods.The results were compared statistically.Results: The live and degenerated taste bud-like structures and degenerated neuron densities of pudendal ganglia (mean±standard deviation, n/mm3) were estimated as 198±24/mm3,4±1/mm3,and 5±1/mm3 in Group I;8±3/mm3,174±21/mm3,and 24±7/mm3 in Group II;and 21±5/mm3,137±14/mm3,and 95±12/mm3 in Group III,respectively.Neurodegeneration of taste buds and pudendal ganglia was significantly different between groups.Conclusion: Intact spinal cord and normal parasympathetic and thoracolumbar sympathetic networks are crucial for human sexual function.The present study indicates that the glans penis injury by using electrocautery may lead to pudendal ganglia degeneration.Iatrogenic damage to taste rosea and retrograde degeneration of the pudendal nerve may be the cause of sexual dysfunction responsible mechanism.
1.Introduction
Hemostasis is of utmost importance in glans penile surgery.Since its introduction in the early 20th century,electrocautery has been urological surgery to achieve hemostasis and facilitate dissection.It also has been used in glans penile procedures,and an electric current may disperse to surrounding tissue during using this procedure [1].Besides,unintended effects such as iatrogenic injuries of urethral mucosa may occur.No doubt erectile dysfunction(ED)has a significant impact on the quality of life of men and their partners [2].Its overall prevalence is not low.It has been assumed that it affects nearly 100 million men worldwide [2].
Control of the lower urinary and reproductive tract is maintained by the autonomic nervous system.The apex of penile tissue is innervated by the pelvic nerves.The hypogastric and pudendal nerves provide the sympathetic fibers and the parasympathetic innervation,consecutively.The parasympathetic innervation of penile organs comes from the sacral spinal parasympathetic center [3].Aydın et al.[4]reported a new structure named taste bud-like structure in the male urethra.Erectile and ejaculatory dysfunctions may occur following spinal cord injury [5].During urogenital surgeries,these penile nerves and pelvic ganglia may be injured.In addition,in urogenital surgeries,monopolar cautery and bipolar cautery have been used.In benign prostatic hyperplasia,the electrocautery-based transurethral resection of the prostate is an established gold standard treatment,but transurethral resection of the prostate is associated with several complications and side effects [6].Therefore,there is an increasing interest in several emerging minimally invasive therapies as alternative treatment options [6].
We should know some neuron-related medical hypotheses.
(a) Both anterograde and retrograde transport occur in the neuron [7].Especially,retrograde transport is thought to play a critical role in neuronal survival and maintenance [8].
(b) An abundant and continuous supply of oxygen is an important issue for the proper functioning of neurons [9].
(c) The pelvic ganglia provide autonomic innervation to urogenital organs.The pudendal ganglion is a dorsal root ganglion (DRG).Severe spinal cord ischemia causing neurodegeneration in the DRG via vasospasm of DRG-supplying arteries has been reported [10,11].DRG neurons show the same characteristics of retrograde signaling as do sympathetic neurons.The pudendal motoneurons are located in the Onuf’s nucleus(ON),which plays an important role in urinary and sexual function[12].These neurons are susceptible to injury during surgery of this area,and yet their regenerative mechanisms are not well-known.An injury during glans penis injury by incision surgery may affect the anatomic structure of the penis.Cavernous nerve damage after radical prostatectomy may occur and be accepted as a neurogenic cause of ED.
Sexual dysfunction may be seen in aging men with lower urinary tract symptoms [13].The underlying mechanisms for the relationship between lower urinary tract symptoms and ED have not been fully elucidated [13],and current treatments for ED are not effective.Several new management tools such as neuromodulation devices have been used.These devices are known to act to normalize or modulate nerve activity through the targeted delivery of electrical stimuli.In case sacral neuromodulation has failed,pudendal neuromodulation may be used,but there is much to learn about the mechanisms of retrograde degeneration of the pudendal nerve and ganglia during neuromodulation procedures.
There is a fine coordination between the urinary bladder and the urethral sphincter.The normal function of the lower urinary tract is required.ED is one of the most common sexual disorders in men.Phosphodiesterase (PDE) inhibitors are commonly used to treat ED [14].However,an intact nitric oxide supply from the nerves and endothelium is required to ensure the efficacy of PDE5 inhibitors.Denervation of the cavernous nerve due to pelvic surgeries,or disease such as diabetes mellitus will likely reduce its effectiveness.Therefore,the efficacy of PDE5 inhibitors in these patients may not be as high as in the general population of ED patients.The present study will also add a new dimension to understanding the importance of urethral taste-bud-like structure-related sexual dysfunctions.In this study,we hypothesized that the cautery used in penile surgery animal model could disrupt the network between the pudendal nerve and taste roseas of the urethra.There have been major changes in the medical practice in the last decades,but this subject has not previously been researched.We aimed to investigate the effect of electrocautery usage on the network between the pudendal nerve and taste roseas of the urethra during penile incisions.
2.Materials and methods
Eighteen male New Zealand rabbits were studied.Animals were approximately 1.5 years old and weighed 3.3 (standard deviation: 0.6) kg.The experiment was approved by the ethical committee of Ataturk University(17.09.2018/10).Each group was housed within stainless cages (3-4 animals per cage) under standard conditions including room condition (12-hour light/dark cycles,temperature 23±2°C,light intensity 350-400 lux,and humidity 30%-60%),and free access to water and food.Animals were housed under controlled environmental conditions.Animals were divided into three groups:five in the control(Group I,n=5),five in the penile surgery without using electrocautery (sham group,Group II,n=5),and eight in the monopolar cautery (study group,Group III,n=8).The penile organ was disinfected with a povidone-iodine solution.An anteroposterior midline incision was made in the glans.A urethral catheter was inserted in the urethra of rabbits.This procedure was performed without using electrocautery in the Group II,but cautery was used in the Group III.The cautery energy level was preferred as 15 W,lasting 5 s on an approximately 2 mm area of the ventral side and dorsal side of the apex of the penile shaft,as in the study of Gunal et al.[1].The cautery was applied after the penile incision.Afterward,the incision was closed with Vicryl (7/0) using the continuous suture technique.In the postoperative period,the antibiotic and analgesic regimen was given.The animals were kept for 3 weeks after surgery in cages in a standard laboratory room.The animals were anesthetized via the subcutaneous injection of a mixture of ketamine hydrochloride (25 mg/kg),lidocaine hydrochloride(15 mg/kg),and acepromazine(1 mg/kg).Glans penile incision was done with surgical scissors and monopolar cautery (20 W/400 kHz,Petas-Petkot 600 [Petas,Ankara,Turkey]) following surgical region sterilization with local antiseptics.After general anesthesia,all animals were then sacrificed.Exposure of the pudendal nerves was accomplished by dividing the gluteus maximus and medius muscles.Then sacral 1 (S1) level to sacral 3 (S3) level laminectomy was done,and pudendal ganglia were extracted at the sacral 2 (S2) level of spinal cord.Besides,the margins of the incised ventral urethra were dissected from the tunica and were removed with the neurovascular compartments.All extracted tissue was embedded in paraffin blocks after fixated in a 10% formalin solution for 1 week.Blocks were cut into five-micrometer sections and stained with hematoxylin and eosin and TUNEL method.Preparations were examined under a microscope at 4×and 40× magnification.Primary antibodies (Jackson Immunoresearch Laboratories Cambridgeshire,CB7 4EX,UK) were rabbit anti-gustducin at 1:1000,mouse anti-NCAMat 1:200,and rabbit anti-PGP 9.5 at 1:1000,all of which were diluted in blocking buffer as in the study of Landin et al.[15].After allowing primary antibody binding for 1 h,taste buds were washed in PBS and then were incubated with secondary antibody (donkey anti-rabbit Cy3 at 1:650) for 1 h [15].After extensive rinsing,the cultured taste buds or epithelia were mounted in Fluoromount-G (Thermo Fisher Scientific,Waltham,MA,USA)[15].The degree of the degeneration of taste roseas and pudendal nerve ganglia were analyzed.Pathological examinations were done using a light microscope (Nikon Co.,Tokyo,Japan) by a pathologist (Ozmen S).To estimate the density of the neurons in pudendal ganglia and penile taste roseas,the stereological method was used according to the study of Aydın et al.[4],and taste rosea estimation method was also done in the study of Aydin et al.[16].Statistical analysis was undertaken using the one-way ANOVA test in SPSS 20.0 for Windows (SPSS Inc.,Chicago,IL,USA).Ap-value of <0.05 was considered statistically significant.
3.Results
3.1.Histopathological results
Fig.1A showed sacral spinal cord including ON;in Fig.1B,S2 DRG with normal and degenerated ganglion cells and S2 nerve root were seen;S2 DRG with deformed ganglion cell,nerve root,and spinal cord of an animal in whom electrocautery was applied during penile incision could be seen in Fig.1C.In Fig.2A,histopathological view of urethral taste buds or normal taste bud-like structures named as taste roseas was seen;Fig.2B showed the intraluminal scattered located taste rosea;Fig.2C showed the DTR after application of electrocautery during penile incision in an animal of the Group III.
3.2.Numerical results
The mean live and degenerated taste bud-like structures and degenerated neuron densities of pudendal ganglia(mean±standard deviation,n/mm3) were estimated as 198±24/mm3,4±1/mm3,and 5±1/mm3in Group I;8±3/mm3,174±21/mm3,and 24±7/mm3in Group II;21±5/mm3,137±14/mm3,and 95±12/mm3in Group III,respectively.Neurodegeneration of taste buds and pudendal ganglia was significantly different between Group I and Group II(p<0.005);Group II and Group III (p<0.0005);Group I and Group III(p<0.000 01).
Table 1 shows that the number of live taste bud-like structures (n/mm3) were significantly decreased also in the sham group with no additional damage in the electrocautery group;the number of degenerated taste bud-like structures(n/mm3) was also increased in the sham group with no additional damage in the electrocautery group;the number of degenerated neuron densities of pudendal ganglia was the only parameter that was increased in electrocautery group compared to both controls and the sham group.

Table 1 The values of taste bud-like structures (n/mm3).
3.3.Statistical results
The degenerated neuron numbers of each group were compared.The differences were compared between control and surgical penile incision (p<0.005),electrocautery and without electrocautery surgical incision (p<0.0005),and the control and electrocautery group (p<0.000 01).
4.Discussion
This study investigated the effect of penile surgery with and without electrocautery on taste bud-like structures and pudendal ganglia in a rabbit model,and showed that using electrocautery during surgery of the apex of the penile organ led to the degeneration of urethral taste roseas and pudendal nerve ganglia.An increased number of degenerated neurons in pudendal ganglia after penile surgery with the use of electrocautery compared with control animals were observed.

Figure 1 Histopathology of the effect of electrocautery.(A) Sacral spinal cord including ON (LM,GFAP,4×);(B) S2 DRG with normal and degenerated ganglion cell and S2 NR (LM,H &E,4×);(C) S2 DRG with deformed neurons,NR,and spinal cord of an animal in whom electrocautery was applied during penile incision (LM,GFAP,4x).ON,Onuf’s nucleus;GFAP,glial fibrillary acidic protein;LM,light microscopy;DRG,dorsal root ganglion;NR,nerve root;H&E,hematoxylin and eosin;S2,sacral 2;SC,spinal cord.
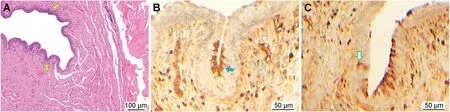
Figure 2 Changes of histopathologic view of urethral taste roseas.(A) Urethral taste buds or normal taste bud-like structures named as taste roseas(yellow arrows,LM,H&E,10×);(B)Intraluminal scattered located taste rosea(blue arrow,LM,gustducin,40×);(C) the DTR after application of electrocautery during penile incision in an animal of the study group (green arrow,LM,gustducin,40×).DTR,degenerated taste rosea;LM,light microscopy;H &E,hematoxylin and eosin.
4.1.Electrocautery and urologic surgery
Using electrocautery during the glans penile procedure may be a cause of ED after urological surgeries even if the surgery is performed with the nerve-sparing surgical technique.In these surgeries,different energy sources have been used.In classical cautery,the electric circuit can penetrate deeper tissue and damage the glans penile tissue.The energy source is generally delivered transurethrally;during this procedure,pudendal nerve or ganglia,later the taste roseas of the urethra can be damaged.It should be noted that transurethral prostate and bladder surgery may lead to iatrogenic injury of the ureter and its orifice.Urethral stricture may occur after penis surgical procedures.As a result,many men may confront sexual complications after urological surgeries.In this study,pudendal ganglia damage has been observed after penile incision.Our findings support that ED could be secondary to pudendal nerve degeneration or disruption of orchestral equilibrium.If we consider the nervous system as a great orchestra that can express a complete range of rhythms and melodies and the most complex harmonic combinations[17],we will find it easier to understand how any lesion will be translated into an alteration of the rhythmic systems in the urogenital tract.
4.2.What is already known on this subject?
The urogenital system is innervated by the sacral parasympathetic,thoracolumbar sympathetic,and somatosensation nerves.The pudendal nerve carries motor,sensory,and autonomic fibers,arising from the ventral rami of the sacral spinal nerves S2 to sacral 4 (S4) [18].The dorsal nerve of the penis is the terminal branch of the pudendal nerve.This nerve includes afferent fibers that innervate the penile skin,including the glans penis [19].This nerve as an afferent nerve transmits signals to the central nervous system[19].The pudendal nerve is involved in penile and clitoral erection and ejaculation in males[18].ON is an important structure in the sacral spinal cord which was described in 1899 by Onufrowicz [20].Neuronal morphology and functions of ON-connected lumbosacral plexus nearly resemble anatomical and functional features in both rabbits and humans.In this center,ON is an important component.For that reason,sacral parasympathetic and thoracolumbar sympathetic network disruption by penile surgery may result in orgasmic dysfunction.The human sexual function requires an intact spinal cord [21]and also intact sacral parasympathetic and thoracolumbar sympathetic networks.
4.3.What does this study add to current knowledge?
In medical practice,understanding the mechanism of complications is crucial to developing preventative measures [12].We aimed to investigate the effect of electrocautery knives during the apex of the penile surgery procedure on the taste roseas and pudendal nerve.Our result is not surprising because intraurethral stimulation at 2.5 Hz may activate the hypogastric and pudendal nerves[22].It has been previously reported that electrocauterization also produced neurodegeneration in the urethral sphincter of rats [23].In the present paper,we used 20 W/400 kHz.The urethra ends as a urinary meatus at the tip of the penile organ;we think that urethral injury by electrocautery could be occurred by penile surgical procedures.According to this present pilot study,monopolar cautery in penile surgery may be omitted because of pudendal nerve damage.The dorsal nerve of the penis may be damaged during penile cauterization.Finally,it was found significantly different neurodegeneration of taste buds and pudendal ganglia in the electro-cautery used and unused group (p<0.000 01).The subject of the effect of lower urinary tract surgery on erectile function is of interest.In this study,taste roseas can be degenerated by incision-related injury;the disconnection between glans and the dorsal nerve of the penis has likely be occurred,and resulted in the degeneration of pudendal nerve ganglia.
4.4.What can we learn from this study?
In this study,an experimental procedure was conducted on the 1.5 years old young rabbits.The average life expectancy of a laboratory rabbit is about 8-12 years [24],whereas the life expectancy of humans globally is about 70 years.Approximately 1.5 years old rabbits in this study are equal to 10 years old humans.Rabbits attain sexual maturity approximately at 1 year of age and the average age of this period in humans is about 11.5 years [24].We hypothesized that damaging taste rosea by using electrocautery during penile surgery may be responsible for this event.Etiological speculations could be better explained by an experimental animal model [25].This study indicates considering the potential for iatrogenic damage to taste rosea and retrograde degeneration of the pudendal nerve in surgical procedures involving the penile organ.According to the finding of the present study,the post-procedure sexual dysfunction following penile incision may be related to pudendal ganglia or nerve injury by heat generated during the procedure.
A comprehensive understanding of the pathophysiology is an important issue in medical practice [26,27].Our findings can be explained by the balance between cell proliferation and cell death which is crucial in all tissue,particularly in the nervous system [28].The neurodegeneration by using electrocautery during penile surgery led to changes in pudendal ganglia neurons.If hedonic sensations are regulated by a network between taste bud-like structure and pudendal nerve,the insults that disrupt this network following penile organ surgical procedures may be responsible for sexual dysfunctions and sexual anhedonia.If this theory takes place,orgasmic pleasure,sexual pathologies,infertility,and all aspects of marriage,causes,and treatment methods will be reconsidered according to previous penile surgery.
4.5.Limitations
Despite extensive research in this area,no other group has yet concluded that there are taste buds in the urethra.For that reason,this study should be done again with more advanced techniques and methods.In addition,this study does not represent the human model,and the biomechanical,molecular,and cellular changes after penile surgery can be seen only in experimental animal studies.We did not evaluate intrinsic or extrinsic sphincters.Intrinsic sphincter deficiency can occur by aging,also be acquired secondary to prior incontinence surgery,urethral trauma,or sacral spinal cord injury [23].Given rabbit is the study subject with no parameter of erectile function such as intracorporeal pressure,our conclusion might be overstated and merely speculated to human beings.Another limitation is about the taste roseas in the male urethra.Currently,no genito-urethral reconstructive surgeries are referable and implicated in this subject.The sample size may be not adequate.As can be seen from the result,some changes have been observed in the sham group,and the difference was statistically significant (p<0.05).It is necessary to confirm the finding of this study in human being.
5.Conclusion
The only proven effect of electrocautery is the increased degeneration of the pudendal ganglia.Electrocauterization applied during penile surgery is hazardous to pudendal ganglia neurons and should not be applied unless required.A better understanding of the pathophysiological processes in the development of electro-cautery-induced pudendal nerve damage or degeneration would be beneficial for the management of patients with ED.More studies are required in this subject.
Author contributions
Study concept and design: Mehmet Dumlu Aydin.Data acquisition: Ozgur Caglar.
Data analysis: Sevilay Ozmen.
Drafting of manuscript: Ayhan Kanat.
Critical revision of the manuscript: Nezih Akca.
Conflicts of interest
The authors declare no conflict of interest.
杂志排行
Asian Journal of Urology的其它文章
- Primary unifocal penile follicular center non-Hodgkin lymphoma:Report of a rare case and review of the literature
- Efficacy of tadalafil on improvement of men with erectile dysfunction caused by COVID-19:A randomized placebo-controlled trial
- Robotic-assisted retroperitoneal lymph node dissection for stage II testicular cancer
- Percutaneous antegrade management of large proximal ureteral stones using non-papillary puncture
- Predictive factors for percutaneous nephrolithotomy bleeding risks
- Can we predict the incidence of high-grade Clavien-Dindo complications in patients with forgotten encrusted stents undergoing endourologic management?
